Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Mastozoología neotropical
Print version ISSN 0327-9383On-line version ISSN 1666-0536
Mastozool. neotrop. vol.12 no.2 Mendoza July/Dec. 2005
Hoof growth in neonatal Patagonian huemul (Hippocamelus bisulcus): a tentative tool for aging
Werner T. Flueck1 and Jo Anne M. Smith-Flueck2
1 CONICET, DeerLab, CC 176, 8400 San Carlos de Bariloche, Argentina. deerlab@baritel.com.ar. 2 DeerLab, CC 176, 8400 San Carlos de Bariloche, Argentina.
Key words. Aging model. Hippocamelus bisulcus. Huemul. Neonate. Morphometry.
The endangered Patagonian huemul (Hippocamelus bisulcus) is the only large native herbivore to reside in most of the sub-antarctic false-beech (Nothofagus spp.) forest habitat occurring in Chile and Argentina. It asserts that this deer species has a distinctly important role at the community level. During the past century, numbers of huemul in Chile and Argentina have declined perhaps as much as a 99% from levels before arrival of Europeans (Redford and Eisenberg, 1992).There are likely less than 600 deer left in Argentina fragmented along about 1850 km of the Andes mountain range, and maybe 1500 in Chile (Smith-Flueck, 2000). Recently, conservation efforts for this species have increased substantially. Consequently, more people work in areas where huemul still survive and come into contact with both live and dead animals. This report results from such a recent encounter which permitted us to examine a dead female huemul fawn for the first time. There are only two previous accounts of observations of newborn huemul (Smith-Flueck, 2000). Franke (1952) referred to his experiences with captive huemul, which included the births of two fawns. He kept detailed scientific notes, but unfortunately, these were lost after his death. The other account involved the birth of a fawn which died after 35 hours (Texera, 1974). The mother, which had lost about 50% of her body weight during a brief time in captivity, died shortly after her fawn. Measurements of her fawn indicated it was born underdeveloped. Due to the complete lack of information on newborn huemul, we report on morphological features which might assist in aging alive or dead neonatal huemul fawns. The possibility to age young fawns in a consistent manner is paramount considering that problems with recruitment may be at the heart of huemul recovery (Smith-Flueck, 2000; Smith-Flueck and Flueck, 2001).
A huemul fawn was found by hikers on 17 November, 1999, near the Argentine National Park Lago Puelo (42° S, 71° 30' W). It remained in the cervid freeze and erroneously assuming it to be abandoned, the hikers took it home and tried to raise it, but it died on November 20. We collected morphometric data and documented physiognomic features photographically. We measured hoof length from the hair line along the abaxial ridge to the tip of the outer hoof. We measured hoof growth from the hair line along the abaxial ridge to the growth line (Haugen and Speake, 1958).
Several observations indicate that the huemul fawn was found shortly after birth. First, when found it remained in a cervid freeze, typical for neonates less than a week old (McCullough, 1969; White et al., 1972). Adult females of Odocoileus spp., closest relatives to huemul, are similar in size and their single fawns represent about 7% of adult body weight (Robinette et al., 1973). Huemul fawns are always born singly and thus would weigh approximately 5 kg at birth (Smith-Flueck, 2000). Neonatal growth rates for fawns in Odocoileus spp. are about 250 g/day (Cowan and Wood, 1955; Robbins and Moen, 1975). Based on these estimates, the weight of 6.3 kg at death could account for approximately 5-6 days of growth. The amount of hoof growth also corroborates young age when captured. An additional feature typical for very young fawns was the presence of a navel scab.
The hoof physiognomy in huemul fawns (Fig. 1) has the same features as found in white-tailed (O. virginianus), black-tailed (O. hemionus columbianus) and mule deer (O. h. hemionus) (Haugen and Speake, 1958; Robinette et al., 1973; Flueck, unpubl. data). There are enough similarities between huemul and other members of the Odocoilines regarding size, weight at birth, and growth pattern to allow comparison of neonatal hoof growth. The hoof exhibits a ridged line separating hoof growth which occurred prenatally and it shows proximal-distal streaks. Postnatal growth occurs proximally (near the hair line), and thus the ridged growth line grows toward the tip of the hoof until disappearing. The length of all hooves of the female huemul fawn was 26 mm, and hoof growth at death was 4 mm. Our data of a neonate and several subadults confirms that the change in hoof physiognomy in huemul occurs as described in other cervids, based on several huemul carcasses aged between 9 and 18 months (Smith-Flueck and Flueck, 2001). These hooves clearly show that the proximal-distal streaks have grown out, being replaced by a smooth surface which may show some streaking, but parallel to the hair line. It is reasonable to generalize this developmental process to occur in huemul as this developmental pattern has been shown to be fixed and homogenous in other cervids (see Johnson, 2002).
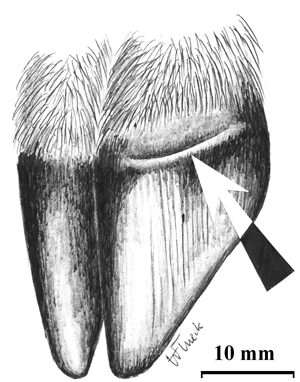
Fig. 1. Hoof of a huemul fawn showing the ridged growth line (arrow) and the proximal-distal streaking (drawn after photo).
Of several morphometric measures examined, Sams et al. (1996) found that hoof growth provided the most reliable and accurate aging model for white-tailed fawns, was least affected by gender and maternal nutrition, and was best represented by linear regression. Hoof growth rates have been reported as 0.45 mm/day (Haugen and Speake, 1958) and 0.27 mm/day (Sams et al., 1996) for white-tailed deer fawns, 0.40 mm/day for mule deer fawns (Robinette et al., 1973), and 0.31 mm/day (95% C.L. = 0.02) for black-tailed deer fawns (Flueck, unpubl. data). Using the aging model proposed by Haugen and Speake (1958), a one-day old white-tailed fawn has a hoof growth of 0.15 mm. Sams et al. (1996) reported an average hoof growth for one-day old white-tailed fawns of 2.44 mm, whereas Robinette et al. (1973) reported 2.5 mm growth at birth for mule deer. Black-tailed deer fawns have practically no hoof growth between the hairline and the ridged growth line at birth. Based on vaginal telemetry used to determine parturition (Flueck, 1998), fawns evaluated at 1 day of age had a mean hoof growth of 0.125 mm (SE = 0.089, n = 12) and a mean hoof length of 22.1 mm (SE = 0.41, n = 5) (Flueck, unpubl. data).
The similarity between weights of adult females and single fawns among Odocoileus spp. and huemul and the unlikelihood studies in the near future on hoof growth in relation to age in huemul warrants the formulation of a tentative working model for aging huemul fawns, based on hoof growth (also see Schmidt-Nielsen, 1984). In the case that huemul have no growth at birth, the measured 4 mm would represent 9, 11 or 13 days of age, according to reported growth rates of 0.45, 0.35 or 0.31 mm/day, respectively. If on the other hand we assume the age of 6 days at the time of death as corroborated by the presence of a navel scab and the observed cervid freeze, then the measured 4 mm would indicate the presence at birth of 1.3, 1.9 or 2.1 mm of growth, according to reported growth rates of 0.45, 0.35 or 0.31 mm/day, respectively.
Given the presence of a navel scab and the observed cervid freeze, we propose to use 1.5 mm hoof growth at day one and an average daily growth rate of 0.35 mm (Haugen and Speake, 1958; Robinette et al., 1973; Sams et al., 1996; Flueck, unpubl. data). An approximate age of huemul fawns can thus be obtained as follows: AGE = [hoof growth (mm) - 1.5] / 0.35.
In the present case with 4 mm hoof growth, the fawn would thus be estimated approximately 7 days old, or 3 days old when captured. Although model predictions might be biased until more data is available for validating age, estimations can be made uniformly by all workers encountering life or dead huemul fawn. This is especially important because huemul fawns are unspotted and their age is thus more difficult to assess without having this criteria available, and information on other methods like tooth irruption and wear have not been established. Furthermore, measurements commonly used on neonatal jaws require fresh specimens to measure the gum line (Severinghaus, 1949).
So far only subjective, unreliable, unreplicable and uncomparable estimates of aging huemul neonates are available. The proposed model allows accurate estimation of age for about the first three months (Robinette et al., 1973) by reducing the relative error of measurements by providing an easily measurable variable (Kleinbaum et al., 1982). This method applies to dead fawns as well, because the feet and hooves of carcasses tend to remain intact for several months, and even up to a year in semi-arid regions. Hence, relative random sampling error would be reduced considerably among different observers, resulting in increased precision of reported estimates. It would present the fundamental condition for subsequent analysis of such meta-replication studies as the most reliable method to advance in knowledge, assuming that no controlled experiments are available beforehand to establish relationships based on known-aged fawns (Johnson, 2002). Of course, concerns remain regarding the internal validity of the model with respect to the possible introduced systematic error (bias) of choosing model parameters from studies on other species.
As there are no other established methods to estimate age of huemul fawns, particularly easily measurable criteria, the proposed model allows a unified although possible biased way of addressing issues where age of neonates is important. The possibly introduced systematic bias, such as the age differing by some days, is judged unimportant considering the informed analysis made possible of factors affecting a given phase of neonatal survival. As indicated elsewhere (Smith-Flueck, 2000; Smith-Flueck and Flueck, 2001), a general problem with this critically endangered species appears to be related to problems of recruitment, hence a consistent method of aging neonatal fawns, albeit biased in the present case, is urgently needed.
LITERATURE CITED
COWAN IM and AJ WOOD. 1955. The growth rate of the black-tailed deer (Odocoileus hemionus columbianus). Journal of Wildlife Management 19:331-336. [ Links ]
FLUECK WT. 1998. Telemetrical monitoring of the peripartum period in free-ranging cervids through vaginal implants. Pp. 282, in: Recent developments in deer biology (JA Milne, ed.). Moredun Research Institute, Edinburgh, UK. [ Links ]
FRANKE FR. 1952. Mein Inselparadies. R. Piper & Co. Verlag, München, Germany. [ Links ]
HAUGEN AO and DW SPEAKE. 1958. Determining age of young fawn white-tailed deer. Journal of Wildlife Management 22:319-321. [ Links ]
JOHNSON DH. 2002. The importance of replication in wildlife research. Journal of Wildlife Management 66:919-932. [ Links ]
KLEINBAUM DG, LL KUPPER, and H MORGENSTERN. 1982. Epidemiologic research: principles and quantitative methods. Lifetime Learning Publ., Belmont, California. [ Links ]
McCULLOUGH D. 1969. Tule Elk. University of California Publications in Zoology, Vol. 88. University of California Press, Berkeley, CA, USA. [ Links ]
REDFORD KH and JF EISENBERG. 1992. Mammals of the Neotropics: The Southern Cone. Volume 2. Chile, Argentina, Uruguay, Paraguay. The University of Chicago Press, Chicago and London. [ Links ]
ROBBINS CT and AN MOEN. 1975. Milk consumption and weight gain of white-tailed deer. Journal of Wildlife Management 39:355-360. [ Links ]
ROBINETTE WL, CH BAER, RE PILLMORE, and CE KNITTLE. 1973. Effects of nutritional change on captive mule deer. Journal of Wildlife Management 37:312-326. [ Links ]
SAMS MG, RL LOCHMILLER, EC HELLGREN, WD WARDE, and LW VARNER. 1996. Morphometric predictors of neonatal age for white-tailed deer. Wildlife Society Bulletin 24:53-57. [ Links ]
SEVERINGHAUS CW. 1949. Tooth development and wear as criteria of age in white-tailed deer. Journal of Wildlife Management 13:195-216. [ Links ]
SCHMIDT-NIELSEN K. 1984. Scaling: why is animal size so important? Cambridge University Press, Cambridge. [ Links ]
SMITH-FLUECK JM. 2000. The current situation of the Patagonian Huemul. Pp. 67-146, in : The Patagonian huemul: A mysterious deer on the brink of extinction (N Díaz and JM Smith-Flueck, eds). Literature of Latin America, Buenos Aires, Argentina. [ Links ]
SMITH-FLUECK JM and WT FLUECK. 2001. Natural mortality patterns in a population of southern Argentina huemul (Hippocamelus bisulcus), an endangered Andean cervid. European Journal of Wildlife Research 47:178-188. [ Links ]
TEXERA WA. 1974. Algunos aspectos de la biologia del huemul (Hippocamelus bisulcus) (Mammalia: Artiodactyla, Cervidae) en cautividad. Anales del Instituto Patagónico, Punta Arenas (Chile) 5:155-188. [ Links ]
WHITE M, FF KNOWLTON, and WC GLAZENER. 1972. Effects of dam-newborn fawn behavior on capture and mortality. Journal of Wildlife Management 36:897-906 [ Links ]
Recibido 13 junio 2004.
Aceptación final 23 agosto 2005.














