Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Latin American applied research
versión impresa ISSN 0327-0793versión On-line ISSN 1851-8796
Lat. Am. appl. res. vol.44 no.2 Bahía Blanca abr. 2014
A new utilization approach of natural soda ash: to manufacture sodium percarbonate
† College of Chemistry & Pharmacy of Qingdao Agricultural University, Qingdao, 266109, China. dch1218@163.com
‡ Institute of Trona, Inner Mongolia University of Technology, Hohhot 010051, China. liujr@imut.edu.cn
Abstract- Until now, natural soda ash ore is only used for manufacturing sodium carbonate and other common chemicals. As a fine chemical, the traditional method of sodium percarbonate takes sodium carbonate and hydrogen peroxide as raw materials. The primary goal of the present research is to explore a holistic route to manufacture sodium percarbonate from natural soda ash. The integrated approach avoids the operations of deep evaporation and calcination in the traditional manufacture process of sodium carbonate from natural soda ash. It also avoids the dissolution of merchandise sodium carbonate and any addition of salting-out agents in the production of sodium percarbonate. Taking Chagannuoer natural soda ash as raw material, the operating conditions were optimized by means of an orthogonal design L32(49). The results indicate that the availability ratios of H2O2 and Na2CO3 are higher than 75% and 81% respectively under optimal conditions. The IR and XRD characterizations denote that the major component of the prepared product is Na2CO3·1.5H2O2. The research provides a case study for processes integration, and possibly contributes to the investigation of utilizing other natural resources.
Keywords- Natural Soda Ash; Sodium Percarbonate; Process Integration; Multi-index Orthogonal Design; Natural Resource.
I. INTRODUCTION
Natural soda ash ore, is an mineral consisting mainly of soda ash (sodium carbonate, Na2CO3). Sodium bicarbonate (NaHCO3), sodium sulphate (Na2SO4), and sodium chloride (NaCl) usually occur with Na2CO3 in natural soda ash. Vast natural soda ash deposits are widely distributed in nature, such as the Green River Basin of America, Inner Mongolia plateau and Henan province of China, the rift valley system of East Africa, and other areas around the world. Natural soda ash has been widely used to produce Na2CO3, NaHCO3, NaOH, Na2SO4, and other common chemical products in the past several years. However, the high value-added fine chemical prepared from natural soda ash has not been reported.
The production processes of sodium carbonate from natural soda ash are usually classified as "monohydrate process", "carbonation process", and "sodium sesquicarbonate process"(China Soda Industry Association, 1990). These processes comprise a deep evaporation followed by a calcination step to convert monohydrate sodium carbonate (Na2CO3·H2O), sodium bicarbonates, or sodium sesquicarbonate (Na2CO3.NaHCO3.2H2O) into anhydrous sodium carbonate, thus require the use of large quantities of coal fuel, gas or mixtures thereof. A typical monohydrate process is shown in Fig. 1.
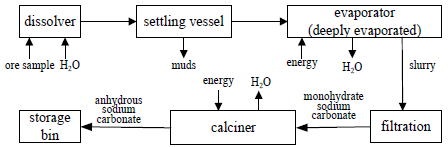
Fig. 1. Typical preparation process of sodium carbonate from natural soda ash
Sodium percarbonate (SPC) is a non-toxic, water-soluble, crystalline peroxygen compound with the molecular formula Na2CO3·1.5H2O2. It has a theoretical active oxygen concentration (AO) of 15.28% by weight. Sodium carbonate and hydrogen peroxide (H2O2) are released when SPC is dissolved in water. Owing to this characteristic, SPC finds widespread applications as bleaching agent, oxidizing agent, oxygen producing agent, disinfector and so on (Chen and Chen, 2009).
The preparation methods of SPC, normally employing a reaction between hydrogen peroxide and sodium carbonate, are often classified as "dry process" and "wet process" and the latter is more widely used (Chen and Chen, 2009; Zhang and Zeng, 2005). To restrain the catalytic effects of transition metal irons in merchandise sodium carbonate on the decomposition of peroxide, stabilizing agents are usually introduced into the reaction system. The steps of wet process can be listed as: 1) mixing aqueous solutions of sodium carbonate and hydrogen peroxide under controlled reaction conditions, which include the concentrations, temperature, reaction time, and stabilizer; 2) after that SPC will crystallize out from the reaction system and then be separated by filtration or centrifugation. According to several published researches (Zhang and Zeng, 2005; Zhao et al.,2006a,b), sodium sulphate and sodium chloride are usually used as salting-out agents in the production of SPC.
Process integration pays attention to the interactions between different unit operations and emphasizes a holistic approach to process design and allows industrial productions to be more economic, efficient and environmental friendly (El-Halwagi Mahmoud, 2006). In order to avoid the steps of deep evaporation and calcination in the manufacturing processes of sodium carbonate from natural soda ash, and the salting-out agents addition in the production of SPC, this investigation tries to obtain SPC from natural soda ash and hydrogen peroxide without adding extra salting-agents.
II. EXPERIMENTAL SECTION
A. Investigation System
Chagannuoer natural soda ash ore is located in Inner Mongolia of China contains more than 40 billion metric tons of fine ore, with the average composition shown in Table 1. Containing many kinds of typical associated salts, the ore is a representative raw material. The total content of Na2SO4 and NaCl is relatively high, helpful to salt-out the SPC product.
Table 1 Mass composition of Chagannuoer natural soda ash ore, sample and clean liquid
B. Overall Idea
Our overall idea to produce SPC from natural soda ash is shown in Fig. 2, and the equation for main reaction is listed as Eq.1. The sample collected from Chagannuoer natural soda ash ore is crushed and then dissolved in water. To obtain clean natural soda ash solution, the cloudy suspension is flocculently flocculated. To fully utilize NaHCO3, stoichiometric sodium hydroxide (NaOH) is added into the clear solution to convert NaHCO3 into Na2CO3, and the reaction equation is shown in Eq.2. To improve the consistency, the weight ratio of Na2CO3 : Na2SO4 : NaCl component ratio in the solution will be adjusted to the same as that of the neutralized average composition of the ore, which is given in Table 1. We call the operation as "matching-salts". Then the liquid reacts with H2O2, and the SPC crystal will be obtained after filtrating and drying operations. It should be noted that the process is designed only for experimental investigation. In large-scale production, if the concentrations of solutes could be high enough to satisfy the demand of the subsequent reaction, the evaporation operation can be elided.
 | (1) |
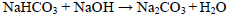 | (2) |

Fig. 2. The preparation route of SPC from natural soda ash
C. Reagents and instruments
Sodium carbonate, sodium sulphate, sodium chloride, sodium silicate, Ethylenediaminetetraacetic acid disodium salt and hydrogen peroxide are of analytical grade. The SPC product was analyzed by X-Ray diffraction analyzer (D/MAX-IIIA, Kigaku, Japan) and FT-IR (FT-IR1730, Perkin-Elmer, America).
D. Investigation method
Preparation of natural soda ash solution or suspension
The chemistry analysis of the ore sample is reported in Table 1. The ore sample crushed by a wooden hammer was dissolved in water within a stainless barrel at 80±1°C. After 3 hours' agitation, the suspension was settled under the presence of anion flocculant (CW-0704) for more than 12 hours at 68±1°C, and then the clear solution was carefully transferred into another stainless barrel. According to Table 2, matching-salts was performed to adjust the weight ratio of Na2CO3: Na2SO4 : NaCl to 22.5:8.74:1. To investigate the effect of the concentration of Na2CO3, the clear solution was concentrated by evaporation.
Preparation of SPC
Natural soda ash solution or suspension containing 50 g sodium carbonate was allowed to react with H2O2 added at a certain feed rate. The wet SPC product was obtained from the reaction slurry by vacuum filtration and then was dried in a vacuum oven at 50°C under the pressure of 160 mmHg. The yield (mass, g) and AO value of SPC are used to evaluate the experimental performance. The L32(49) orthogonal table (Ma et al, 1989) is employed to investigate the following eight factors: (A) reaction temperature, (B) reaction time, (C) molar ratio of H2O2:Na2CO3, (D) feeding time of H2O2, (E) concentration of Na2CO3, (F) concentration of H2O2, (G) dosage of sodium silicate (stabilizer), and (H) dosage of Ethylenediaminetetraacetic acid disodium salt (stabilizer). The factor and level settings are shown in Table 2. The experiment conditions and results are listed in Table 3. The significance tests of different factors on yield and AO are evaluated by means of the F-test, and the final optimized conditions are determined by the multi-index comprehensive evaluation method (Li and Hu, 2009).
Chemical analysis methods
Analysis for ore sample and natural soda ash solution
The total alkalinity (involving sodium bicarbonate and sodium bicarbonate) was titrated using methyl orange solution as indicator (with a mass fraction uncertainty of 1.2%). Sodium bicarbonate was determined using phenolphthalein solution as the indicator (with a mass fraction uncertainty of 1.5%). Sodium carbonate was determined by the quantity difference between total alkaline and sodium bicarbonate. Sodium sulphate was determined by gravimetric method. Sodium chloride was determined by Mohr's method.
Analysis for SPC
The AO of SPC was titrated using potassium permanganate standard solution (with a mass fraction uncertainty of 0.2%). The carbonate was determined by sulfuric acid solution using phenolphthalein solution as the indicator as before (with a mass fraction uncertainty of 1.2%).
III. RESULTS AND DISCUSSION
A. Preparation of clean natural soda ash liquid
According to the mass compositions of the ore sample and clean liquid listed in Table 1, the content of Na2CO3 is slightly higher but those of other constituents are slightly lower than the mean ore composition (shown in Table 1). After introducing stoichiometric NaOH to turn NaHCO3 into Na2CO3, a given quantity of Na2SO4 and NaCl are added to make the mass ratio of Na2CO3 : Na2SO4 : NaCl equal to 22.5:8.74:1.
B. Preparation of SPC
The orthogonal design scheme, experimental results and the consequences of statistical analysis for yield and AO indexes are presented in Table 2-4, respectively.
Table 2. Experimental factors and levels
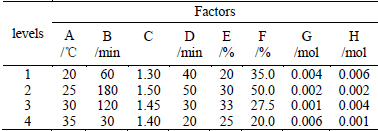
Table 3. Results of L32(49) orthogonal experiments
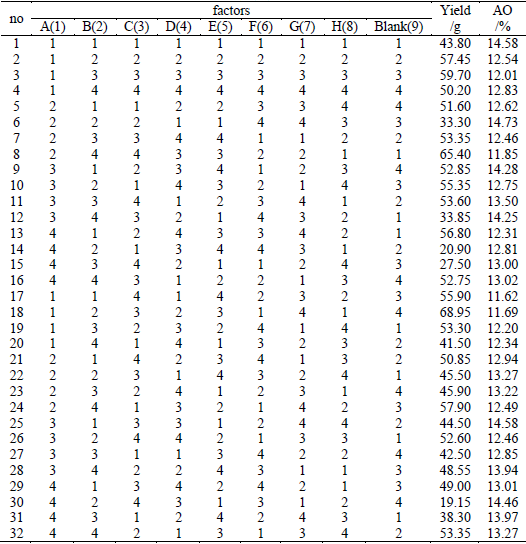
Table 4. Statistical analysis for the orthogonal experimental results
The yield of obtained SPC appears to vary markedly from 19.15 to 68.95 g. K1, K2, K3, and K4 represent the total yield of Level 1, Level 2, Level 3 and Level 4 of a certain factor, respectively. If F value of a factor is higher than the F-critical at the significance level of 0.01, 0.05, or 0.10, the factor is significant at the confidence level of 0.99, 0.95, or 0.90 and is given the significance symbol of ***, **, or *, respectively. In Table 4, the variance analysis shows that at the 99% confidence level, the effects of the reaction temperature (A), concentration of Na2CO3 (E), and concentration of H2O2 (F) are confirmed. At the 95% confidence level, the effects of the reaction time (B) and molar ratio (C) are acknowledged. According to K1, K2, K3 and K4, considering the production cost, the optimal conditions merely for yield index can be taken as A1B1C3D4E3F2G3H4, which are: reaction temperature, 20°C; reaction time, 60 min; mole ratio of H2O2:Na2CO3, 1.45; feeding time of H2O2, 20 min; concentration of Na2CO3, 33%; concentration of H2O2, 50%; dosage of sodium silicate, 0.001 mol; dosage of Ethylenediaminetetraacetic acid disodium salt, 0.001 mol.
The AO of obtained SPC is relatively high and appears to vary from 11.62 to 14.73. K1, K2, K3,and K4 represent AO sum of Level 1, Level 2, Level 3 and Level 4 of a certain factor, respectively. Table 4 indicates the summary of the variance analysis. From the table, it is clear that the effects of reaction temperature (A) and concentration of Na2CO3 (E) are confirmed at the 99% confidence level. At the 95% confidence level, the effects of the feed time of H2O2 (D) and dosage of sodium silicate (G) are acknowledged. The molar ratio(C) is also of consequence, which is statistically significant at the 90% confidence level. According to K1, K2, K3 and K4, with consideration of the production costs, the optimal conditions only for AO index can be taken as A3B4C2D1E1F4G1H4, which are: reaction temperature, 30 C; reaction time, 30 min; mole ratio of H2O2:Na2CO3, 1.5; feeding time of H2O2, 40 min; concentration of Na2CO3, 20%; concentration of H2O2, 20%; dosage of sodium silicate, 0.004 mol; dosage of Ethylenediaminetetraacetic acid disodium salt, 0.001 mol.
According to Table 4 and the above analysis, dosage of Ethylenediaminetetraacetic acid disodium salt (H) isn't statistically significant for any index and thus can be picked as H4. Since feed time of H2O2 (D) and dosage of sodium silicate (G) only have significant effect on AO index, they can be taken as D1 and G1 respectively. Reaction time (B) and molar ratio (C) can be selected as B1 and C3, respectively, as they are more significant for yield index than for AO index. There are larger gaps between the F values of Factor A or E and critical value used in F test for index yield than for AO index at the same confidence level, that means the two factors have more significance for yield index than for AO index, thus factor A and E can be chosen as A1 and E3, respectively. Based on these analyses, without involving the concentration of H2O2, the optimized condition can be determined as A1B1C3D1E3G1H4.
Although concentration of H2O2 is significant only for yield index, with a view to the cost of various concentrations of H2O2, the determination of Factor F needs further investigation and economic evaluation. The supplementary experimental results investigating the effect of the concentrations of H2O2 under the above optimized condition (A1B1C3D1E3G1H4) are shown in Table 5. It can be seen that the utilization rates of H2O2 and Na2CO3 grow with the increase of the H2O2 concentrations but there is only slightly increase when the concentration rises from 35% to 50%. However, the AO decreases evidently with the increase of H2O2 concentration. Taking into consideration of the cost, the concentration of H2O2 can be chosen as from 27.5% to 35%.
Table 5. The effects of concentration of H2O2

According to the above analysis, the final optimized conditions are as follows: temperature 20°C, reaction time 60 min, mole ratio of H2O2:Na2CO3 1.45, concentration of Na2CO3 33%, dosage of sodium silicate 0.004 mol, dosage of Ethylenediaminetetraacetic acid disodium salt 0.001 mol, feed time of H2O2 40 min, and concentration of H2O2 27.5-35%.
As shown in Table 5, under the optimized conditions, the availability ratios of H2O2 and Na2CO3 are higher than 75% and 81% respectively. The product prepared with 27.5% H2O2 was used for IR and XRD identification.
C. Identification of SPC product
IR spectra of the obtained sample was investigated in KBr pellets and recorded in the range of 450-4000cm-1. The comparison in Table 6 shows that the observed bands of the sample are in agreement with the values of Na2CO3·1.5H2O2 published by Jones and Griffith (1980). It should be noted that the splitting of the torsion of O-H is not observed, which may be related with the crystal quality or measure conditions. The IR data of Na2CO3·1.5H2O2 at 25°C reported by Carrondo et al. (1977) also does not involve the splitting of the torsion of O-H.
Table 6. Comparison of IR analysis of SPC sample and literature

**According to reference (Jones and Griffith,1980)
The most intense peaks should be pay attention to when manipulating X-ray diffraction data (Warren, 1990). The d values of eight most intense peaks and maximum d value at scattering angles (2θ) of 34.95o, 37.09o, 32.64o, 32.85o, 45.72o, 26.51o, 23.58o, 24.57o, and 11.22o, are 2.57, 2.42, 2.75, 2.73, 1.98, 3.37, 3.78, 3.63,and 7.92, respectively, which are in relative agreement with the data reported by PDF card (no 11-656) corresponding to Na2CO3·1.5H2O2.
According to the above discussion, the major component of the sample is Na2CO3·1.5H2O2.
IV. CONCLUSIONS
An efficient route to manufacture sodium percarbonate from natural soda ash instead of sodium carbonate is explored. The process conditions were optimized by means of multi-factor orthogonal test method. Taking Chagannuoer natural soda ash as raw material, the optimal conditions are easy control and described as follows: reaction temperature, 20°C; reaction time, 60 min; mole ratio of H2O2:Na2CO3, 1.45;concentration of Na2CO3, 33%; dosage of sodium silicate, 0.004 mol;dosage of Ethylenediaminetetraacetic acid disodium salt, 0.001 mol; feeding time of H2O2, 40 min; concentration of H2O2, 27.5-35%. The availability ratios of H2O2 and Na2CO3 are higher than 75% and 81% respectively. IR and XRD identification denote that the major component of the prepared product is Na2CO3·1.5 H2O2. The research provides a new approach to produce fine chemical from natural soda ash.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the financial supports from the National Science Foundation of China (No. 29966002).
REFERENCES
1. Carrondo, C.T., W.P. Griffith, D.P. Jones and A.C. Skapski, "X-Ray Crystal Structure of the Industrial Bleaching Agent 'Sodium Percarbonate'[Sodium Carbonate-hydrogen Peroxide (2/3)]," J. Chem. Soc.Dalton Trans., 23, 2323-2327 (1977). [ Links ]
2. Chen, X. and D. Chen, "Synthesis and Stabilization of Low-bulk-density Sodium Percarbonate," Chemical Industry and Engineering Progress (In Chinese), 28, 292-296 (2009). [ Links ]
3. China Soda Industry Association, Soda Engineering Science, Chemical Industry Press, Beijing (In Chinese) (1990). [ Links ]
4. El-Halwagi Mahmoud, M., Process Integration, Elsevier Academic Press, London (2006). [ Links ]
5. Jones, D.P. and W.P. Griffith, "Alkali-metal Peroxocarbonates, M2[CO3]·nH2O2, M2[C2O6], M[HCO4]·nH2O, and Li2[CO4]·H2O," J. Chem. Soc. Dalton Trans., 12, 2526-2532 (1980). [ Links ]
6. Li, Y. and C. Hu, Experiment Design and Data Processing, Chemical Industry Press, Beijing (In Chinese) (2009). [ Links ]
7. Ma, F., L. He, M. Yu and J. Fan, Applied Probability and Statistics, Higher Education Press, Beijing (In Chinese) (1989). [ Links ]
8. Warren, B. E., X-ray diffraction, Dover Publications, New York (1990) [ Links ]
9. Zhang, K. and F. Zeng, "The Production and Research Progress of Sodium Percarbonate," Inorganic Chemicals Industry, 37, 18-20 (In Chinese) (2005). [ Links ]
10. Zhao, H., C. Tang, D. Zhang, W. Xu and Y. Wang, "Solid−Liquid Equilibrium for the Quaternary System of Sodium Carbonate + Sodium Chloride + Hydrogen Peroxide + Water at 293.15 K," J. Chem. Eng. Data, 51, 676-679 (2006a). [ Links ]
11. Zhao, H., C. Tang, D. Zhang, W. Xu, Y. Wang and P. Jian, "Solubility and Phase Diagram for the Quaternary System Na2CO3 + Na2SO4 + H2O2 + H2O at 293 K," J. Chem. Eng. Data, 51, 1567-1570 (2006b). [ Links ]
Received: March 24, 2013
Accepted: October 31, 2013
Recommended by Subject Editor: Marcelo Martins Seckler














