Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Mastozoología neotropical
versión impresa ISSN 0327-9383versión On-line ISSN 1666-0536
Mastozool. neotrop. v.11 n.1 Mendoza ene./jun. 2004
Cytogenetic Analysis of Ctenomys Opimus (Rodentia, Octodontidae) from Argentina
Ariel C. Toloza1, Orlando Scaglia2, and Alicia I. Massarini1
1Grupo de Investigaciones en Biología Evolutiva (GIBE), Departamento de Ecología, Genética y Evolución, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Pabellón II, 4º piso, Ciudad Universitaria, Núñez, 1428, Buenos Aires, Argentina. Tel: 0114576-3348. Fax:0114576-3384.<ariel@bg.fcen.uba.ar>. 2Museo Municipal de Ciencias Naturales de Mar del Plata Lorenzo Scaglia, Mar del Plata, Argentina.
Abstract. Comparison of the normal karyotype, C and G-banding patterns of Ctenomys opimus from Argentina, with karyotypes from Chile and Bolivia, do not distinguish Argentinean from Chilean or Bolivian forms of this species.
Key words: Ctenomys opimus, Tuco-tucos, cytogenetics, Argentina.
Resumen. Análisis citogenética de Ctenomys opimus (Rodentia . Octodontidae) de la Argentina La comparación del cariotipo normal, las bandas C y G de Ctenomys opimus de Argentina, con cariotipos de Chile y Bolivia, no permite distinguir, desde el punto de vista citogenético, las formas argentinas de las bolivianas o chilenas.
Palabras clave: Ctenomys opimus, Tuco-tucos, citogenética, Argentina.
Subterranean rodents of the genus Ctenomys, commonly known as tuco-tucos, are endemic to the southern half of South America. This genus is by far the most speciose group of extant subterranean rodents, with more than 56 recognized living species (Reig et al., 1992; Lacey et al., 2000). The earliest fossils of this genus are known from Pleistocene deposits (Reig, 1989; Cook et al., 2000), and molecular findings suggest an early burst of cladogenesis following the appearance of the genus in the early Pleistocene (Lessa and Cook, 1998; Slamovits et al., 2001).
The subterranean life-style of tuco-tucos is likely to have favoured rapid fixation of chromosomal rearrangements: small, semi-isolated demes constrained by the soils they inhabit, low vagility due to morphological modifications for digging and highly territorial behaviour. It has been suggested that chromosome rearrangements together with population structure have played an important role in the explosive diversification of Ctenomys (Reig and Kiblisky, 1969; Reig, 1989). Diploid numbers for tuco-tucos range between 10 and 70 (Anderson et al., 1987) while the fundamental numbers vary from 16 to 90 (Reig et al., 1992). Interspecific conservation of karyotypes is uncommon (Gallardo, 1979; Cook et al., 1990; Massarini et al., 1991) and most species display karyotypic heterogeneity, polymorphisms and polytiphisms (Reig et al., 1990). These characteristics make Ctenomys an important model for the study of chromosomal evolution in subterranean rodents and Vrba and Gould (1986) mentioned that tuco-tucos are a relevant model for the analysis of the species selection approach.
Ctenomys opimus is distributed in four countries: Peru, Bolivia, Chile and Argentina (Sanborn and Pearson, 1947; Pearson, 1959; Reig and Kiblisky, 1969; Gallardo, 1979; Cook et al., 1990; Reig et al., 1990). Three subspecies have been proposed for this species: C. o. opimus for Chile and Bolivia (Gallardo, 1979; Cook et al., 1990), C. o. nigriceps for Perú (Sanborn and Pearson, 1947; Pearson, 1959) and C. o. luteolus for Argentina (Reig and Kiblisky, 1969)
In this study we describe the gross, C and G- banded karyotype of Ctenomys opimus from two populations of Salta Province, Argentina, and we compare these results with previous information about the karyotypes of populations belonging to the three recognized subspecies. Moreover, in the present study we test the idea proposed by Reig et al. (1992) about the sub-specific distinction between C. o. opimus and C. o. luteolus. The sample studied consists of 3 specimens: one female and one male from Las Cuevas (24º18’ 66º10’), and one female from Santa Rosa de Tastil (24º23’ 66º00’), both localities belonging to Salta Province, Argentina, in the heights of 3,500 and 2,000 meters, respectively. The animals were live-trapped with Oneida Victor Nr. 0 snap traps. Chromosomal preparations were obtained from bone marrow of animals injected with yeast, one day before sacrifice (Lee and Elder, 1980). G-bands and C-bands were obtained by the methods of Seabright (1971) and Hsu (1974), respectively. Chromosomal nomenclature follows Levan et al. (1964) and nomenclature relating to chromosome size and arrangements follows Massarini et al. (1991). Skin and skull voucher specimens were deposited at the Municipal Museum of Natural Sciences of Mar del Plata Lorenzo Scaglia.
The gross morphology of karyotypes for the studied animals is similar to that for specimens of C. opimus collected in Tres Cruces, Jujuy Province, Argentina (Reig and Kiblisky, 1969; Reig et al., 1992), as well as for specimens belonging to Chilean populations (Gallardo, 1979) and for Bolivian populations (Cook et al., 1990; Cook and Salazar-Bravo, 2003).
Specimens from both, Santa Rosa de Tastil and Las Cuevas populations, had a fully biarmed karyotype with 2n = 26 and FN = 48. This karyotype consists of three pairs of large metacentric, three pairs of medium-sized submetacentric, three pairs of medium-sized metacentric, one small submetacentric and two small metacentric autosomes. The X chromosome is a medium-sized submetacentric. This does not agree with the metacentric condition of the X chromosome reported for C. opimus by Reig and Kiblisky (1969), but is similar to the submetacentric condition reported by Gallardo (1979) and by Cook et al. (1990). The Y chromosome is a small subtelocentric (Fig. 1a). C-band patterns revealed that pairs A1 to A3 showed faintly stained centric C-bands, whereas seven pairs of biarmed autosomes show intense pericentromeric heterochromatin. In pair A-7 and the X chromosome, heterochromatin was absent although the Y has an entire heterochromatic q arm (Fig. 1b). The G-band pattern (Fig. 1c) is similar to that of C. o. luteolus from Tres Cruces, Jujuy, Argentina (Ortells, 1995), the only G- band pattern reported at the present time for this species.
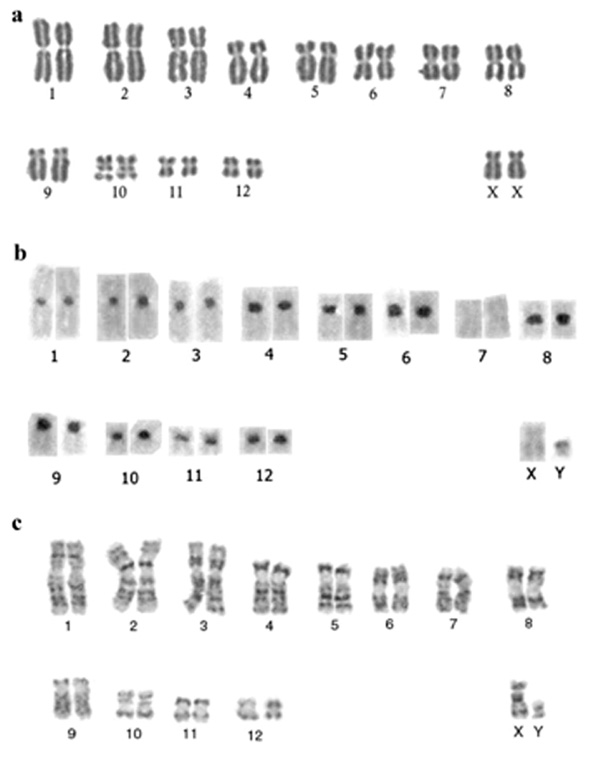
Fig. 1.Karyotype of Ctenomys opimus.a) Standard karyotype of a female from Santa Rosa de Tastil; b) C-bands of a male from Las Cuevas; c) G-bands of a male from Las Cuevas.
Cariotipo de Ctenomys opimus. a) Cariotipo estándar de una hembra proveniente de Santa Rosa de Tastil. b) Bandas C de un macho proveniente de Las Cuevas. c) bandas G de un macho proveniente de Las Cuevas.
According to Reig et al. (1992), the main difference between the karyotypes of C. o. opimus and C. o. luteolus is the amount of heterochromatin in pairs A1 to A3, which show faintly stained centric C-bands in C. o. luteoleus and heavily stained pericentromeric blocks in C. o. opimus.
The location and the amount of heterochromatin from the Salta population karyotypes reported here agree with the C- bands patterns reported for C. o. luteolus (Reig et al., 1992) and with those reported by Cook et al. (1990) for the Bolivian populations of C. o. opimus. No qualitative differences were found when C-band patterns of the populations studied here were compared with those of Chilean populations. Moreover, in this study a low number of metaphasesshowed stronger signals in the pairs A1 to A3.
A comparative analysis of our results and previous data suggest that the C-band patterns of the two subspecies do not differ and that previously described differences may be artefacts rather than consistent chromosomal differences. In this sense, we stand that cytogenetic evidences do not support the sub-specific distinction proposed by Reig et al. (1992).
The karyotypes of Peruvian populations of C. opimus are still unknown. The only studies of Peruvian populations of Ctenomys opimus nigriceps showed that some morphological characters were similar to those reported for C. o. opimus and C. fulvus from Chile (Sanborn and Pearson, 1947; Pearson, 1959). Pearson (1959) referred to these populations as Ctenomys opimus excluding the sub-specific classification and suggesting that additional studies are necessary to assign sub-specific status to Peruvian tuco-tucos. Cook et al. (1990) studied the cytogenetics and morphology of six different populations of C. opimus from Bolivia and concluded that, among these populations, is not possible to recognize Bolivian subspecies. At the same time, this author suggests that the subspecies C. o. luteolus from Argentina, C. o. opimus from Chile, and C.o. nigriceps from Peru need to be added to an integrated analysis in order to evaluate the status of subspecies throughout the species range.
It is remarkable that the karyotypes of previously studied populations of Ctenomys opimus were identical in number, gross morphology and C- band pattern to those belonging to Ctenomys fulvus from Chile (Gallardo, 1979; Gallardo, 1991; Reig et al., 1992). In addition, C. opimus and C. fulvus show karyotypic similarities (Gallardo, 1979; Reig et al. 1992), belong to a well-supported clade in phylogenetic trees based on cytocrome b, and also share patterns and low copy number of satDNA (RPCS) (Slamovits et al., 2001). All these facts suggest that these two taxa may be conspecific. Gallardo (personal communication) has mentioned that the main morphological differences between these two species are based on the pelage coloration, which can vary considerably within and between species.
The description and the comparison of new populations of Ctenomys opimus and Ctenomys fulvus involving cytogenetic, morphology and molecular studies will help us to solve the status of subspecies and species in this lineage of subterranean rodents.
ACKNOWLEDGMENTS
We thank Raúl Fernandez-Donoso, Joe Cook and Jorge Salazar-Bravo for sharing with us information of Ctenomys opimus from Chile and Bolivia. Enrique Prina helped us to collect the animals employed in this paper. We wish to express our gratitude to Eileen Lacey for insightful comments on the manuscript.
LITERATURE CITED
ANDERSON, S., T.L. YATES, and J.A. COOK. 1987. Notes on Bolivian mammals 4: The genus Ctenomys (Rodentia: Ctenomyidae) in the eastern lowlands. American Museum Novitates, 2891:1-20. [ Links ]
COOK, J.A., S. ANDERSON and T.L. YATES. 1990. Notes on Bolivian mammals. 6. The genus Ctenomys (Rodentia, Ctenomydae) in the highlands. American Museum Novitates, 2980:1-27. [ Links ]
COOK, J.A., E. LESSA and E. HADLY. 2000. Paleontology, phylogenetics patterns, and macroevolutionary process in subterranean rodents. Pp. 332-369. In: Life underground: The Biology of Subterranean Rodents (Lacey, E.A., J.L. Patton, and G.N. Cameron, eds.). University of Chicago Press, Chicago, 449 pp. [ Links ]
COOK, J.A. and J. SALAZAR-BRAVO. 2003. Heterochromatin variation in the chromosomally diverse Tuco-tucos (Rodentia: Ctenomyidae) of South America. In: Contributions in honour to Bernardo Villa (Medellin, R. and V. Sanchez-Cordero, eds.). Fondo de Cultura económico, México DF, México. [ Links ]
GALLARDO, M. 1979. Las especies Chilenas de Ctenomys (Rodentia, Octodontidae). I. Estabilidad Cariotípica. Archivos de Biología y Medicina experimental., 12:71-82. [ Links ]
GALLARDO, M. 1991. Karyotypic evolution in Ctenomys (Rodentia, Ctenomyidae). Journal of Mammalogy, 72(1):11-21. [ Links ]
HSU, T.C. 1974. Procedures for inducing C-bands and G-bands in mammalian chromosomes. Mammalian Chromosome Newsletter, 15:88-96. [ Links ]
LACEY, E.A., J.L. PATTON and G.N. CAMERON (eds.). 2000. Life underground: The Biology of Subterranean Rodents. University of Chicago Press, Chicago. 449 pp. [ Links ]
LEE, M.R. and F. ELDER. 1980. Yeast stimulation of bone marrow mitosis for cytogenetic investigations. Cytogenetic and Cell Genetics, 26:36-40. [ Links ]
LESSA, E. and J.A. COOK. 1998. The molecular phylogenetics of tuco-tucos (genus: Ctenomys, Rodentia: Octodontidae) suggests an early burst of speciation. Molecular Phylogenetics and Evolution, 9(1):88-99. [ Links ]
LEVAN, A., K. FREDGA, and A. SANDBERG. 1964. Nomenclature for centromeric position on chromosomes. Hereditas, 52:1-11. [ Links ]
MASSARINI, A.,M.A. BARROS, M.O. ORTELLS, and O.A REIG. 1991. Evolutionary biology of fossorial Ctenomyidae rodents. (Caviomorpha: Octodontidae). I. Chromosomal polymorphism and small karyotypic differentiation in Central Argentinean populations of tuco-tucos. Genetica, 83:131-144. [ Links ]
ORTELLS, M.O. 1995. Phylogenetic analysis of G-banded karyotypes among the South American subterranean rodents of the genus Ctenomys (Caviomorpha: Octodontidae), with special reference to chromosomal evolution and speciation. Biological Journal of the Linnean Society, 54:43-70. [ Links ]
PEARSON, O.P. 1959. Biology of the subterranean rodents, Ctenomys, in Perú. Memorias del Museo de Historia Natural " Javier Prado". Universidad Nacional Mayor de San Marcos, Lima 9, 1-56. [ Links ]
REIG, O.A. 1989. Karyotypic repatterning as one triggering factor in cases of explosive speciation. Pp. 246-289. In: Evolutionary Biology of Transient, Unstable Populations (Fontdevila, A. eds.). Springer Verlag, Berlin, New York. [ Links ]
REIG, O.A. and P. KIBLISKY. 1969. Chromosome multiformity in the genus Ctenomys (Rodentia, Octodontidae). A progress report. Chromosoma, 28:211-244. [ Links ]
REIG, O.A., C. BUSCH, M.O. ORTELLS, and J.R CONTRERAS. 1990. An overview of evolution, systematics, population biology and speciation in Ctenomys. Pp. 97-128. In: Biology of subterranean mammals at the organismal and molecular levels (Nevo, E. and O.A. Reig eds.).Wiley-Liss, New York. [ Links ]
REIG, O.A., A.I. MASSARINI, M.O. ORTELLS, M.A. BARROS, S.I. TIRANTI, and F.J. DYZENCHAUZ. 1992. New karyotypes and C-banding patterns of the subterranean rodents of the genus Ctenomys (Caviomorpha, Octodontidae) from Argentina. Mammalia, 56:603-623. [ Links ]
SANBORN, C.C. and O.P. PEARSON. 1947. The Tuco-tucos of Perú (Genus Ctenomys). Proceedings of the Biological Society of Washington, 60:135-138. [ Links ]
SEABRIGHT, M. 1971. A rapid banding technique for human chromosomes. Lance, 2:971-972. [ Links ]
SLAMOVITS, C.H., J.A COOK, E. LESSA, and M.S. ROSSI. 2001. Recurrent amplifications and deletions of Satellite DNA accompanied chromosomal diversification in South American tuco-tucos (Genus Ctenomys, Rodentia: Octodontidae): A phylogenetic approach. Molecular Biology and Evolution, 18(9):1708-1719. [ Links ]
VRBA, E.S. and S.J. GOULD. 1986. The hierarchical expansion of sorting and selection: Sorting and selection cannot be equated. Paleobiology, 12:217-228 [ Links ]
Recibido 16 marzo 2004.
Aceptación final 5 julio 2004.














