Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793versión On-line ISSN 1851-8796
Lat. Am. appl. res. vol.43 no.4 Bahía Blanca oct./dic. 2013
Copolymerization of Styrene and n-Butyl Acrylate with Itaconic Acid: Influence OF Carboxylic Groups Distribution on Performance of Decorative Paints
M.P. Oliveira and C.R. Silva
BASF S.A. - Research and Development, Guaratinguetá, São Paulo - Brazi. mauricio.oliveira@basf.com
Abstract— The main aim of this study is to understand the influence of itaconic acid (IA) on the colloidal properties of the latexes made by semi-batch emulsion copolymerization of styrene (St) with n-butyl acrylate (BA). A series of copolymerization were carried out with different IA concentrations to produce latexes for decorative paints. The effect of functional monomer on the conversion, colloidal properties of the latexes and the distribution of the carboxylic groups (buried, particle surface and serum) were investigated. The behavior of IA on wet scrub resistance of highly pigmented paints for architectural coatings was examined. The results obtained showed that the number of particles and polymerization rate both decreased with increase of the amount of IA. The properties of decorative paints are greatly dependent on the amount and carboxylic acid distribution. The carboxylic acid distributed in the aqueous phase has a strong influence on the characteristics of the final products.
Keywords— Emulsion Copolymerization; Carboxylic Acid; Itaconic Acid; Scrub Resistance; Paints.
I. INTRODUCTION
Industrial emulsion polymerization formulations frequently involve monomers that are relatively water insoluble, such as styrene (St), n-butyl acrylate (BA), 2-ethylhexyl acrylate (2-EHA), and a small amount of carboxylic monomers such as acrylic (AA), methacrylic (MAA), itaconic (IA) and fumaric (FA) acids (Oliveira et al., 2006; Wildeson et al., 2008). These carboxylic monomers are typically incorporated in formulations of emulsion polymerization to improve the colloidal stability of the latexes during and after the production when compared with latexes stabilized only by physically adsorbed soaps. The enhanced colloidal stability and freeze-thaw stability result from the presence of carboxylic groups on the outer surface of the latex particles, providing both steric and electrostatic stabilizations (Vorwerg and Gilbert, 2000; Fritz et al., 2002). These properties justify the use of small amounts of the carboxylic acids in industrial formulations used essentially as bonding agents in paper coatings, carpet backing, adhesives, architectural paints and for adjusting the latex viscosity by varying the degree of neutralization (Oliveira et al., 2006; Wildeson et al., 2008; Kumthekar and Kolekar 2011). A significant generic problem for carboxylated latexes is the polymerization of the carboxylic monomers in the aqueous phase. Some of the factors that influence the final distribution of carboxylic groups in latex products, such as partitioning of carboxylic monomers between the organic phase and the continuous phase (water), the reactivity ratios of the monomers, the pH of the polymerization, the degree of dissociation of the functional monomer and the amount of the functional monomer (Santos et al., 1997; Slawinski et al., 2000; Oliveira et al., 2006; Kumthekar and Kolekar 2011).
A few fundamental studies have been conducted on the behavior of IA in emulsion polymerization (Vijayendran, 1979; Lock et al., 1991; Václavová et al., 1993; Oliveira et al., 2006; Kumthekar and Kolekar 2011). Oliveira et al., 2006 demonstrated that the kinetics of the emulsion polymerization of methyl methacrylate with n-butyl acrylate in the presence of IA was slower than the copolymerization without IA and a small fraction of this acid was found buried inside the particles. The behaviors of IA in the emulsion polymerization have been investigated including both the application performance and particle growth (Wildeson et al., 2008; Václavová et al., 1993; Lock et al., 1991).
One of the most important ingredients in decorative paints is the latex, which is responsible for forming the paint films, scrub resistance of the dry paint film and many of it is final properties. For this reason, styrene-acrylic latexes are widely used for interior and exterior paints due to good durability, but the scrub resistance results of the paint film depends on the method, type, distribution and amount of IA employed to prepare the latexes (Wildeson et al., 2008; Khorassani et al., 2009; Kumthekar and Kolekar 2011). These carboxylic groups anchored to the latex particle can interact directly with the pigment particle and an additional steric barrier can be created that can further prevent pigment agglomeration. The ionic strength of the extent of carboxylation on the latex particle surface changes the way the latex particles consolidate with themselves and with the particles in the paint formulation. Wildeson et al. (2008) investigated the influence of IA and degree of neutralization on wet scrub resistance. They showed that the degree of neutralization before the emulsion copolymerization greatly influences the ability of the latexes to prevent titanium dioxide particle aggregation. Kumthekar and Kolekar (2011) verified that the latex having higher amount of free acid (IA) in the aqueous phase had better pigment dispersing ability than the latex which had less free acid in the aqueous phase.
The main objective of this study was to determine the optimal conditions regarding IA concentration and wet scrub resistance to produce decorative paints. In the first part of this article, the effects of the amount and carboxylic acid distribution was presented. The objective was to understand the effects of IA concentrations on the overall conversion, on the colloidal properties of the latexes, as well as on the distribution of the carboxylic groups to produce latex with the minimum amount of carboxylic acid in the water phase. In the second part, the objective was to understand the effect of IA distribution and different types of dispersing agents to produce highly pigmented paints.
II. MATERIALS AND METHODS
A. Materials
Industrial grade-inhibited monomers, St and BA, were received from BASF and used throughout the reactions. Itaconic acid (>99.0%) was used as received. Analytical grade potassium persulfate (99.0%,Merk) was purified by recrystallization before use. The emulsifier, sodium lauryl sulfate (SLS) and modified ethoxylated fatty alcohols (Disponil® A 3065, BASF) were used as received. All polymerizations and analysis of carboxylic groups concentration were carried out with deionized water. The latex samples were cleaned using a mixed-bed ion-exchange resin (Dowex-MR3,Dow). Decorative paints were formulated using industrial grade products.
B. Synthesis of the latexes
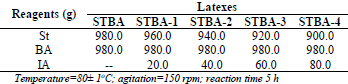
The latexes were prepared by semi-batch emulsion copolymerization process employing a 5L glass reactor equipped with a mechanical stirrer and a reflux condenser, under nitrogen atmosphere. The fixed part of the formulation contained water (1000.0g) and SLS (12.0g) were charged into reactor under vigorously stirring at 80°C for 15 min. After the pre-emulsion containing water (636.6g), SLS (21.6g), Disponil® A 3065 (13.0g) and the variable part of the pre-emulsion St, BA and IA (Tab. 1) were added at a constant feed rate over a period of 4h. Potassium persulfate solution (303.8g at 1.25% w/w) was added at a constant feed rate over a period of 5 h. The temperature of the reaction medium in all the polymerizations was carried out at 80±1°C at 50% solid content. The variable parts of the formulation considered in this work are reported in Tab. 1. The solid content and monomer conversion at each time were measured by gravimetric analysis on the samples collected from the reaction mixture. The reaction was stopped immediately after sampling by using a 1% w/w hydroquinone solution in combination with an ice-water bath. The contents were dried at 105°C in an oven to attain a constant weight (about 2h). The conversion was calculated according to Eq. 1:
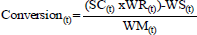 | (1) |
Table 1. Experimental conditions and variable emulsion polymerization recipe
where SC(t), WR(t), WS(t) and WM(t) are solids content, weight of the reactor contents, weight of the solids (emulsifier, initiator) and weight of the monomers in the reactor at time (t) respectively. After monomer conversion analyses the unreacted monomers were removed at 50°C with 10 wt% aqueous solution of t-butyl hydro peroxide (28.0g) and 10 wt% aqueous solution of sodium formaldehyde sulfoxylate (25.0g). The reaction mixture was cooled and characterized.
C. Paint formulation
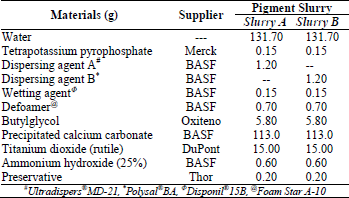
Decorative paints were formulated using a standard pigment slurry formulation (Tab. 2) with TiO2 as pigment and precipitated calcium carbonate as filler. To prepare a white base, both pigment and filler were dispersed using a typical high-speed disperser (700 rpm) having a double bottom container. Paints were formulated with two different polymer dispersing agents: sodium salt of a copolymer of diisobutene and maleic acid (Ultradispers® MD-21) and poly(acrylic acid) sodium salt (Polysal® BA). To understand the effect of the amount and carboxylic acid distribution on wet scrub resistance eight decorative paints were prepared with a pigment volume concentration (PVC) of 80.0%. The latex was added to the pigment slurry followed by viscosity adjustment using an associative thickener. The combinations for each formulation are shown in Tab. 2 and 3.
Table 2. Pigment slurry formulations used in this study.
Tab. 3 Decorative paint formulations used in this study

D. Characterization
The latexes were characterized in terms of average particle diameter by the light scattering technique, using the Mastersizer 2000G instrument (Malvern) at 25°C. The number of polymer particles was calculated through conversion and average particle diameter data. The latex viscosity was measured in a Brookfield viscosimeter at 25°C (method LVT with spindle number 1 at 60 rpm). The pH was measured in a Metrohm pH meter at 25°C. The amount of coagulum after the polymerizations was measured by screening out the coagulum using a stainless steel screen (125 mesh) and calculated by means of gravimetric analyses. The freeze-thaw stability was determined by placing the latex in a freezer at -18°C for 17 h and then removing it and leaving it at room temperature for another 17 h to complete one freeze-thaw cycle. Then whether the emulsion gelled or not was checked.
Acid group distribution in the latexes products was measured by combination of two methods: conducto-metry to evaluate the number of carboxylic groups on the surface of the latex particles and titration with NaOH in methanol solution to determine the number of carboxylic groups buried and on the surface of the latex particles (Santos et al., 1997, Slawinski et al., 2000, Oliveira et al., 2006). The carboxylic groups located buried in the particles could be computed by the difference between the conductometric and potentio-metric titrations. The concentration of carboxylic groups in the serum was determined by conductometric titration of the serum obtained after centrifugation and by means of a mass balance, i.e., deduced from the initial amount of carboxylic monomer introduced and the determined amounts of carboxylic groups buried and at the surface of the latex particles.
Water absorption tests were carried out according to ASTM D570-8 (ASTM, 1998). The latex films were prepared by casting and making the water evaporate slowly at room temperature for 7 days. For these tests, ten samples(2.0 x 2.0 x 0.10) cm were extracted from the latexes film, immersed for 8.0 hours in deionized water at room temperature and then taken out. The excess water at their surface was gently removed and the samples were weighed. Wet scrub resistance of the paints was evaluated quantitatively in according to ABNT-NBR 15078 (ABNT, 2006). The paints were coated on Leneta Co. foil with a dry film thickness of 175±5µm. The sample was allowed to dry for 7 days at 23°C and 55-65% relative humidity. After drying, the test was performed, since during the test a soap solution drops onto the coating. The final scrub resistance was reached when the first spot of Leneta foil appeared indicating that the paint layer had been removed and so a higher number of cycles show a more scrub-resistant paint. Three panels were tested for each paints and the average was rated.
III. RESULTS AND DISCUSION
A. Overall conversion and particle number
To better understand the influence of IA on semi-batch emulsion copolymerization of St with BA a series of experiments at different IA concentration were prepared. The effect of IA concentration on the overall conversion-time curves for the semi-batch emulsion polymerization of St with BA can be clearly seen from the Fig. 1a. The presence of IA had a negative influence on the polymerization rate as well as the copolymer conversion. The polymerizations performed by increasing the carboxylic monomer concentration from 0.5% to 2.0% show a successively stronger reduction in the polymerization rate (Fig. 1a).

Fig. 1. Overall conversion (A) and particles number (B) vs time for the semi-batch emulsion copolymerizations of St and BA in the presence of different IA concentrations.
In this study, a water soluble initiator was used to form radicals and these radicals react with monomer dissolved in the aqueous phase to form soluble oligomeric radicals. These oligomeric radicals grow up to a critical chain length and generate precursor particles. These particles are unstable due to colloidal aspects and a small portion of these particles have to coagulate to form the primary particles. The use of carboxylic monomers with high solubility in water normally leads to the formation of water soluble polymer and the polarity of radical formed in the core of the particles by a chain transfer mechanism can cause their migration to the aqueous phase, resulting in the reduction of the average number of radicals per particle, and consequently, in the reduction of the overall rate of polymerization (Bajaj et al., 1994). In the case of IA, it is proposed that a radical of IA form a sterically hindered propagating radical and that this radical is unlikely to react with another IA molecule. It is postulated that a radical of IA can terminate the propagation step in a homopolymerization reaction (Václavová et al., 1993).
The effect of IA on the rate of copolymerization has been discussed in the literature (Ceska, 1974, Lock et al., 1991; Václavová et al., 1993; Oliveira et al., 2006). Václavová et al. (1993) demonstrated that the IA has a negative influence on the rates of the copolymerization of BA/IA and St/IA. According to those authors, this influence increased significantly with increasing the amount of IA in the monomer feed. A similar behavior was observed by Ceska (1974) in the St/butadiene copolymerization performed in the presence of IA. Oliveira et al. (2006) demonstrated that the kinetics of the copolymerization of MMA/BA with IA was slower than the copolymerization without IA.
The results presented in Fig. 1a are in good agreement with the particles number evolution during the polymerizations, as shown in Fig. 1b. The major effect of IA is to decrease the number of particles nucleated during the polymerization with increasing IA content in the monomer feed. During the course of polymerization was possible to observe that all polymerizations conducted with IA showed lower particle number when compared to the polymerization without IA (STBA). This decrease can be attributed by formation of oligomeric radicals in the water phase with increasing IA content in the monomer feed. This behavior can be attributed to a problem in the nucleation of the polymer particles and coagulation problems of polymer particles by some destabilization process could destabilize the latex, such as the polymerization of STBA-4 with 2.0% of IA. The low reactivity ratio of IA during the polymerization with St/BA (rSt=0.301; rIA=0.201 and rBA=1.10; rIA=0.23) (Lock et al., 1991; Fordyce and Ham, 1947) and also the solubility of IA in the water phase leads to the formation of polymer particles in the aqueous phase by homogeneous nucleation and as a consequence, higher particle size was obtained (Tab. 4). The decrease in the particle number from the earliest stage of polymerization was also observed by Lock et al., 1991 in the copolymerization of methyl acrylate with BA using different concentrations of IA. Václavová et al., 1993 have showed that the increase on the amount of IA in the monomer feed has no effect on particle number in the copolymerization of St/IA, but during the copolymerization of BA/IA an increased concentration of IA led to a decreasing number of particles. According to the authors these results could be explained by the mechanism in which the nucleation of particles occurs. On the other hand, a different behavior was observed by Oliveira et al. (2006) in the polymerization of MMA/BA with IA and Ceska (1974) in the copolymerization of St/butadiene carried out in the presence of IA.
Tab. 4. Physical properties of the final latexes

The physical properties of the final latexes obtained in this study are listed in Tab. 4. The polymerizations performed by increasing the carboxylic monomer concentration from 0.5% to 2.0% show a reduction in the pH during the polymerization (Tab. 4). The results showed that the pH values for the latexes that contained IA ranged from 4.0 to 3.0, whereas that for the latex made without IA was 5.7. This behavior can be explained by the decomposition of the initiator and IA in the water phase. The lowest values of pH measured in this reaction could be an evidence of the presence of residual IA in the end of the polymerization. The pH is important since the rate of consumption of the IA depends on the pH of the reaction medium, and it will define the ionic nature that will, in turn, influence directly the reactivity ratios as well as the Kp of the IA (Santos et al., 1997). The viscosity of the latexes decreased with increased IA concentration at low pH values (Tab. 4). It was also observed that viscosity of the latex is dependent of the incorporation of IA in the polymer latex. The decrease in viscosity is related with an increase of the particle size and an increased probability of an IA reaction with a propagating radical. According to the results showed in Tab. 4, the concentration of coagulum formation increased significantly with increasing IA content in the monomer feed. Increasing the concentration of functional monomer (2.0% w/w) leads to the increase of the coagulum formation. These results showed that IA was not able to produce a stable emulsion, even when its concentration was increased from 0.5% to 2.0% w/w of the monomer.
The freeze-thaw stability of polymer latexes is of considerable industrial importance, because of the need to store and transport these products within cold areas during winter. Figure 2 shows that the most of the latexes made without neutralization coagulated completely after the second cycle, except for the latexes prepared with 0% and 2% w/w of IA (latexes STBA and STBA-4) that coagulated in the first cycle of freeze-thaw.

Fig. 2. Freeze-thaw stability of functionalized latexes (neutralized and non-neutralized).
These results showed that the amount of SLS and nonionic surfactant were insufficient to produce latexes with good freeze-thaw stability. A little change was observed between carboxylated latexes and non-carboxylated latex (STBA). There was no improvement in the latex stability with increasing IA concentration, but the latex stability has been improved when the latexes were neutralized with ammonium hydroxide (Fig. 2). At a low pH (high IA content), the latexes were even
less stable than the latex without IA (latex SBA). The steric stabilization effect is lowered at lower pH because the solubility of water-soluble polymer is less than that at high pH. Increasing the pH leads to an increase in the ionic strength of the latex and contributes to a contraction of the electronic double layer, which has a certain influence on the stability. A similar behavior was observed by Xie et al. (2011) in the MMA/BA copolymerizations performed in the presence of monobutyl itaconate (MBI).
The water absorption of the latex films with different amount of IA can be seen in Fig. 3. It is clear from this figure the more hydrophilic behavior of the latex films with increase of IA content. The water absorption of the latex films prepared with 2.0 wt% of IA was about 17 times more than latex films without IA. The water absorption for STBA-1 (0.5 wt% of IA) was lower than that for STBA-4 (with 2.0 wt%). It can be speculated that the different amount of carboxyl groups distributed in the serum plays a role in the water absorption. The existence of hydrophilic domains inside the film and the capacity of these domains for water absorption was the main factor of the water absorption. The large amount of carboxylic groups on the particle surface and aqueous phase suggests the presence of channels which may favor the penetration of water into the film.

Fig. 3. Water absorption of the latex films at different IA concentrations.
Figure 4 shows the carboxyl group distribution at different phases of the latex. The standard deviation of the measurements was <5%. In accordance with Fig. 4, the IA is differently distributed in the three phases of the system (buried/surface/serum). The acid groups were predominantly located in the aqueous phase and on the particle surface, with only a small proportion buried in the polymer particles. The concentration of carboxyl groups in the serum and buried in the polymer particles increase with the increase of the amount of carboxylic monomers. At a concentration of 0.5% w/w of IA, the final pH was 4.0 (Tab. 4), and the concentration of carboxylic acid buried in the latex particles was less when compared with the fraction of carboxylic groups for the other latexes.
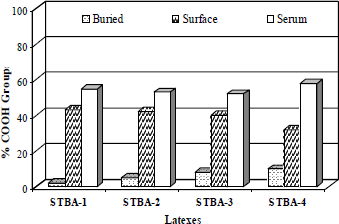
Fig. 4. Carboxyl group distribution (% w/w) at different phases of the latex (buried, surface of particles and serum) prepared from different IA concentration.
In the case of the latex with 2.0% w/w of IA (latex STBA-4), the analysis showed a very large amount of IA in the serum (59% w/w) and on the particle surface (32% w/w). This great amount of IA in the serum can be explained by solubility in water this carboxylic acid and of pH of the reaction medium since it will define the ionic nature of these molecules, what will in turn, influence directly the reactivity ratios. In all experiments with IA, the pH was low, varying between 4.0 and 3.0 (Table 4). In the case of the latex STBA-4, the pH was 3.0 and in this pH range (pH< pKa), suggest that the IA exists in its non-dissociated form. In this form, IA is highly hydrophilic and tends to be found in the water phase, forming hydrosoluble compounds or generating new particles by homogeneous nucleation (Ceska, 1974; Oliveira et al., 2006; Lock et al., 1991). These results are in good agreement with the partition coefficient of IA between organic phase (MMA/BA) and water phase at 70°C obtained by Oliveira et al. (2006). The partition coefficient value obtained was 0,08 at pH 3.0. This value consolidate that IA prefer to be in the aqueous phase than the in the organic phase.
The distribution of carboxylic groups in the final latex products is governed by some of the factors that influence the properties of the final latexes. Understanding the effects of the amount and especially the location of the hydrophilic monomer (IA) in the polymer latex would provide information about the mechanisms of wet scrub resistance of highly pigmented paints. The increase of the amount of carboxylic acid buried in the latex particles can be explained by reactivity ratio differences between St/IA and BA/IA. This difference on reactivity ratio would justify its increase of carboxylic acid buried in the latex particles. The IA copolymerizes with other monomers, i.e., St or BA and tends to make random copolymers. The copolymerization of IA with BA is three to four times more favorable than its homopolymerization and the homopolimerization of BA (Lock et al., 1991). In this case, we could expect that the formation of particles with a hairy-layer-like morphology, depending of course, on the amount of IA used in the monomer feed.
B. Scrub resistance
The influence of IA concentration and dispersing agents on wet scrub resistance of highly pigmented paints are shown in Fig. 5. The results indicate that, generally, the IA gives better wet scrub resistance for lower IA concentrations when compared with high IA concentrations. It can be seen that lower IA monomer contents (0.5-1.0% w/w) resulted in a higher scrub resistance than the higher level (2.0% w/w) for both dispersing agents. A more likely explanation for the differences in the latexes that could account for the abrasion results are the varying hydrophilicities of the latex film. The high concentration of IA in the aqueous phase, possibly present in the form of hydrosoluble compounds, could justify this decrease on the scrub resistance. These water soluble polymers increases with increasing IA content in the monomer feed (Fig. 4), and lead to low wet scrub resistance, which has a negative effect on end-use properties, such as the water resistance of the coating. On the other hand, the presence of IA in to polymer latex give rise to improved wetting properties and is effective in breaking up filler and pigment agglomeration (Wildeson et al., 2008).

Fig. 5. Scrub resistance for different carboxylated latexes and dispersing agents. Dispersing agent A (copolymer of diisobutene/maleic acid) and dispersing agent B (poly(acrylic acid) sodium salt).
The abrasion test was performed under wet conditions, and so the main factors affecting the results will be the pigment binding ability of the latex, the hydrophilicity of the latex and amount and type of carboxylic acid. As described before, the copolymerization of IA with BA is three to four times more favorable than its homopolymerization to form oligomers. In this case, we could expect that the formation of particles with a hairy-layer-like morphology would be expected to be more favorable than the oligomers formed in the aqueous phase to improve the scrub resistance. The results obtained in this study revealed that the amount of IA and especially the carboxylic acid distribution in the water phase were more dominating than the latex particle structure to increase the wet scrub resistance. Kumthekar and Kolekar (2011) showed that the latex having higher amount of free acid in the serum had better dispersing ability than the latex which had less free acid in the serum but latexes with more buried carboxylic acid showed higher scrub resistance.
Finally, the effect of dispersing agent on abrasion resistance was examined (Fig. 5) and the results showed that those paints that contain the dispersing agent A (copolymer of diisobutene/maleic acid) had better scrub resistance than those that contain the dispersing agent B (poly(acrylic acid) sodium salt). The best wet scrub result was obtained using dispersing agent A and latex STBA-1 (paint P1). This result could be attributed to the better interaction with the pigment and filler of the maleic acid groups in pigment dispersant A compared to dispersant B. The dispersing agent is not able to provide complete separation of all pigment and filler particles. As an effect of water-soluble oligomers on the pigment distribution and on scrub resistance were shown by Wildeson et al., (2008); Kumthekar and Kolekar (2011).
According to the results showed in Fig. 5 and considering the carboxylic acid distribution (Fig. 4), we could make the following correlation to explain the scrub resistance results: differences in the hydrophilicity of the polymer film caused by polymerization of IA in the aqueous phase were dominant, paints prepared with latexes with a high amount of polymerizable carboxylic acid are more easily abraded than the particle surface, all paints prepared with hydrophobic dispersing agent (dispersant A) were more resistant on abrasion than the paints prepared with hydrophilic dispersing agent (dispersant B). The influence of the employed dispersing agent is clear and an understanding on properties of the latexes should be realized to produce paints. The combination of latex with low carboxylic acid concentration in the aqueous phase and an effective dispersing agent could be promising for good pigment dispersion and scrub resistance.
IV. CONCLUSIONS
The presence of IA had a negative effect on the overall kinetics of the semi-batch emulsion polymerization of St and BA. The increase of IA concentration from 0.5 to 2.0% leads to the decrease of the polymerization rate and a strong influence on both the nucleation process and the acid incorporation efficiency. The particle number decreased significantly with increasing of IA content during the polymerizations. The amount of IA used in emulsion polymerization affects numerous factors, including particle size, freeze-thaw stability, kinetics, coagulum formation, wet scrub resistance and water sensitivity. There was no improvement in the latex stability with increasing IA concentration, but the latex stability has been improved when the latexes were neutralized.
The IA is distributed differently in the three phases of the emulsion system (particle buried, particle surface and serum). A small fraction of this acid was found buried in the particles, and the remaining was distributed between the particle surface and in higher concentration in the aqueous phase.
Paints prepared with latexes having lower amount of IA in the monomer feed had better scrub resistance than the latex which had higher IA concentration. Latex prepared with a concentration of 0.5% w/w in recipe was found to be the best to prepare decorative paints. The water-soluble polymers present in higher concentration in the aqueous phase led to low wet scrub resistance. All paints prepared with hydrophobic dispersing agent (dispersant A) were more resistant on scrub resistance than the paints prepared with hydrophilic dispersing agent (dispersant B). It may be concluded that to obtain latexes with a high wet scrub resistance, information about the distribution of carboxylic groups, amount of IA in the monomer feed and type of dispersing agent are parameter important to control.
REFERENCES
1. ABNT, NBR 15078, Método para avaliação do desempenho de tintas para edificações não industriais - Determinação da resistência à abrasão úmida sem pasta abrasiva, Rio de Janeiro (2006). [ Links ]
2. ASTM, American Society for Testing and Materials, Norm ASTM D570. Standard test method for water absorption of plastics, Philadelphia (1998). [ Links ]
3. Bajaj, P., M. Goyal and R.B. Chavan, "Synthesis and characterization of methacrylic acid-ethyl acrylate copolymers," J. Appl. Polym. Sci., 53, 1771-1783 (1994). [ Links ]
4. Ceska, G.W., "Carboxyl-stabilized emulsion polymers," J. Appl. Polym. Sci., 18, 2493-2499 (1974). [ Links ]
5. Fordyce, R.G. and G.E. Ham, "Copolymerization II. The mechanism of emulsion copolymerization of styrene and itaconic acid," J. Am. Chem. Soc., 69, 695-696 (1947). [ Links ]
6. Fritz, G., V. Schädler, N. Willenbacher and N.J. Wagner, "Electrosteric stabilization of colloidal dispersions," Langmuir, 18, 6381-6390 (2002). [ Links ]
7. Khorassani, M., F. Afshar-Taromi, M. Mohseni, and S. Pourmahdian, "The role of auxiliary monomers and emulsifiers on wet scrub resistance of various latex paints at different pigment volume concentrations," J. Appl. Polym. Sci., 113, 3264-3268 (2009). [ Links ]
8. Kumthekar, V. and S. Kolekar, "Attributes of the latex emulsion processing and its role in morphology and performance in paints," Prog. Org. Coat., 72, 380-386 (2011). [ Links ]
9. Lock, M.R., M.S. El-Aasser, A. Klein and J.W. Vanderhoff, "Role of itaconic acid in latex particle nucleation," J. Appl. Polym. Sci., 42, 1065-1072 (1991). [ Links ]
10. Oliveira, M.P., D.S. Giordani and A.M. Santos, "The role of itaconic and fumaric acid in the emulsion copolymerization of methyl methacrylate and n-butyl acrylate," Eur. Polym. J., 42, 1196-1205 (2006). [ Links ]
11. Santos, A.M., T.F. McKenna and J. Guillot, "Emulsion copolymerization of styrene and n-butyl acrylate in presence of acrylic and methacrylic acids: Effect of pH on kinetics and carboxyl group distribution," J. Appl. Polym. Sci., 65, 2343-2355 (1997). [ Links ]
12. Slawinski, M., M.A.J. Schellekens, J. Meuldijk, A.M. van Herk and A.L. German, "Seeded emulsion polymerization of styrene: Influence of acrylic acid on the particle growth process," J. Appl. Polym. Sci., 20, 1186-1196 (2000). [ Links ]
13. Václavová, E., A. Hrivík and V. Chrástová, "Emulsion copolymerization of styrene and butyl acrylate with itaconic acid," Makromol. Chem. and Phys., 192, 2243-2250 (1993). [ Links ]
14. Vijayendran, B.R., "Effect of carboxylic monomers on acid distribution in carboxylated polystyrene lattices," J. Appl. Polym. Sci., 23, 893-901 (1979). [ Links ]
15. Vorwerg, L. and R.G. Gilbert, "Electrosteric stabilization with poly(acrylic acid) in emulsion polymerization: Effect on kinetics and secondary particle formation," Macromolecules, 33, 6693-6703 (2000). [ Links ]
16. Wildeson, J., A. Smith, X. Gong, H.T. Davis and L.E. Scriven, "Understanding and improvement of TiO2 efficiency in waterborne paints through latex design," J. Coat. Tech., 5, 32-39 (2008). [ Links ]
17. Xie, J., D. Lu, Q. Zhao, T. Yuan and R. Guan, "Carboxylic-group distribution of carboxylated acrylate copolymer latexes with their properties in the presence of acrylic acid and monobutyl itaconate," Polym. Adv. Technol., 23, 929-937 (2012). [ Links ]
Received: July 19, 2012
Accepted: December 10, 2012.
Recommended by Subject Editor: María Luján Ferreira.














