Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Mastozoología neotropical
Print version ISSN 0327-9383On-line version ISSN 1666-0536
Mastozool. neotrop. vol.13 no.1 Mendoza Jan./June 2006
Habitat use and natural history of small mammals in the Central Paraguayan Chaco
Christopher J. Yahnke
Department of Biology and Museum of Natural History, University of Wisconsin - Stevens Point, 800 Reserve Street, CNR 167, Stevens Point, WI 54481 USA, Telephone: 715-346-2455. <cyahnke@uwsp.edu>
ABSTRACT: I conducted a small mammal trapping study in the central Paraguayan Chaco region of South America to investigate habitat selection by small mammals at different spatial scales. Small mammals were collected in forest, successional thorn scrub, pasture, and crop fields representing both relatively undisturbed habitats and agroecosystems. A total of 1089 small mammals representing 13 species were captured during 23 296 trap nights. Pastures had the highest species richness as well as the highest number of captures. Some small mammal species such as Calomys laucha and Akodon toba were captured in a variety of habitats whereas others like Holochilus chacarius and Bolomys lasiurus were captured almost exclusively in pastures. Principal components analysis distinguished small mammal species primarily associated with agricultural habitats (e.g. Calomys spp.) from those associated with more wooded habitats (e.g. Graomys griseoflavus and Oligoryzomys chacoensis). These results corroborate other studies on habitat use by small mammals in this region, but with some notable exceptions, such as the first documentation of high densities of Calomys musculinus in western Paraguay.
Key words. Chaco. Habitat selection. Natural history. Paraguay. Small mammals.
INTRODUCTION
A striking diversity of small mammals can be found in the variety of habitats scattered throughout the central Paraguayan Chaco. The central Chaco presents a broad array of habitats ranging from undisturbed forest to grasslands, riparian habitats, and agroecosystems. Although studies of wildlife-habitat relationships in South America historically have been conducted in natural, relatively undisturbed ecosystems (Meserve et al., 1988; Patterson et al., 1990; Kelt et al., 1994), interest is increasing regarding studying small mammal communities in agroecosystems. Much of this research has focused on small mammal reservoirs of infectious diseases. For example, a number of studies have been conducted in agroecosystems in central Argentina in the endemic region of Argentine Hemorrhagic Fever (Mills et al., 1991a; Mills et al., 1992; Ellis et al., 1997; Ellis et al., 1998), whereas other studies have looked at agroecosystems associated with hantavirus reservoirs (Calderon et al., 1999; Yahnke et al., 2001; Carroll et al., 2005).
Habitat selection, which affects nearly all of an individual's subsequent choices concerning food, shelter, risk of predation, and mating opportunities, can be viewed as a hierarchical process in which organisms first choose a general place in which to live (a habitat) and then make subsequent decisions about the use of different patches or microhabitats (Orians and Wittenberger, 1991). The spatial distribution of patches within a habitat affects the ability of different species to coexist in a region. A necessary requirement for coexistence is that each species possesses a habitat or patch in which it is the most efficient forager (Kotler and Brown, 1988).
Most natural assemblages comprise competing species that range from those with narrow habitat requirements (specialists) to others with wider habitat requirements (generalists), with specialists competitively excluding less well-adapted species (Morris, 1996). Brown (1996) demonstrated that widespread habitat generalists might coexist with competing habitat specialists if they exploit the shared environment at a larger spatial scale. The generalist's strategy is to exploit whichever habitats are unused or underused by more specialized species. Because habitat selection can occur anywhere along a continuum of spatial scales from the level of microhabitat to macrohabitat (Kotler and Brown, 1988), attempts were made to sample small mammals at multiple spatial scales for this study. I sought to investigate habitat selection in a variety of habitats, including agroecosystems, at different spatial scales as a mechanism of coexistence among small mammals in the central Paraguayan Chaco.
MATERIALS AND METHODS
The Chaco of Paraguay covers an area of 246 925 km 2 (61% of the land in Paraguay) and has about 3% of the human population in Paraguay (Raidán, 1985). The 2002 census listed 142 501 people living in the three departments of the Chaco (DGEEC, 2002). Many of these are Mennonites, who live in three large colonies in the central Paraguayan Chaco. The Mennonites are ardent farmers with large plantations of peanuts, castor beans, sorghum and cotton (Benirschke et al., 1989). This area of the central Chaco is dominated by extensive agriculture and cattle production. Although a large number of cattle ranches exist throughout the Chaco, it is only in the vicinity of Mennonite colonies that dry farming of crops exists on a large scale (Kleinpenning, 1984). Prior to the colonization of the central Chaco by the Mennonites in the 1930s, most of this area consisted of low, dry thorn forest and native grassland. Remnants of these habitat types, together with agricultural habitats and successional communities, have created a landscape mosaic.
The central Chaco is subtropical and semixeric, with a pronounced seasonality in precipitation. Annual rainfall averages 850 mm, and temperatures range from a mean maximum during the hottest month, January, of ca. 35°C, to a mean minimum temperature of ca. 23°C during the coldest month, July (Fig. 1). A predictable dry season occurs from April through September, with most of the rain occurring during the months of November to February (Fig. 1). Precipitation also can be patchy in distribution. For example, one of the trapping sessions during the wet season was rescheduled because the site received over 300 mm of rain during a two-day period, whereas most other sites received between 30- 60 mm during the same two days.

Fig. 1. Mean monthly maximum and minimum temperatures, and total monthly rainfall, during the study period. Bars represent standard deviation about the mean. Measurements were taken at Fortin Toledo (S 22 o 20'23", W 60 o 20'22").
Gross habitat utilization
Four habitats common to the central Chaco were chosen for trapping of small mammals. Two of these habitats (pastureland and cropland) represented the predominant agricultural land use, whereas the other two habitats (thorn forest and thorn scrub) represented the dominant undisturbed and successional habitats, respectively. Three sites for each habitat type were chosen for a total of 12 sites (Fig. 2). The elevation of the 12 sites ranged from 120 to 149 meters above sea level. Trapping was concentrated in Fortin Toledo (including Corrales), Filadelfia (Chacra Experimental Filidelfia), and Cruce de Los Pioneros (Chacra Experimental Chaco Central, Isla Poi). Because thorn scrub represented a successional habitat, identifying a uniform patch of similar age and sufficient size in each of the trapping centers was difficult. To maximize similarity of sites representing this habitat type, all were located near Estancia Toledo, a large cattle ranch that was abandoned about fifteen years ago.
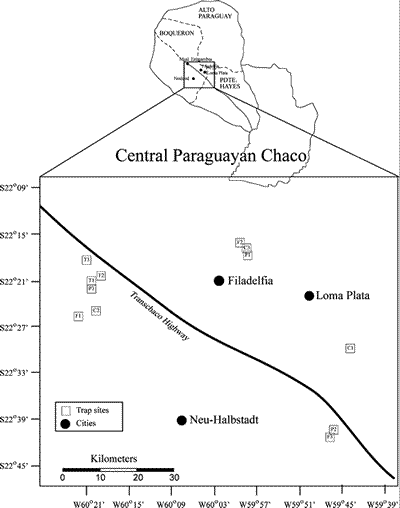
Fig. 2. Map of the central Paraguayan Chaco showing trapping centers near Fortin Toledo, Filadelfia, and Cruce Los Pioneros. Boxes represent trap sites. Letters in boxes represent habitat (P = Pasture, C = Cropland, T = Thorn scrub, F = Forest), and numbers correspond to sites in Table I.

Sorghum was chosen to represent the common cropland habitat in the central Chaco. Bland or unstructured soil and a lack of trees and shrubs characterized cropland habitats. These habitats were disturbed seasonally when crops were harvested. Generally, two to three crops are harvested during the wet season. If the last crop is not productive, the field usually will be left uncut until it is prepared for planting in spring (about mid-September). The typical growing season in the central Chaco is from October through March.
Pasture is the most common land use in the central Chaco and extensive variation occurs from ranch to ranch in the quality and type of pasture. The pastures that I sampled were moderately grazed to ungrazed. In general, these pastures were not devoid of trees and shrubs; with a few large trees that were left by ranchers to provide shade for cattle. These trees are usually red and white quebracho (Schinopsis quebracho-colorado, Aspidosperma quebracho-blanco), although the endemic palo santo (Bulnesia sarmientoi) is commonly found in pastures. The soil was generally hard or more structured than that found in cropland habitats.
Thorn scrub represents the major successional habitat in the central Chaco. The habitat is characterized by hard, sun-baked soils with high clay content, low herbaceous ground cover and a high diversity of cacti and shrubs including Opuntia spp., Acacia spp., and Capparis spp. Moreover pioneering trees such as mesquite (Prosopis spp.) and verde oliva (Cercidium praecox) are characteristic of this habitat. Most of the trees and shrubs range from three to five meters in height, although large trees (15 meters) such as red and white quebracho are scattered throughout this habitat.
Thorn forest is the major forest type in the central Chaco. It is characterized by medium soil hardness, a high diversity of shrubs and trees, a mixed understory of herbs, bromeliads (Bromelia spp.) and cacti (Cleistocactus spp., Eriocerus spp.), and a low canopy (five to ten meters) consisting mainly of Rupretchia triflora with occasional tall trees (15- 20 meters) such as Schinopsis quebracho-colorado, Aspidosperma quebracho-blanco, and the bottle tree Chorisia insignis.
Small mammal trapping was conducted from July 1996 to September 1997. Animals were captured using Sherman live traps (8 x 9 x 23 cm, baited with a mixture of ground peanuts, cracked corn, and rolled oats), and Victor snap traps (baited with manioc). Trapping stations were placed at 10-meter intervals in four parallel traplines of 25 stations. The shape of the grid and spacing of the traps covered one hectare. In addition, every fourth station contained a Tomahawk trap (14 x 14 x 40 cm, baited with manioc, banana, or fish). After six trapping sessions, the Tomahawk traps were not capturing any unique species, and so they were discontinued. At each site, trapping was done for four consecutive nights, once during the wet season and once during the dry season. Four of these sites (one in each habitat type) were trapped in each of two consecutive dry seasons, yielding a total of 28 trapping sessions (Fig. 2). Trapping duration was determined empirically after preliminary studies showed that no new species were encountered after three nights and trap success decreased dramatically by the forth night. Trap success was calculated as the total number of captures divided by the total number of trap nights (i.e., total number of traps minus one-half of snapped-but-empty traps) (Mills et al., 1991b). Because trapping effort and grid size was constant, relative densities of small mammal species were estimated as total captures during the trapping session on a one hectare grid (Brower et al., 1998).
Microhabitat utilization
Quantitative analysis of vegetation was conducted to characterize structural microhabitat within a five-meter radius of each trapping station where animals were captured (Patterson et al., 1990; Kelt et al., 1994). Measurements included distance, diameter, and height of nearest shrub, number of species of shrubs and trees, number of shrubs over 100 cm in height, number of trees over 5 cm in diameter, number of trees over 15 cm in diameter, maximum height of trees, mean percent ground cover (bare, herbaceous, tree plus shrub), percent litter cover, and soil hardness (measured with a soil penetrometer). In addition, numerically dominant trees and shrubs were identified to species at each trap station using descriptions in Arenas (1981) and Lopez et al. (1987). The term "microhabitat" refers to the structural characteristics within a five-meter radius of each trapping stations, whereas the term "habitat" refers to the habitat where a trapping grid was located (e.g. cropland, pasture, thorn scrub, thorn forest).
Data concerning vegetation were tested for normality using the Shapiro-Wilk statistic (Shapiro and Wilk, 1965), as well as the g 1 and g 2 statistics for skewness and kurtosis (Zar, 1984), and transformed where necessary to meet the assumptions of normality. Transformations were not necessary for most data; a square-root transformation was used for distance to nearest shrub, diameter of nearest shrub, number of trees over 5 cm in diameter, number of trees over 15 cm in diameter, and soil hardness, and square-root transformations followed by an arcsine transformation were used for litter cover and percent herbaceous ground cover. All statistical analyses were performed with SAS version 6 (SAS Institute, 1990) or SPSS version 10.0 (SPSS, 1999).
Multivariate analysis of variance (MANOVA) was conducted on vegetative parameters to determine which differed significantly among mammalian species. Vegetative parameters deemed significant using the Student-Neuman-Keuls post-hoc test were used in a principal components analysis (PCA), in order to reduce the multidimensional variation to fewer axes. Correlation matrices were used to equalize the influence of variables with highly different ranges. Captures of rodents at trapping stations (mean and 95% confidence interval) subsequently were superimposed onto reduced dimensions to visualize habitat separation.
RESULTS
Gross habitat utilization
Total rainfall during the 14 month study at Fortin Toledo was 1052 mm, with 727 mm (69%) of precipitation occurring between November and February (Fig. 1). A total of 1089 small mammals representing 13 species was captured during 23 296 trap nights (Table 1). Trap success averaged 5.55% for the entire study but ranged considerably from 0.4% to 50.5% (Fig. 3). Trap success was higher during the dry season (mean = 8.78%, n = 16) than during the wet season (mean = 1.25%, n = 12) (Fig. 3). Each small mammal species was more abundant during the dry season. Only Monodelphis domestica was captured more often during the wet season. Only 118 small mammals were captured during the entire wet season, representing 10.8% of all captured mammals during the 14 month study. Holochilus chacarius and Bolomys lasiurus exhibit a strong preference for pastures, whereas species such as Calomys laucha and Akodon toba were captured in all habitats (Table 1). Species of Calomys were more common in agricultural habitats, whereas Graomys, Oligoryzomys, and marsupials were more common in wooded habitats (Table 1).
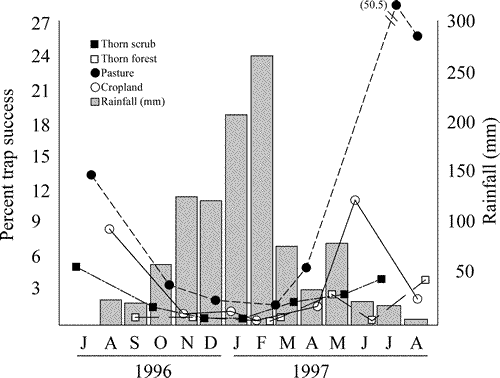
Fig. 3. Total rainfall (measured at Fortin Toledo) and percent trap success in each of four habitats sampled in the central Paraguayan Chaco. Trap success was calculated as the total number of captures divided by the total number of trap nights (i.e., total number of traps minus one-half of snapped-but-empty traps), and each point represents total trap success during a four day trapping session.
Microhabitat utilization
Based on vegetation metrics at all trapping sites (n = 837), species differed significantly (Table 2). However, no difference existed among species for soil hardness (Table 2), so this variable was excluded from the principal components analysis. Three eigenvalues were greater than unity, and accounted for 81.0% of the variability among capture sites (Table 3). Univariate ANOVAs conducted on PC axes scores revealed significant segregation of species along axis 1 (F = 27.64, df = 11, P < 0.001) and axis 2 (F = 5.41, df = 11, P < 0.001). PC 1 loaded heavily on variables associated with trees and shrubs, whereas PC 2 was strongly associated with herb height and litter (Table 3). When captures of small mammals were mapped onto PC axes, considerable overlap characterized species (Fig. 4). However, Student-Newman-Keuls' a posteriori tests on component scores identified separation of small mammals found in agricultural habitats versus those in wooded habitats (Fig. 4). Multiple captures at a trap site violates the assumption of independence in the species data since the same vegetation measurements were used more than once. The data were analyzed discarding trap sites with multiple captures (n=201) and the results for both MANOVA and PCA were almost identical.


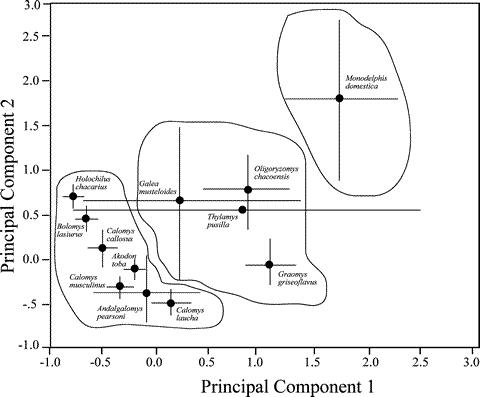
Fig. 4. Results of principal components analysis of habitat variables associated with capture sites of small mammals in the central Paraguayan Chaco. Projections are means with 95% confidence intervals. Ellipses represent groupings along PC 1 identified by Student-Newman-Keuls' a posteriori tests.
DISCUSSION
Published reports of habitat use by small mammals in the Chaco are generally in agreement with these results, although with noteworthy exceptions. Research concerning the morphological and behavioral specialization of these species elsewhere in South America provides some insights into their use of habitat in the central Chaco.
Akodon toba: is endemic to the Chaco of western Paraguay, eastern Bolivia, and northwestern Argentina (Myers, 1989). In the western Chaco, individuals were captured in thorn forests and semi-desert scrublands. Although A. toba reached its highest densities in pasture, it also was captured in wooded habitats and cropland. In multivariate space, it occupied an intermediate position with respect to PC1 and PC2 (Fig. 4). Although it does not exhibit strong microhabitat associations within agricultural habitats, it is associated with high herbaceous ground cover in wooded habitats (Yahnke, 1999). An average of 4.5 embryos (n = 4, range 1-7) was observed, with all pregnant females captured during December and January.
Andalgalomys pearsoni: is endemic to the western Paraguayan Chaco and was first described by Myers (1977). It inhabits dry grasslands in islands of the western Chaco, leading Myers (1982) to suggest that it probably is restricted to the drier, sandier portions of the western Chaco (along with Calomys laucha). The majority of captures during this study were in cropland habitats, with occasional captures in thorn scrub. Andalgalomys pearsoni, along with C. laucha and Graomys griseoflavus, are associated with microhabitats that have relatively little ground cover (Table 2). It was never trapped in forested habitats. One pregnant female with five embryos was captured in January.
Bolomys lasiurus: is a grassland species, although it is trapped occasionally in forest. In the Caatinga of Brazil, B. lasiurus was the most abundant species, with densities as high as 187 individuals per hectare (Alho, 1982). Bolomys lasiurus was seasonally rare in the Chaco, but during the dry season of 1997, grew to densities of 208 individuals per hectare at one pasture site. Bolomys lasiurus was captured almost exclusively in agricultural habitats, and with Holochilus chacarius, was associated most strongly with high herbaceous ground cover and litter (Fig. 4). This is consistent with work by Streilein (1982) who found B. lasiurus exclusively in agricultural habitats in the Caatinga. Pregnant females averaged 5.8 embryos (n = 5, range 5-7) and were captured in the months of January, April and May.
Calomys callosus: in Paraguay, Calomys callosus is found exclusively in the Chaco, where it is widely distributed in grassland and arid thorn scrub (Myers, 1982). In this study, the highest densities were in pasture, with occasional captures in other habitats. Only one individual was captured in thorn scrub (Table 1). Along with B. lasiurus and H. chacarius, it associated most strongly with high amounts of litter and high herbaceous ground cover (Fig. 4). Pregnant females had an average of 6.25 embryos (n = 4, range 4-11) and were captured in the months of May, August, and December. Calomys callosus (or a close relative, see Salazar-Bravo et al., 2002) was identified as the reservoir for Machupo virus, the etiological agent for Bolivian Hemorrhagic Fever (Johnson et al., 1966). At the time Machupo virus was identified, C. callosus inhabited the moist forest regions in the Beni, but clearing of forests for agriculture during the 1950s provided a more productive habitat, and populations of C. callosus increased dramatically (Johnson et al., 1966).
Calomys laucha: this species along with Akodon azarae and C. musculinus has been studied extensively in the pampas and agroecosystems of Argentina (Bilenca et al., 1992; Busch and Kravetz, 1992). In Argentina C. laucha is most common in croplands (corn and wheat) and may be excluded from border habitats by the larger A. azarae. Mills et al. (1991b) suggested that Calomys were able to quickly take advantage of unstable, temporarily suitable habitats such as crop fields. In the Chaco, high densities of C. laucha were recorded in agricultural habitats as well as one thorn scrub site (Table 1). In both agricultural and successional habitats, C. laucha was associated with less herbaceous ground cover than were most other small mammal species (Table 2). Pregnant females had an average of 5.15 embryos (n = 38, range 3-8), and were encountered most frequently during the austral fall and winter months. In the central Chaco, populations of C. laucha began to increase in April whereas increases for other small mammal species were not detected until May or June (Yahnke, 1999).
Calomys musculinus: in Argentine agroecosystems, C. musculinus was associated strongly with cropland. Similar to C. laucha, it takes advantage of temporarily unstable habitats for rapid reproduction (Mills et al., 1991b). Pregnant females had an average of 6.07 embryos (n = 14, range 2-8) with captures restricted to May and June. Myers (1982) listed C. musculinus as occurring strictly in eastern Paraguay. This is the first of the species in large numbers from the central Chaco. In the Chaco, it was captured commonly in cropland and occurred at high densities at one pasture site (Table 1).
Galea musteloides: seven Galea musteloides were captured, mostly in pasture or forest. In the Salta region of Argentina, Galea is common in croplands as well as along stream edges (Redford and Eisenberg, 1989). In the Caatinga it is found in a variety of naturally occurring and agricultural habitats (Streilein, 1982). Captures reported in this study probably under represent its relative abundance in the central Chaco. Although no captures were recorded in thorn scrub, Galea was observed daily in this habitat utilizing its above ground runways.
Graomys griseoflavus: this was the only species to maintain high population numbers throughout the wet season (Yahnke, 1999). It has been captured in diverse habitats, including cultivated areas in northern Argentina (Mares et al., 1989), and is distributed widely in grasslands and thorn scrub in the Paraguayan Chaco (Myers, 1982). In this study, it was more associated strongly with forest and successional habitats, although it occurred in high densities at one of the pasture sites (Table 1). This pasture site, near Estancia Toledo, had a high amount of shrubs and trees relative to the other pasture sites. PCA corroborated the association of G. griseoflavus with tree and shrub variables (Fig. 4). Pregnant females averaged 5.8 embryos (n = 5, range 4-8) and were captured between January and March.
Holochilus chacarius: marsh rats of the genus Holochilus are specialized for semi-aquatic life and are found commonly in swamps, grasslands, and other moist, unforested habitats throughout most of South America, east of the Andes (Hershkovitz, 1955). In the Paraguayan Chaco, H. chacarius was associated strongly with pasturelands, with few captures in the other three habitats sampled (Table 1). Along with B. lasiurus, it preferred areas with high amounts of litter and high herbaceous ground cover (Fig. 4). Numbers were highest during the dry season and there was no evidence of standing water near any pastures sampled, although in many of the pastures in this region, cattle ponds have been dug to provide water throughout the dry season. H. chacarius may utilize these habitats during the dry season, when standing water is not present in other regions. Of 44 captured individuals, only three were pregnant. These included both individuals from the cropland habitat in May. The average number of embryos was 7 (n = 3, range 4-9).
Oligoryzomys chacoensis: this species was described by Myers and Carleton (1981). The holotype was collected in an area of dry grass surrounded by thorn scrub, about 20 km from trapping grids at the Chacra Experimental Chaco Central. Myers and Carleton (1981) found O. chacoensis to be ubiquitous in thorn scrub, but found that its highest densities were in grassland. Although this species occurred in each of the four habitats in this study, its highest density (21 individuals per hectare) was in forest. This is consistent with the observation that the short hind feet of O. chacoensis indicate a semi-arboreal existence (Myers and Carleton, 1981). O. chacoensis has an affinity for sites with tree and shrub characters (Fig. 4). Myers and Carleton (1981) found an average of 4.6 embryos (range 2-5) with reproduction primarily occurring in January and February. The two pregnant individuals in this study (2 and 5 embryos) were trapped in April and July.
Mouse opossums
Fourteen individuals representing three species of marsupials were trapped during this study, 12 in forest (Table 1). Each of the three species was associated with forest, although Monodelphis domestica has been captured in grassy areas, brush piles, and domestic settings (Myers and Wetzel, 1979). Marsupials evince strong microhabitat associations with tree and shrub height as well as high amounts of litter (Fig. 4). Because many mouse opossums are primarily carnivorous or insectivorous, marsupials may have been underrepresented in the traps, in part because of choice of bait. Also, all trapping was conducted at ground level, which could underestimate the abundance of small arboreal mammals.
Community-level patterns
The distribution and abundance of small mammals in the central Paraguayan Chaco is complex. Populations of most small mammal species in this region were higher during the d ry season than during the wet season (Fig. 3). Mammalian assemblages were diverse, with compositional variation between similar habitats from different locations (Table 1). In general, pastures exhibited the highest species richness and greatest abundance of small mammals. In contrast, species richness varied widely in the other three habitat types, with as few as two species at one of the forest sites and as many as nine species at one of the cropland sites (Table 1). Only B. lasiurus and H. chacarius demonstrated a strong association with a single habitat type (pasture), although each was captured occasionally in cropland (Table 1).
Relative distance between sites may be more useful than gross habitat designations in predicting community composition in the central Chaco since different gross habitat patches in close proximity to one another appeared to share a greater proportion of species than apparently similar gross habitat patches that were not close to one another. The pasture site at Toledo shared 91% of the small mammal species with the cropland site in nearby Corrales (about 2 km). In contrast, the three thorn scrub sites, which are close to each other in both distance and vegetative structure, never shared more than 50% of the small mammal species. Although factor analysis demonstrated differences among gross habitats based on selected vegetation metrics (Yahnke, 1999), these broad habitat categories were not necessarily good predictors of small mammal community composition.
Implications for coexistence of small mammals
Small mammals may partition microhabitats within wooded and agricultural habitats. In wooded habitats, marsupials and Oligoryzomys chacoensis were captured at sites with tall trees and shrubs, suggesting an arboreal association. Coexistence of O. chacoensis and marsupials within these microhabitats is likely due to partitioning of food, with marsupials feeding primarily on invertebrates and small vertebrates and O. chacoensis being more granivorous (Meserve et al., 1988).
Akodon toba is the only species in this woodland community with a strong preference for high herbaceous ground cover. Graomys griseoflavus is a habitat generalist without microhabitat preference in wooded habitats, whereas Calomys laucha is captured at sites with low amounts of litter and hard soils. These preferences are likely due to higher densities of C. laucha in thorn scrub habitats with harder, sun-baked soils and less litter than in forested habitats. However, the preference for hard soils may be significant in wooded habitats. Hodara et al. (1997) found that C. laucha dug burrows under laboratory conditions, and that hard, compacted soils as opposed to loose, unstructured soils are best for digging burrows.
Partitioning of microhabitats is more complex in agroecosystems that have higher species richness and less habitat complexity. A number of important environmental parameters likely were not measured in this study, although inferences are possible with available data. Holochilus chacarius and B. lasiurus were associated strongly with microhabitats with high herbaceous ground cover. Additionally, H. chacarius was associated with sites containing high litter. Four species (Akodon toba, Calomys callosus, C. musculinus, and Graomys griseoflavus) did not display microhabitat preferences within agroecosytems (Yahnke, 1999). Coexistence of these species is due probably to partitioning of food resources or time. Priotto and Polop (1997) found that Akodon azarae was mainly active during daytime and crepuscular hours, whereas Calomys venustus was particularly active during crepuscular and night hours in agroecosystems in Cordoba, Argentina. Ellis et al. (1998) found that Akodon and Bolomys were primarily entomophagous in Argentina, feeding on arthropods, whereas other rodent species ate dicot seeds. Further, C. laucha and C. musculinus had nearly identical diets throughout the year, but exhibited high spatial segregation in Argentine agroecosystems (Ellis et al., 1998). That finding corroborates this study, in which C. laucha and Andalgalomys pearsoni were restricted to microhabitats with low herbaceous ground cover. It is unclear whether C. laucha prefers microhabitats with bare soils, or it is excluded from more preferred sites. Calomys laucha is the only species that demonstrates negative pair-wise correlations with all other species, suggesting avoidance behavior (Yahnke, 1999). In central Argentina, habitat segregation between species may be attributable to interspecific competition that causes C. laucha to shift to use of less preferred cropfields (Hodara et al., 1997). Shifting to less preferred gross habitats such as cropfields or even less preferred microhabitats may be an adaptive behavior for subordinate species in a two-species system when densities are low (Rozenzweig, 1979). Thus, C. laucha may avoid direct competition with other species of small mammals in the central Chaco by exploiting less preferred gross habitats (i.e. cropfields) as well as less preferred microhabitats such as those with little herbaceous cover and litter.
ACKNOWLEDGEMENTS
I am indebted to W. Schmidt, R. Mueller, A. Glatzle, and the staffs at the experimental farms in Isla Poi, Filadelfia, and Chaco Central, who allowed me to set traps and moved cows. This work would not have been possible without the financial support of the Zoological Society of San Diego, the Centers for Disease Control and Prevention, and the Graduate School at Northern Illinois University. I thank P. Meserve, B. Patterson, B. Stanley, J. Mills, P. Myers, and M. Mares for support and encouragement during my time in Paraguay. I am grateful for the thoughtful comments of Mike Willig and three anonymous reviewers. Finally, I extend my deepest gratitude to my wife, Bridget, who ran the tagua project at Fortin Toledo while I was trapping and processing animals.
LITERATURE CITED
ALHO CJR. 1982. Brazilian rodents: their habitats and habits. Pp. 143-166, in: Mammalian biology in South America (MA Mares and HH Genoways, eds.). Special Publication Series, Pymatuning Laboratory of Ecology. [ Links ]
ARENAS P. 1981. Etnobotanica Lengua-Maskoy. Fundación para la educación, la ciencia, y la cultura, Buenos Aires, Argentina. [ Links ]
BENIRSCHKE K, ML BYRD and RJ LOWE. 1989. The Chaco region of Paraguay: peccaries and Mennonites. Interdiscipinary Science Reviews 14:144-147. [ Links ]
BILENCA DN, FO KRAVETZ and GA ZULETA. 1992. Food habits of Akodon azarae and Calomys laucha (Cricetidae, Rodentia) in agroecosystems of central Argentina. Mammalia 56:371-383. [ Links ]
BROWER JE, JH ZAR and CN von ENDE. 1998. Field and Laboratory Methods for General Ecology. Fourth ed. WCB/McGraw-Hill, Dubuque. [ Links ]
BROWN JS. 1996. Coevolution and community organization in three habitats. Oikos 75:193-206. [ Links ]
BUSCH M and FO KRAVETZ. 1992. Competitive interactions among rodents (Akodon azarae, Calomys laucha, C. musculinus, and Oligoryzomys flavescens) in a two-habitat system. I. Spatial and numerical relationships. Mammalia 56:45-56. [ Links ]
CALDERON G, N PINI, J BOLPE, S LEVIS, J MILLS, E SEGURA, N GUTHMANN, G CANTONI, B J ECKER, A FONOLLAT, C RIPOLL, M BORTMAN, R BENEDETTI, and D ENRIA. 1999. Hantavirus reservoir hosts associated with peridomestic habitats in Argentina. Emerging Infectious Diseases 5:792-7. [ Links ]
CARROLL DS, JN MILLS, MJ MONTGOMERY, DG BAUSCH, PJ BLAIR, JP BURANS, V FELICES, A GIANELLA, N IIHOSHI, ST NICHOL, JG OLSON, DS ROGERS, M SALAZAR, and TG KSIAZEK. 2005. Hantavirus pulmonary syndrome in central Bolivia: relationships between reservoir hosts, habitats, and viral genotypes. American Journal of Tropical Medicine and Hygiene 72:42-46. [ Links ]
DGEEC. 2002. Resultados finales censo nacional de población y viviendas, año 2002, total país. Dirección General de Estadística, Encuestas, y Censos, Asunción, Paraguay. [ Links ]
ELLIS BA, JN MILLS, JE CHILDS, MC MUZZINI, KT MCKEE, JR., DA ENRIA, and GE GLASS. 1997. Structure and floristics of habitats associated with five rodent species in an agroecosystem in Central Argentina. Journal of Zoology (London) 243:437-460. [ Links ]
ELLIS BA, JN MILLS, GE GLASS, KT MCKEE, JR., DA ENRIA, and JE CHILDS. 1998. Dietary habits of the common rodents in an agroecosystem in Argentina. Journal of Mammalogy 79:1203-1220. [ Links ]
HERSHKOVITZ P. 1955. South American marsh rats of the genus Holochilus with a summary of Sigmodont rodents. Fieldiana: Zoology 37:639-673. [ Links ]
HODARA K, OV SUAREZ and FO KRAVETZ. 1997. Nesting and digging behavior in two rodent species (Akodon azarae and Calomys laucha) under laboratory and field conditions. Zeitschrift für Säugetierkunde 62:23-29. [ Links ]
JOHNSON K, M KUNS, R MACKENZIE, P WEBB, and C YUNKER. 1966. Isolation of Machupo virus from wild rodent Calomys callosus. American Journal of Tropical Medicine and Hygiene 15:103-106. [ Links ]
KELT DA, PL MESERVE and BK LANG. 1994. Quantitative habitat associations of small mammals in a temperature rainforest in southern Chile: Empirical patterns and the importance of ecological scale. Journal of Mammalogy 75:890-904. [ Links ]
KLEINPENNING J. 1984. The integration and colonization of the Paraguayan Chaco. Geografisch en Planologisch Instituut, Haag, Holland. [ Links ]
KOTLER BP and JS BROWN. 1988. Environmental heterogeneity and the coexistence of desert rodents. Annual Review of Ecology and Systematics 19:281-307. [ Links ]
LOPEZ J, E LITTLE, G RITZ, J ROMBOLD, and W HAHN. 1987. Árboles comunes del Paraguay, ñande yvyra mata kuera. US Government Printing Office, Asunción. [ Links ]
MARES MA, RA OJEDA and RM BARQUEZ. 1989. Guide to the mammals of Salta. University of Oklahoma Press, Norman. [ Links ]
MESERVE PL, BK LANG and BD PATTERSON. 1988. Trophic relationships of small mammals in a Chilean temperate rainforest. Journal of Mammalogy 69:721-730. [ Links ]
MILLS JN, BA ELLIS, K MCKEE, G CALDERON, J MAIZTEGUI, G NELSON, TG KSIAZEK, CJ PETERS, and JE CHILDS. 1992. A longitudinal study of Junin virus activity in the rodent reservoir of Argentine hemorrhagic fever. American Journal of Tropical Medicine and Hygiene 47:749-63. [ Links ]
MILLS JN, BA ELLIS, K MCKEE, TG KSIAZEK, J ORO, JI MAIZTEGUI, G CALDERON, CJ PETERS, and JE CHILDS. 1991a. Junin virus activity in rodents from endemic and nonendemic loci in central Argentina. American Journal of Tropical Medicine and Hygiene 44:589-97. [ Links ]
MILLS JN, BA ELLIS, KT MCKEE, JI MAIZTEGUI, and JE CHILDS. 1991b. Habitat associations and relative densities of rodent populations in cultivated areas of central Argentina. Journal of Mammalogy 72:470-479. [ Links ]
MORRIS DW. 1996. Coexistence of specialist and generalist rodents via habitat selection. Ecology 77. [ Links ]
MYERS P. 1977. A new phyllotine rodent (genus Graomys) from Paraguay. Occasional papers of the Museum of Zoology, University of Michigan 676:1- 7. [ Links ]
MYERS P. 1982. Origins and affinities of the mammal fauna of Paraguay. Pp. 85-94, in: Mammalian biology in South America (MA Mares and HH Genoways, eds.). Special Publication Series, Pymatuning Laboratory of Ecology. [ Links ]
MYERS P. 1989. A preliminary revision of the varius group of Akodon (A. dayi, dolores, molinae, neocenus, simulator, toba, and varius). Pp. 5-54, in: Advances in Neotropical mammalogy (JF Eisenberg and KH Redford, eds.). Sandhill Press. [ Links ]
MYERS P and MD CARLETON. 1981. The species of Oryzomys (Oligoryzomys) in Paraguay and the identity of Azarae's "rat sixieme ou rat a tarse noir". Miscellaneous Publications Museum of Zoology, University of Michigan 161:1-41. [ Links ]
MYERS P and RM WETZEL. 1979. New records of mammals from Paraguay. Journal of Mammalogy 60:638-641. [ Links ]
ORIANS GH and JF WITTENBERGER. 1991. Spatial and temporal scales in habitat selection. American Naturalist 137:S29-S49. [ Links ]
PATTERSON BD, PL MESERVE and B LANG. 1990. Quantitative habitat associations of small mammals along an elevational transect in temperate rainforests of Chile. Journal of Mammalogy 71:620-633. [ Links ]
PRIOTTO J and J POLOP. 1997. Space and time use in syntopic populations of Akodon azarae and Calomys venustus (Rodentia, Muridae). Zeitschrift für Säugetierkunde 62:30-36. [ Links ]
RAIDÁN G. 1985. Perfil ambiental del Paraguay. Instituto Internacional para el Desarrollo y Medio Ambiente. Secretaria Técnica de Planificación de Paraguay, Agencia Internacional para el desarrollo de los Estados Unidos de Norte América, Asunción. [ Links ]
REDFORD KH and JF EISENBERG. 1989. Mammals of the Neotropics, Volume 2. The southern cone: Chile, Argentina, Uruguay, Paraguay. The University of Chicago Press, Chicago. [ Links ]
ROZENZWEIG ML. 1979. Optimal habitat selection in two-species competitive systems. Fortschritte der Zoologie 25:283-293. [ Links ]
SALAZAR-BRAVO J, JW DRAGOO, MD BOWEN, CJ PETERS, TG KSIAZEK, and TL YATES. 2002. Natural nidality in Bolivian hemorrhagic fever and the systematics of the reservoir species. Infection, Genetics and Evolution 1:191-199. [ Links ]
SAS INSTITUTE Inc. 1990. SAS/STAT user's guide, Version 6. Fourth ed. In. 6 ed. Cary, North Carolina: SAS Institute, Inc. [ Links ]
SHAPIRO SS and MB WILK. 1965. An analysis of variance test for normality (complete samples). Biometrika 52:591-611. [ Links ]
SPSS. 1999. SPSS Base 10.0 for Windows User's Guide. In: SPSS Inc., Chicago IL. [ Links ]
STREILEIN KE. 1982. The ecology of small mammals in the semiarid Brazilian Caatinga. IV. Habitat Selection. Annals of Carnegie Museum 51:331-343. [ Links ]
YAHNKE CJ. 1999. Community ecology and habitat associations of small mammals in the endemic region of Hantavirus Pulmonary Syndrome in the central Paraguayan Chaco. Ph. D. Dissertation. Northern Illinois University, DeKalb. [ Links ]
YAHNKE CJ, PL MESERVE, TG KSIAZEK, and JN MILLS. 2001. Patterns of infection with Laguna Negra virus in wild populations of Calomys laucha in the central Paraguayan Chaco. American Journal of Tropical Medicine and Hygiene 65:768-76. [ Links ]
ZAR J. 1984. Biostatistical analysis. Second ed. Prentice Hall, Inc., Englewood Cliffs, New Jersey. [ Links ]
Recibido 7 julio 2005.
Aceptación final 7 abril 2006.














