Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Mastozoología neotropical
versão impressa ISSN 0327-9383versão On-line ISSN 1666-0536
Mastozool. neotrop. v.13 n.2 Mendoza dez. 2006
A distinctive new cloud-forest rodent (Hystricognathi: Echimyidae) from the Manu Biosphere Reserve, Peru
Bruce D. Patterson1 and Paul M. Velazco1, 2
1Department of Zoology, Field Museum of Natural History, 1400 S. Lake Shore Dr, Chicago IL 60605-2496 USA. 2Department of Biological Sciences, University of Illinois at Chicago, 845 W. Taylor St, Chicago IL 60607 USA
ABSTRACT: Recent surveys in Peru 's Manu National Park and Biosphere Reserve uncovered a new species of hystricognath rodent, a spiny rat (Echimyidae) with dense, soft fur. Inhabiting Andean cloud-forests at 1900 m, the new rodent belongs to a radiation of "brush-tailed tree rats" previously known only from the Amazon, Orinoco, and other lowland river drainages. Phylogenetic analysis of morphology (cranial and dental characters) unambiguously allies the new species with species of Isothrix. While the inclusion of the new taxon does not resolve the "star phylogeny" which underlies the echimyid radiation, it identifies the Andes as a possible theatre for the evolution of some soft-haired members of the group. The new species is described and a revised diagnosis of the genus Isothrix is presented.
RESUMEN: Nueva especie de roedor (Histriocognathi: Echimydae) del bosque nublado en la Reserva de Biosfera del Manu, Perú. Recientes inventarios en el Parque Nacional y Reserva de Biosfera del Manu en Perú revelaron una nueva especie de roedor histricognato, una rata espinosa (Echimyidae) con pelaje denso y suave. Este roedor habita el bosque nublado andino a 1900 m, y pertenece a la radiación de los Echimyidae conocidos previamente de las cuencas de los ríos Amazonas, Orinoco, y de otras cuencas de ríos de bosque bajo. El análisis filogenético morfológico (caracteres craneales y dentales) inequívocamente agrupa la nueva especies con las otras especies de Isothrix. A pesar de que la inclusión del nuevo taxón no resuelve la filogenia en forma de estrella que domina la radiación de los Echimyidae, sugiere a los Andes como posible escenario para la evolución de algunos de los miembros del grupo de pelo suave. Esta nueva especie es descrita y se presenta un diagnóstico revisado del género.
Key words. Andes. Echimyidae. Isothrix. Morphology. Phylogenetics. Species description. Systematics.
Palabras clave. Andes. Echimyidae. Isothrix. Morfología. Filogenética. Descripción de especie. Sistemática.
The Neotropics are home to roughly a quarter of the world's mammal species (Nowak, 1999; Wilson and Reeder, 2005), and scientists working there frequently discover new taxa. On average during the 1990s, scientists described a new genus and 8 new species from the Neotropics each year (Patterson, 2000), and three times that number of species became newly validated by studies in museums and biochemical labs (Patterson, 1996). One of the world's richest concentrations of plants and animals live on the eastern slopes of the Andes Mountains in tropical South America. Towering 3000- 4000 m above humid tropical lowland forests, the Andes are annulated by a succession of habitats stratified by elevation. Bathed by easterly winds and inundated by extensive regions of endemism on either side-Amazonia on the east and the Altiplano on the west, the Andes are also the world's longest continuous mountain chain. This constellation of factors apparently multiplies the richness of species of its regional pools (Rahbek, 1997; Patterson et al., 1998; Fjeldså, 1999).
During a three-year survey to extend inventories of mammals and birds in the Manu Biosphere Reserve, joint expeditions from Field Museum of Natural History, Chicago, and Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Lima, collected various new species of rodents, bats, and a marsupial. Mammals described to date include a previously enigmatic mouse important to resolving sigmodontine relationships (Luna and Patterson, 2003; D'Elía et al., 2006), several broad-nosed bats (Velazco, 2005), and two short-tailed bats (Pacheco et al., 2004; Solari and Baker, 2006). But the most remarkable-looking species was a large nocturnal rodent we encountered in cloud forest at 1900 m elevation. Its heavy build, blockish head and fleshy nose suggested affinities with the caviomorphs, probably either the Abrocomidae (chinchilla rats) or the Echimyidae (spiny rats). Long, lax hair and a noticeable blackish crest on the crown, nape and shoulders of the rodent only added to its striking appearance.
Subsequent examination of the skull and teeth and museum comparisons confirmed the identity of this striking rodent as a distinctive highland species of Isothrix (Echimyidae). Torós or brush-tailed tree rats are otherwise known only from tropical lowland forests, and this new Andean species offers a new context for evaluating the evolution and biogeography of this group. In this paper, we describe the new species, assess its affinities to Isothrix and other related arboreal "spiny rats", and offer an amended diagnosis of the genus.
METHODS
To characterize the cranial and dental morphology of the new species, we denoted cranial features following Woods and Howland (1979) and Bezuidenhout and Evans (2005) and used the dental terminology of Patterson and Wood (1982) and Carvalho and Salles (2004). External measurements were recorded from the original field tags, and external characters are as described by Brown (1971) and Brown and Yalden (1973). Ages were estimated following the criteria of Patton and Rogers (1983). Capitalized color terms are as in Ridgway (1912). Cranial measurements (see Voss et al., 2001) were taken with digital calipers to the nearest 0.01 mm, as follows: CIL, condylo-incisive length; LD, length of diastema; LIF, length of incisive foramina; BIF, breadth of incisive foramina; MTR, maxillary toothrow length; LM, length of the molars; BM1, breadth of M1; ZB, zygomatic breadth; ZL, zygomatic length; LN, length of nasals; LIB, least interorbital breadth; and BB, breadth of braincase. Additionally, we also measured the following, which were described and figured by Voss and Angermann (1997): BP4, breadth of P4; HIF, height of infraorbital foramen; BNA, breadth of nasal aperture; DI, depth of incisor; BIT, breadth of incisor tips. Specimens examined are listed in Appendix 1. Summary statistics for these variables were generated using Statistica 6.0 (StatSoft Inc, 2003).
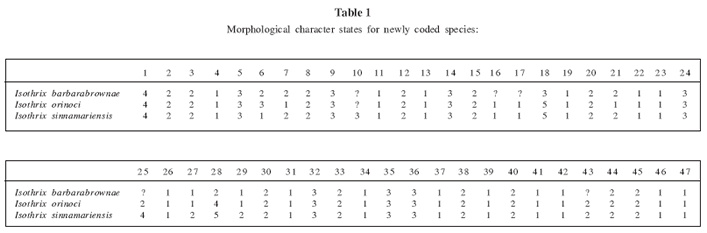
To assess the phylogenetic position of the new species using morphology, we analyzed variation in 47 external, cranial, and dental characters identified by Emmons (2005) as useful in assessing arboreal Echimyidae. Characters and their states are discussed and illustrated in Emmons (2005) and are listed in Appendix 2. Character states for the new species and for I. sinnamariensis and I. orinoci, not included in Emmons's analysis, are presented in Table 1. The resulting matrix of 36 taxa and 47 characters was analyzed with PAUP* version 4.0b10 (Swofford, 2002), using unordered states and the heuristic search specifying the tree bisection-reconnection option. The topology of shortest-length trees was then subjected to bootstrap analysis (1000 replicates).
RESULTS
The cladistic analysis of 47 morphological characters among 36 taxa identified 304 trees of 250 steps (Fig. 1; CI = 0.380; CI excluding uninformative characters = 0.375; RI = 0.668). In all of the most parsimonious trees, the new species was grouped with the 4 examined species of Isothrix (Fig. 1). A monophyletic Isothrix that includes the new species was recovered in 93% of the bootstrap replicates, a clustering of species equaled elsewhere in the tree only by Echimys chrysurus + E. saturnus (97%). The bootstrap-consensus tree placed all Isothrix species in a polytomy. As in Emmons's (2005) analysis, Isothrix was uncertainly grouped in a massive polytomy involving most recognized genera of Echimyinae. On these bases, we feel justified in describing the new form as a species of Isothrix, and regard the genus as sufficiently distinct to obviate the need for comparing the new species to other genera of Echimyidae.
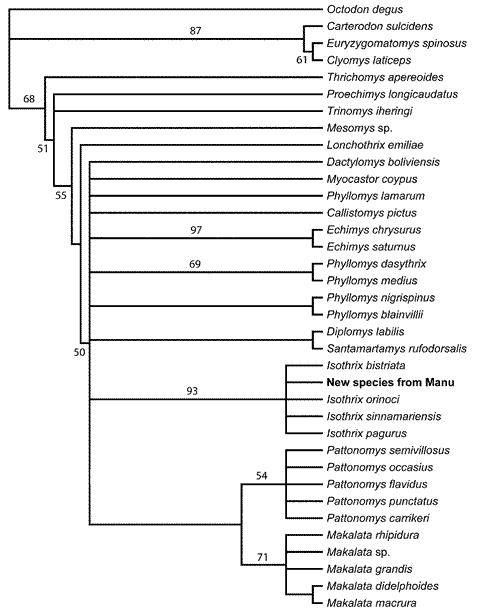
Fig. 1. A strict consensus of 304 trees relating various echimyid rodents by the morphology of 47 external cranial, and dental characters, using Octodon as outgroup. The tree has 250 steps and a consistency index of 0.380 and a retention index of 0.668. Numbers above nodes indicate bootstrap values in excess of 50%. The new species from Manu is unambiguously placed as a species of Isothrix, and Isothrix is one of the best-supported clades in the morphological tree.
Isothrix Wagner, 1845
Synonyms. -Lasiuromys Deville 1852 (based on Lasiuromys villosus Deville, 1852).
Type species. -Isothrix bistriata Wagner, 1845, by subsequent designation (Goldman, 1916).
Included species.- I. bistriata Wagner, 1845 (including boliviensis, molliae, pachyura, villosa); I. negrensis Thomas 1920; I. orinoci (Thomas, 1899); I. pagurus Wagner, 1845 (including crassicaudus); I. sinnamariensis Vie et al. 1996, and the new species from Manu, described below.
Revised diagnosis.-A soft-furred echimyid rodent with parietals ridged, upper cheek-teeth not tending to become separated into transverse plates, fur soft, without bristles or spines, and tail long and densely haired. The following combination of characters appears to be diagnostic: The tooth-rows are relatively long and their occlusal planes are slightly canted laterad; the lower premolar anterolophid is triangular with a flexid opening labially, the labial and lingual flexids of the lower molars are oriented perpendicularly to the axis of the tooth, the metaflexid meeting or almost meeting the hypoflexid in mid-tooth; the squamosotympanic fenestra typically unenclosed, appearing as a large, open slit; the squamosal is scarcely exposed (< 1 mm) at the squamosotympanic foramen; the lateral tube of the auditory meatus is oriented antero-ventrally; the inferior jugal process is weak and located anterior to superior jugal process; the palatal vacuities lining the alisphenoid-basisphenoid region are small but distinct and rounded; the mandibular foramen is located on the mandibular ramus, anterior to the condyloid ridge; the angular process is short, subequal in size to the condyloid process.
Remarks.-This genus was long thought to contain only the two species described by Wagner 160 years ago, I. bistriata and I. pagurus (Emmons and Feer, 1990; Woods, 1993), as well as I. pachyura and I. picta, species later transferred to Trichomys (Goldman, 1916; Cabrera, 1961) and Callistomys (Emmons and Vucetich, 1998), respectively. New appraisals in museums and laboratories (Patton and Emmons, 1985; Patton et al., 2000; Bonvicino et al., 2003) and new field discoveries in French Guiana (Vie et al., 1996) and Peru (new species) have trebled that number and expanded their known distributions (Fig. 2; detailed localities and documentation are available from the senior author). Most are poorly known and are uncommon or rare in collections, but this is true of most arboreal echimyids and may relate as much to their arboreal habitat and folivorous habits as to actual population size. However, it seems likely that additional species remain undiscovered and that the range limits of known species are still incompletely documented.

Fig. 2. Map of Isothrix species, depicting six species and all recorded localities. Manu National Park, home of the new species is depicted with the location of the type locality. Plotted records from Patton and Emmons (1985), Patton et al. (2000), and Bonvicino et al. (2003) and from museum vouchers at NHM, AMNH, FMNH, MVZ, and USNM. Patterns reflect the distribution of Tropical and Subtropical biomes for Moist Broadleaf Forest (dark), Dry Broadleaf Forest (pale), and Grasslands, Savannas and Shrublands (stipple).
Previously, I. orinoci has been considered conspecific with I. bistriata, but we provisionally regard it as distinct, owing to its marked morphological separation from both negrensis and bistriata (cf. Patton and Emmons, 1985). The latter forms are quite similar in morphological terms but differ both chromosomally and in DNA (Bonvicino et al., 2003).
Isothrix barbarabrownae, new species
Holotype.-MUSM 16819 (Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Lima), subadult female, collected on 5 October 1999 by B.D. Patterson (original number BDP 3878) with a shotgun. Specimen preserved as a stuffed skin with single hand and foot, cleaned skull and mandibles, and headless, skinless carcass with intact hand and foot in formalin. Tissue samples were frozen in liquid nitrogen (stored as FMNH 170722 at the Field Museum in Chicago) and the specimen was fumigated with ethyl ether and brushed for ectoparasites before preparation. A tick, a laelapid mite (identified as a small species of Androlaelaps by Don Gettinger, personal communication), and many lice were recovered from the holotype and are currently under study by R.M. Timm.
Type locality.-Km 138.5 on the Carretera Paucartambo-Shintuya, near "Suecia" (a roadside restaurant), 1900 m, Provincia de Paucartambo, Departamento de Cuzco, Peru. The type locality is in the Cultural Zone of the Manu Biosphere Reserve at 13°6.032'S, 71° 34.125'W.
Referred specimens.-Known only from the holotype.
Distribution.-Known only from the type locality, in cloud-forest habitat along the Río Cosñipata drainage (Fig. 2). Given the scarcity of other Isothrix species in museum collections, it seems probable that Isothrix barbarabrownae is distributed more widely in Andean cloud forests and perhaps outside them.
Diagnosis.-A species of Isothrix with the following combination of characters: long, lax fur without conspicuous supraorbital stripes, a blackish dorsal crest or mane, a uniformly haired tri-colored tail with the terminal 5 cm white, compact braincase, broad foramen magnum with distinct dorsal median notch, small auditory bullae, narrow triangle-shaped infraorbital foramen, narrow palate, and well developed maxillary component of septum in incisive foramina.
Description.-The description is based on a subadult, nulliparous specimen and some characters likely vary with age. A medium-sized species of Echimyinae; fur is long and lax, on the back 20 mm long, on the flanks 15 mm, and on the belly more than 10 mm. On the dorsal midline, a crest of blackish hairs 25- 30 mm long extends from the crown back to the shoulders; although indistinct, blackish hairs 30 mm long extend back onto the rump. Pelage is distinctly agouti, grey at the base, reddish-brown or cinnamon for most of its length, with blackish tips. There is a molt line on the pelage covering the right rib-cage, the hair on the rump and left side presumably a replacement for the subadult pelage still visible on the right. The muzzle is distinctly grayish (Pale Neutral Gray), except where pink and violet where vascularized. There is no trace of blackish or whitish markings on the rostrum or in the interorbital area. The cheeks, forehead and temples are washed with Snuff Brown, giving way to the small blackish crest on the dorsal midline medial to the pinnae. The eyelids are fleshy and naked; except for elongate tufts, the ear pinnae are mostly naked and colored Burnt Umber in life.
Ventral coloration is indistinctly separated from that on the flanks, the hairs plumbeus at the base and washed with Russet and Cinnamon-Buff highlights. Tones darken on the sides to Cinnamon Drab and on the rump to Snuff Brown. The pelage clothing the cheridia is same-colored, washed with Light Ochraceus Buff hairs, especially laterally. The tail is densely clothed throughout by hairs 10 mm long oriented perpendicularly to its axis and evenly invested along its length. At its base, the tail is Russet or Cinnamon Brown, as is the rump; 50 mm distally, it begins to blacken, becoming Black 100 mm out, and remains this color until the apical 35 mm, which is White.
Mystacial vibrissae reach 20- 75 mm in length and are arrayed in parallel fields along the sides of the muzzle (Fig. 3). Superciliary vibrissae are few in number but long, reaching 30 mm to behind the ears; subocular vibrissae appear as a dense tuft of hairs, 30- 40 mm long, on a fleshy pad behind the eye. Shorter tufts of hairs 20 mm long anterior to the ear pinnae and behind the notch appear to be genal vibrissae. Submental and interramal vibrissae are either missing or inconspicuous.
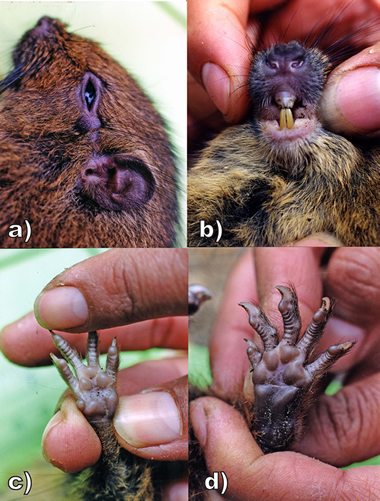
Fig. 3. External characters of the new species: a) lateral view of head, showing genal tuft and dorsal crest; b) anteroventral view of muzzle, mouth and throat, showing the blocky nose, buccal brushes, and lightly pigmented incisors (maxillary teeth broken); c) left manus and d) left pes, each with four interdigital pads, 2 palmar pads, and powerful claws.
Photos by and courtesy of Lucia Luna.
Fore- and hind-feet powerfully built with fleshy digits, four on the manus and five on the pes, each bearing a curved claw 3- 5 mm long. The first interdigital pad of the manus is spherical in shape, the thenar and interthenar pads subequal in size, slightly larger than interdigital pads 2-4.
Skull compact, with long axis bowed, and neither rostrum nor occiput produced (Fig. 4). Zygomatic arches moderately flared, with the superior jugal process distinct when viewed from above. Nasal bones shorter at midline than laterally. Lacrimal bone well developed. Interorbital region broad, expanded posteriorly and extending over orbits in flared, elevated supraorbital shelf. Frontal bones squared posteriorly at level of posterior zygomatic root. Braincase not swollen and temporal fossa not deep. Nuchal crest well developed and arched, running continuously from the paracondylar processes of the exocciptals. Supraocciptal with medial ridge overhanging the foramen magnum and forming the posteriormost extension of the skull.
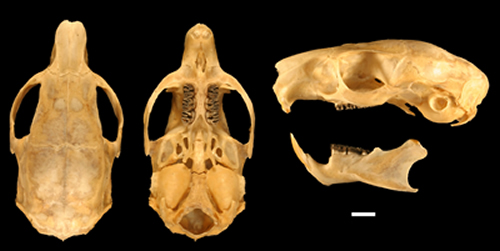
Fig. 4. Skull and mandible of the holotype of Isothrix barbarabrownae (MUSM 16819). Scale bar equals 5 mm.
In lateral view, skull slightly bowed, flexed across the orbits and slightly elevated in the rostrum and frontal-temporal zone. Premaxilla slightly projecting anterior to the plane of the incisors, which appear to be nearly orthodont. Infraorbital foramen large and open laterally, with a nearly vertical rostrum and unflexed superior zygomatic root, so that the foramen appears almost triangular when viewed from the front. Jugal broad and strap-like with a weak, rounded inferior process. Bullae small, rounded, with a raised and anteriorly directed external auditory meatus. Temporals crossed by two longitudinal ridges, one continuous with the supraorbital ridges and the other bounding the squamosal and continuous with the posterior zygomatic root. The squamosotympanic fenestra is slit-like and dorsal to the auditory meatus. The mastoid process is short and blunt, while the condylar processes are long and arched, paralleling the curvature of the bullae but not appressed to them.
In ventral aspect, cranial flexure is marked, with the foramen magnum completely visible and bounded by the occiput. The incisive foramina are short and narrow, flexed in the middle and bounded by elevated ridges running onto the palate, forming on each side a marked fossa on the ventral margins of the zygomatic inferior root. Palate narrow, ridged longitudinally, slightly widening posteriorly, where it is deeply incised by a V-shaped mesopterygoid fossa. Hamular processes of the pterygoids long and delicate, flaring over foramen lacerum and passing the anterior plane of the bullae. Foramina ovale positioned posterior and lateral to the pterygoid fossae. Bullae separated by a distance greater than the greatest palatal breadth and framed posteriorly and laterally by the mastoid. The holotype has a foramen magnum measuring 7.2 mm in breadth, much broader than other similarly-aged and -sized Isothrix and 18% of CIL (not 11.5-13% as in a sample of I. bistriata). The supraoccipital overhangs the foramen magnum and has a medial notch.
The mandible is strongly built, with a short, blunt, dorsally-directed coronoid and an elongate, posteriorly directed angular. The mandibular foramen is tiny, situated behind the coronoid. The pterygoid fossa is shallow and its shelf practically absent, the hystricognathous groove defined only medially; the masseteric fossa is stronger and better defined. Cranial and dental measurements of the holotype are given in Table 2.
Table 2
Means and standard deviations of cranial and dental measurements of age-classes 5-9 (sexes combined) of Isothrix species, with minimum sample size for each species. Although males average slightly smaller than females (mean M:F ratio in a sample of 61 I bistriata was 97.3%, with a range from 92.6 to 102.7%), no variable is sexually dimorphic when Bonferroni corrections are made for experiment-wide error rates (all P>0.05). Accordingly, sexes were pooled in summary statistics and tests. Table includes results of ANOVA tests that species differences for each character were equal to zero; probabilities of F statistics as large or larger than those determined are tabulated. F -tests had 4 treatment d.f. and 59-73 error d.f. Measurements of I. negrensis from Thomas (1920) are tabulated for comparison, but were not included in summary statistics or ANOVAs.
The holotype reflects tooth-wear typical of age 5, using Patton & Rogers (1983) age categories (Fig. 5). The upper incisors were broken near the alveolus when it was shot; they are slim, delicate and weakly pigmented (Cinnamon Buff), as are the unbroken mandibular incisors. On the maxillary premolar, taken to be dP 4, the hypoflexus and mesoflexus are opposite and broadly joined, dividing the tooth into anterior and posterior portions. The paraflexus is transversely oriented and opens laterad, while the metaflexus is enclosed by enamel. As in other Isothrix, there is no neoflexus on any cheektooth. On the M 1, the hypoflexus is large and short, sharply square against the protocone and hypocone. The mure is large and triangular, and the paraflexus and mesoflexus are large and open slightly to the anterior, while the metaflexus is parallel to these but enclosed; the neoloph-posteroloph is small, sized as is the metaloph. On M 2, the broad, square-shaped hypoflexus and mesoflexus are confluent and bisect the tooth, and paraflexus and metaflexus are oriented transversely and completely enclosed.

Fig. 5. Maxillary (upper) and mandibular (lower) occlusal patterns of Isothrix species: a, b- Isothrix barbarabrownae (MUSM 16819), age 5; c, d- I. bistriata (AMNH 73257) age 8; e, f- I. negrensis type, (BMNH 20.7.1.20), as figured by Petter and Cuenca Aguirre [1982], inverted, age 9; g-h- I. orinoci (AMNH 77367), age 8; i, j- I. pagurus (FMNH 140820) age 9; k, l- I. sinnamariensis (V-1708) age 9; n, m- Mesomys hispidus (FMNH 75197) age 9.
On the scarcely-worn mandibular premolar, dp 4, the neoflexid and transversely-oriented anteroflexid are broadly confluent and open labially. The hypoflex and mesoflexid are broadly joined and open to both sides, dividing the tooth into anterior and posterior units. The metaflexid is large and open lingually. Only two molars are at occlusal level; both lack any trace of a neoflexid. On m 1, the hypoflexid and mesoflexid are broadly joined, and the metalophid poorly developed so that the anteroflexid opens posterolingually onto the hypolophid. The posterolophid is short and broad, scarcely reaching the tooth's midline. On m 2, the flexids are oriented and proportioned as in other Isothrix, with a large, well-defined hypoflexid labially, and two large somewhat posteriorally-directed flexids opening lingually.
Comparisons.-The new species of Isothrix can be distinguished from all congeners by its long, lax and saturate pelage. No other species has a dorsal crest or mane of blackish hairs. I. bistriata and I. orinoci share its distinctive coloration of the tail (albeit often without the white tip), while that of I. pagurus is monocolored and I. sinnamariensis sports a tail with elongate curls distally. Cranially, it most closely resembles I. bistriata. Although comparisons are hampered by the lone subadult specimen, it is biometrically distinct from all (Table 2). The foramen magnum is relatively broad (7.2 mm), proportionately broader than other species of Isothrix, and its dorsal margin is notched. The bullae are small and rounded, not inflated as in other species; in I. barbarabrownae, the bullae are separated by distinct gaps from the paraoccipital processes posteriorly and the parapterygoid fossa anteriorly, so that it has the most pronounced middle lacerate foramen.
Etymology.-We are pleased to name this species after Barbara E. Brown, a close friend and colleague, in recognition of her extraordinary commitment and tireless dedication to the Field Museum. It seems fitting to recognize her many contributions to the museum and its programs in mammalogy in the midst of her 37 th year of service, with a great deal of affection and admiration.
Ecology.-The type specimen was encountered during our 1999 field season. At 21:15 h, the senior author heard it traversing a 70° slope of ferns, mosses, and bryophytes covering bedrock along the Paucartambo-Shintuya road. As it moved across the improved gravel road, it was shot with birdshot (#12). The following night, near midnight at ca 1850 m elevation, another individual was seen sitting in the dry grass that lined a rocky crevice above the road-cut, but this specimen eluded capture. During two subsequent years, we established survey camps at 1550 m, 2450 m, and 2850 m along the same road and searched assiduously for this animal, but no further individuals were seen. The species is unknown to residents in the area. Given the overwhelming association of other species of Isothrix with trees and vine tangles, the detection of these two specimens on the ground, albeit on steeply inclined surfaces, seems noteworthy.
DISCUSSION
Analyses of morphological characters unambiguously place the new rodent as a species of Isothrix. A monophyletic Isothrix that includes the new species appears in 93% of the bootstrap replicates, a clustering of taxa equaled elsewhere among echimyines only by Echimys chrysurus + E. saturnus. However, within the genus, there is no phylogenetic structure to the distribution of these morphological characters. The five Isothrix species included in the analysis appear in a polytomy, and their inclusion added no resolution to higher-order relationships within Echimyinae. As Emmons (2005) noted, there is little support for groupings of taxa above the level of genera, and no support for the subfamilial groupings employed by Patton and Reig (1989) and McKenna and Bell (1997). Our analyses with an expanded set of Isothrix species are also consistent with the "star-phylogeny" suggested by the molecular analyses of Lara et al. (1996) and Leite and Patton (2002). Recent analyses of the morphology of fossil and living Echimyidae (Carvalho and Salles, 2004) and mitochondrial and nuclear genes (Galewski et al., 2005) consistently resolve a clade of arboreal taxa-the echimyines plus dactylomyines, as sister to some of the terrestrial heteropsomyine taxa. However, Isothrix remained unassociated with any other genus in this clade, even in the expanded analysis. The morphological characters considered by Carvalho and Salles (2004) and Iack-Ximenes et al. (2005) should be added to future analy ses.
Whatever its eventual position, the new species raises interesting adaptive and biogeographic scenarios. Most Isothrix species are associated with mesic riverine habitats, including seasonally flooded forests and swamps (Fig. 2), although I. pagurus inhabits lowland terra firme forest in the eastern Amazon Basin. The soft pelage of the genus contrasts with that of all other echimyids in the lowlands, which ranges from relatively coarse (Dactylomys) to remarkably spiny (Lonchothrix). The discovery of Isothrix barbarabrownae shows this genus also has Andean associations, where long, soft fur has obvious adaptive value in the cool, damp habitats found on the Andean slopes. Isothrix possesses the softest hair of any Echimyidae, although it is rivaled in this regard by Callistomys. Other non-spiny echimyids that approach them in hair texture and density are Diplomys, Thrichomys, some species of Phyllomys, and the dactylomyines (Dactylomys, Olallamys, and Kannabateomys). Callistomys, Phyllomys and Kannabateomys inhabit the Atlantic Forest and Serra do Mar of coastal Brazil while the remaining genera have ranges that include the central and northern Andes. Of these forms, only Thrichomys, endemic to the Cerrado, is distributed independent of montane habitats.
Long, lax hair is adaptive for life in the cool, wet eastern slopes of the Andes, and groups as divergent as bats (Sturnira tildae-S. erythromos, Anoura geoffroyi), primates (Lagothrix cana), and rodents (Oxymycterus inca-O. paramensis, Nephelomys [formerly Oryzomys; Weksler et al., 2006] keaysi-N. levipes) show increased hairiness at higher elevations. Modest differences in hirsuteness can be attributable to acclimatization, as hair density is known to increase predictably in response to cold, as shown by latitudinal variation among populations of Macaca fuscata (Fooden and Aimi, 2005). However, the gross differences seen among echimyids appear to be adaptations to prevailing climates, represented by different norms of reactions (Dobzhansky, 1970). For echimyid genera that range over both montane and lowland areas, montane species (Isothrix barbarabrownae and Dactylomys peruanus) are distinctly hairier than nearby congeners in the lowlands (I. bistriata and D. dactylinus).
Phylogenetic analyses have not yet resolved evolutionary relationships among the echimyid genera, clouding interpretation of this pelage variation. On distributional grounds, most of the soft-furred echimyine groups occur in mountains as well as the tropical lowlands. At issue is whether they arose in the mountains ringing the Amazon Basin (and lining the coastal plain in Brazil). Peri-Amazonian highlands have been implicated as a nursery for Amazonian diversity, and Hershkovitz (1977) developed this argument in detail using metachromism for callitrichid primates (see also Jacobs Cropp et al., 1999). Alternatively, the recency of Andean uplift supports the alternative view that Andean taxa have lowland ancestry. Molecular dating currently suggests that both the origin of the arboreal clade and the diversification of its genera took place in the middle Miocene (Galewski et al., 2005). It will require more fully resolved phylogenies and detailed character analyses to determine whether lax fur is a synapomorphy of the group, and one indicative of its biogeographic origins, or instead represents convergent responses of different lineages to varied environments.
Specimens examined
Isothrix barbarabrownae
PERU: Cuzco; Paucartambo, Suecia, km 138.5 Carretera Shintuya, 1900 m (MUSM 16819/ FMNH 170722).
Isothrix bistriata
BOLIVIA: El Beni; Río Itenez, frente de Costa Marques (AMNH 210353). BRAZIL: Amazonas; Rio Jurua, Eirunepe (=João Pessoa), 130 m (FMNH 140818-9). PERU: Loreto; Boca Río Curaray (AMNH 71905); Boca, Río Masan (BMNH 1932.8.4.20a); Lago Mirano, Rio Napo (BMNH 1932.8.4.19-20); Maynas, Iquitos, Pampa Chica, 116 m (FMNH 87253-7); Orosa, Río Amazonas (AMNH 73788-9, AMNH 74071-3, AMNH 73227-30, AMNH 73246-57, AMNH 73259, AMNH 73261-6, AMNH 73787, AMNH 73790); Pebas (BMNH 1869.3.31.20; BMNH 1928.7.21.85-88); Río Itaya near Iquitos (AMNH 98245); Rio Maniti, Zarate (FMNH 112566); Río Nanay, Santa Rita, 120 m (FMNH 87258-63); Río Samiria, Santa Elena, 130 m (FMNH 87264); Río Tigre, 1 km below Rio Tigrillo, 150 m (FMNH 122994); Río Tigrillo, 150 m (FMNH 122995); Río Ucayali, Sarayacu (AMNH 75268-72, AMNH 76435-40, AMNH 76453-5); Alto Amazonas, San Lorenzo, 180 m (FMNH 88955-6); Madre de Dios; Reserva Cuzco Amazonico, 200 m (KU 144527); Ucayali, Coronel Portillo, Yarinacocha (FMNH 55487); Lagarto, Alto Ucayali (AMNH 78933-6); Tushemo, Masisea, Rio Ucayali (BMNH 1924.2.22.24-6-holotype of molliae); mouth of Urubamba River (AMNH 98246).
Isothrix orinoci
VENEZUELA: Amazonas; 68 km SSE Esmeraldas, Boca Mavaca, 138 m (USNM 406373-5); Casiquiare Canal, Capibara, 130 m (USNM 4151930); Maipures (BMNH 1899.9.11.42-44; 1899.9.11.45-holotype); Monduapo (BMNH 1899.9.11.46); Mt. Duida, Esmeralda, 143 m (AMNH 77367-76); Río Casiquiare, Orilla Izquierda, El Merey (AMNH 78118, AMNH 78120); Río Orinoco; mouth of Río Ocama (=Ocamo) (AMNH 78113-4; AMNH 78116-7); Sierra Duida, Río Orinoco, Caño Leon (AMNH 77377, 77379-83).
Isothrix pagurus
BRAZIL: Amazonas; Fazenda Esteio (USNM 555639); Para; Boim, Rio Tapajoz (BMNH 1914.6.10.2); Inajatuba (AMNH 95644); Rio Tapajós, west bank, opposite Iroçanga (FMNH 140820); Rio Tapajós, Inajatuba (AMNH 95651-2).
Isothrix sinnamariensis
FRENCH GUIANA: Regina; Les Nouragues, 120 m (ISE Montpellier V-1708).
Characters and states used in the phylogenetic placement of the new species. All character determinations follow Emmons (2005), which should be consulted for character analyses, discussions of polarity, and character states for other species and genera of Echimyinae.
C1. Pelage of lower back-spiny (1); bristly (2); stiff (3); soft (4).
C2. Guard hair structure-without dorsal sulcus (1); with sulcus (2).
C3. Crest of longer hair on crown and nape-absent (1); present (2).
C4. Guard hair pigmentation-banded (1); unbanded (2).
C5. Tail hairiness-"naked" (1); slightly hairy (2); well clothed with hair, usually with a terminal tuft (3).
C6. Coloration of tail tip-same as basal third of tail (1); sharply paler (2); sharply contrasting black or darker (3).
C7. Extension of body fur onto tail base-less than two cm (1); more than two cm (2).
C8. Tiny tubercles-covering naked plantar soles of feet (1); only present between raised, smooth, well-developed pads (2); absent (3).
C9. Hind foot-without raised, smooth, firm, well-developed plantar pads (1); with five pads, lateral metatarsal pad and first digital pads joined as a single pad (2); six pads, lateral metatarsal pad and first digital not joined (3).
C10. Mammae-arranged in two pairs (1); three pairs (2); four or more pairs (3).
C11. Maxillary cheekteeth-brachydont, with 4 roots (1); hypselodont, with 3 roots (2); hypsodont and unrooted (3).
C12. Inclination of occlusal plane relative to palate-subparallel (1); weakly inclined (2); strongly inclined (3).
C13. Origin of upper incisor root-within or posterior to the maxillary root of zygoma (1); level with or outside zygomatic root (2).
C14. Origin of lower incisor root-posterior to m3, high in coronoid process (1); below m3 (2); anterior to m3 (3).
C15. Occlusal surface of maxillary cheekteeth with one short lingual flexus/fossette and two labial flexi/fossettes (1); one lingual flexus and three labial flexi (2); two lingual flexi, two labial flexi (3); four separate and parallel laminae (4); none of these (5); 1 lingual flexus, 4 labial flexi/fossettes (6);or polymorphic for (2) and (3), (7).
C16. M3 with four or more well-developed lophs (1); or with reduced posteroloph (2); or with three or fewer lophs (3); polymorphic for (1) and (2) (4); polymorphic for (2) and (3) (5).
C17. M3 mesoloph-similar in size to protoloph (1); much shorter than protoloph (2).
C18. Anteroloph of lower premolar-not triangular (1); triangular with a flexid opening lingually or apparently so (2); triangular with flexid open labially (3); triangular with flexid open posteriorly (4); or an enclosed triangle with a central fossetid (5); loph triangular or oblong, with no fossetid (6).
C19. Metalophid of lower premolar-absent (1); present as bar in middle of tooth (2); homologies unclear (3).
C20. Anterior edge of the crowns of m2-3- almost straight, at right angles to tooth axis (1); curved with radius like part of a circle encompassing tooth or caret shaped (2l); straight line diagonal to tooth axis (3).
C21. Labial and lingual flexids of lower molars-slanted forward, medial end anterior to labial end, m2-3 metaflexid well separated from hypoflexid (1); labial and lingual flexids about 90Ú to tooth axis, metaflexid meets or almost meets hypoflexid in mid-tooth (2).
C22. Laminar lophs of lower molars-no separate lophs (1); with one separate anteroloph (2); with three separate lophs (3); with one separate posteroloph (4).
C23. Orientation of hypoflexid-slants posteriorly (1); slants weakly anteriorly (2); slants strongly anteriorly (3).
C24. Orientation of mandibular toothrows-strongly convergent anteriorly (1); slightly convergent anteriorly (2); parallel or divergent (3).
C25. Ratio of length of upper toothrow to basilar length of Hensel-very short, 20-22% (1); short 23-24.4% (2); intermediate 24.4-25.7% (3); long, 26.9-31% (4).
C26. Curvature of lower incisors-strongly curved (1); straightened (2).
C27. Squamosotympanic fenestra-a large open slit (1); a tube enclosed in bone (2).
C28. Masticatory foramen and foramen ovale acessorius-not separated by a bony strut or no masticatory foramen (1); a narrow strut (2); a broader strut (3); a wide strut (4); polymorphic for 2 and 3 (5).
C29. Interparietal foramen between foramen ovale acessorius and masticatory foramen-present but small (1); absent (2); polymorphic for 1 and 2 (3).
C30. Orientation of lateral tube of auditory meatus-perpendicular or slightly forward of cranial axis (1); angled strongly forward or downward (2); angled upward and backward (3).
C31. Septum within incisive foramen-premaxillary and maxillary portions separate, maxillary portion dipping in dorsally (1); portions fused, maxillary portion dipping in dorsally (2); broadly fused, in the same plane as rim of foramen (3).
C32. Depth of anterior jugal-hugely expanded, more than 1/2 width of infraorbital foramen (1); broad, but less than 1/2 width of infraorbital foramen (2); narrow (3).
C33. Shape of jugal fossa-anterior point diffuse and broad (1); sharply pointed (2).
C34. Inferior jugal process-inconspicuous and forward of superior process; (1); elongate and opposite or posterior to superior process (2).
C35. Width of jugal fossa-angle from upper rim to lower border of inferior process 10Ú or less (1); about 20Ú (2); about 30Ú (3); about 40Ú or more (4).
C36. Infraorbital canal-well developed with sharp crest or closed beneath a bony shelf (1); present only as a groove (2); completely absent (3); polymorphic for 2 and 3 (4).
C37. Ventral lip of squamosotympanic fenestra-smooth, without a beaded rim or a depression ventrad (1); raised as a beaded rim, with distinct depression below it (2).
C38. Palatal vacuities present in alisphenoid-basisphenoid region-large, such that parapterygoids are freestanding (1); small, distinct round openings of unfused sutures persisting to adulthood (2); sutures completely or practically fused in adults (3).
C39. Buccinator foramen-without medial wall, open below pterygoid (1); with a bony shelf on foramen floor beside alisphenoid (2); with a shelf and medial wall or partial wall enclosing foramen (3).
C40. Position of mandibular foramen-near top of the condylar process (1); on low or mid ramus anterior to a bladelike condyloid ridge (2); at the base of ramus near toothrow posterior to condyloid ridge (3); at base of ramus near toothrow anterior to condyloid ridge (4).
C41. Length of angular process of mandible-short, subequal to condylar process (1); long, much longer than condylar process (2).
C42. Position of dorsal rim of auditory meatus-approaches squamosal suture to within width of auditory meatus (1); separated from it by at least the width of the meatus (2).
C43. Posterior maxillary notch of maxillary and palatine behind M3-enclosed as a foramen, with maxillary fused with suture to alisphenoid (1); open as notch, alisphenoid not fused to maxillary (2); polymorphic for 1 and 2 (3).
C44. Elevation of coronoid process of mandible-above condylar process (1); below condylar process (2).
C45. Fourth premolar-deciduous (1); not deciduous (2).
C46. Elevation of squamosoparietal suture-raised into a ridge across parietal (1); smooth, with no raised ridge (2).
C47. Squamosal width at squamosotympanic foramen- d" 1 mm (1); 1d" 2 mm (2); 2d" 3 mm (3).
ACKNOWLEDGMENTS
The Manu surveys were supported by the NSF (DEB-9870191), the Field Museum (including the Marshall Field III Fund, the Barbara E. Brown Fund for Mammal Research, and a gift from J. and C. Jacobus), and the Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Lima, Peru. We thank S. Solari, project coordinator and inveterate fieldworker, and V. Pacheco, I. Franke, and N. Valencia for their support in hosting the teams. L. Luna, J. Amanzo, U. Paredes, E. Suazo, C. Dick, D. Ge ttinger, D. Stotz, T. Pequeño, J. Tello, T. Gnoske, and B. O'Shea all helped record or collect mammals in the field. We are particularly grateful for Máximo Careche's outstanding field skills. The Instituto de Recursos Naturales, Peru (INRENA) and the Jefatura of Parque Nacional del Manu issued permits for the collection and exportation of this material (Autorización No. 64-99 INRENA, and 003-2001 INRENA-J-DGANPFS). During preparation of this report, the junior author held graduate assistantships funded by the Barbara E. Brown Fund and the Ellen Thorne Smith Fund and received tuition-fee waivers from the Department of Biological Sciences at the University of Illinois at Chicago. Study visits were granted by D.E. Wilson, A.L. Gardner and H. Kafka (USNM) and P. Jenkins (NHM London); Paula also compiled collection notes. R.S. Voss (AMNH), R.M. Timm (KUMNH), and F. Catzeflis (Montpellier) loaned us study material, which was managed by J. Phelps. L. Luna (UMMZ) permitted use of her photos of the holotype and D. Gettinger his preliminary identifications of ectoparasites. We are most grateful to L.H. Emmons for sharing her then-unpublished analysis of echimyines with us. Velizar Simeonovski and Nancy Halliday painted reconstructions of the new species in life and J. Knittle helped compile the literature used in making this and other reports. Insightful and constructive comments of J. L. Patton and A. Percequillo helped focus and improve the text.
LITERATURE CITED
BEZUIDENHOUT AJ and HE EVANS. 2005. Anatomy of the woodchuck (Marmota monax). American Society of Mammalogists Special Publication 13:x + 180 pp. [ Links ]
BONVICINO CR, AREAN DE MENEZES, and JA DE OLIVEIRA. 2003. Molecular and karyologic variation in the genus Isothrix (Rodentia, Echimyidae). Hereditas 139:206-211. [ Links ]
BROWN JC. 1971. The description of mammals. 1. The external characters of the head. Mammal Review 1:151-167. [ Links ]
BROWN JC and DW YALDEN. 1973. The description of mammals. 2. Limbs and locomotion of terrestrial mammals. Mammal Review 3:107-134. [ Links ]
CABRERA A. 1961. Catálogo de los Mamíferos de America del Sur. Vol. 2. Revista del Museo Argentino de Ciencias Naturales "Bernardino Rivadavia", Instituto Nacional de Investigación Ciencias Naturales, Ciencias Zoológicas 4:308-732. [ Links ]
CARVALHO GAS and LO SALLES. 2004. Relationships among extant and fossil echimyids (Rodentia: Hystricognathi). Zoological Journal of the Linnean Society 142:445-477. [ Links ]
D'ELÍA G, L LUNA, EM GONZÁLEZ, and BD PATTERSON. 2006. On the structure of the sigmodontine radiation (Rodentia, Cricetidae): an appraisal of the phylogenetic position of Rhagomys. Molecular Phylogenetics and Evolution 38:558-564. [ Links ]
DOBZHANSKY TG. 1970. Genetics of the evolutionary process. Columbia University Press, New York. [ Links ]
EMMONS LH. 2005. A revision of the genera of arboreal Echimyidae (Rodentia: Echimyidae, Echimyinae); with descriptions of two new genera. Pp. 247-310, in: Mammalian diversification: from population genetics to phylogeography (EA Lacey and P Myers, eds.). University of California Press, Berkeley, California. [ Links ]
EMMONS LH and F FEER. 1990. Neotropical rainforest mammals: A field guide. University of Chicago Press, Chicago. [ Links ]
EMMONS LH and MG VUCETICH. 1998. The identity of Winge's Lasiuromys villosus and the description of a new genus of echimyid rodent (Rodentia: Echimyidae). American Museum Novitates 3223:1-12. [ Links ]
FJELDSÅ J. 1999. Correlation between endemism and local ecoclimatic stability documented by comparing Andean bird distributions and remotely sensed land surface data. Ecography 22:63-78. [ Links ]
FOODEN J and M AIMI. 2005. Systematic review of the Japanese macaques, Macaca fuscata (Gray, 1870). Fieldiana: Zoology, new series 1533:1-200. [ Links ]
GALEWSKI T, J-F MAUFFREY, YLR LEITE, JL PATTON, and EJP DOUZERY. 2005. Ecomorphological diversification among South American spiny rats (Rodentia; Echimyidae): a phylogenetic and chronological approach. Molecular Phylogenetics and Evolution 34:601-615. [ Links ]
GOLDMAN EA. 1916. Notes on the genera Isothrix Wagner and Phyllomys Lund. Proceedings of the Biological Society of Washington 29:125-126. [ Links ]
HERSHKOVITZ P. 1977. Living New World monkeys (Platyrrhini). With an introduction to Primates. University of Chicago Press, Chicago. [ Links ]
IACK-XIMENES GE, M DE VIVO, and AR PERCEQUILLO. 2005. A new genus for Loncheres grandis Wagner, 1845, with taxonomic comments on other arborial echimyids (Rodentia, Echimyidae). Arquivos do Museu Nacional, Rio de Janeiro 63:89-112. [ Links ]
JACOBS CROPP S, A LARSON, and JM CHEVERUD. 1999. Historical biogeography of tamarins, Genus Saguinus: the molecular phylogenetic evidence. American Journal of Physical Anthropology 108:65-89. [ Links ]
LARA MC, JL PATTON, and MNF DA SILVA. 1996. The simultaneous diversification of South American echimyid rodents (Hystricognathi) based on complete cytochrome b sequences. Molecular Phylogenetics and Evolution 5:403-413. [ Links ]
LEITE YLR and JL PATTON. 2002. Evolution of South American spiny rats (Rodentia, Echimyidae): the star-phylogeny hypothesis revisited. Molecular Phylogenetics and Evolution 25:455-464. [ Links ]
LUNA L and BD PATTERSON. 2003. A remarkable new mouse (Muridae: Sigmodontinae) from southeastern Peru: with comments on the affinities of Rhagomys rufescens (Thomas, 1886). Fieldiana: Zoology, new series 101:1-24. [ Links ]
MCKENNA MC and SK BELL. 1997. Classification of Mammals: above the species level. Columbia University Press, New York. [ Links ]
NOWAK RM. 1999. Walker 's mammals of the world. Johns Hopkins University Press, Baltimore, MD. [ Links ]
PACHECO V, S SOLARI, and PM VELAZCO. 2004. A new species of Carollia (Chiroptera: Phyllostomidae) from the Andes of Peru and Bolivia. Occasional Papers, The Museum, Texas Tech University. [ Links ]
PATTERSON B and AE WOOD. 1982. Rodents from the Deseadan Oligocene of Bolivia and the relationships of the Caviomorpha. Bulletin Museum Comparative Zoology Harvard University 149:371-543. [ Links ]
PATTERSON BD. 1996. The 'species alias' problem. Nature 380:589. [ Links ]
PATTERSON BD. 2000. Patterns and trends in the discovery of new Neotropical mammals. Diversity and Distributions 6:145-151. [ Links ]
PATTERSON BD, DF STOTZ, S SOLARI, JW FITZPATRICK, and V PACHECO. 1998. Contrasting patterns of elevational zonation for birds and mammals in the Andes of southeastern Peru. Journal of Biogeography 25:593-607. [ Links ]
PATTON JL and LH EMMONS. 1985. A review of the genus Isothrix (Rodentia, Echimydae). American Museum Novitates 1-14. [ Links ]
PATTON JL and MA ROGERS. 1983. Systematic implications of non-geographic variation in the spiny rat genus Proechimys (Echimyidae). Zeitschrift für Säugetierkunde 48:363-370. [ Links ]
PATTON JL and OA REIG. 1989. Genetic differentiation among echimyid rodents, with emphasis on spiny rats, genus Proechimys. Pp. 75-96, in: Advances in Neotropical Mammalogy (KH Redford and JF Eisenberg, ed.). Sandhill Crane Press, Gainesville, FL. [ Links ]
PATTON JL, MNF DA SILVA, and JR MALCOLM. 2000. Mammals of the Rio Juruá and the evolutionary and ecological diversification of Amazonia. Bulletin of the American Museum of Natural History 244:1-306. [ Links ]
PETTER F and H CUENCA AGUIRRE. 1982. Un Isothrix nouveau de Bolivie (Rongeurs, Echimyidés). Mammalia 46:191-203. [ Links ]
RAHBEK C. 1997. The relationship among area, elevation, and regional species richness in Neotropical birds. The American Naturalist 149:875-902. [ Links ]
RIDGWAY R. 1912. Color standards and color nomenclature. The author and A. Hoen and Co, Washington, DC. [ Links ]
SOLARI S and RJ BAKER. 2006. Mitochondrial DNA sequence, karyotypic, and morphological variation in the Carollia castanea species complex (Chiroptera: Phyllostomidae) with description of a new species. Occasional Papers, Museum of Texas Tech University 254:1-16. [ Links ]
STATSOFT INC. 2003. Statistica (data analysis software system), version 6. www.statsoft.com. [ Links ]
SWOFFORD D. 2002. PAUP* 4.0b10: Phylogenetic Analysis Using Parsimony. Sinauer Associates, Sunderland, Massachusetts. [ Links ]
THOMAS O. 1920. On mammals from the lower Amazons in the Goeldi Museum, Pará. Annals & Magazine of Natural History series 9, 6:266-283. [ Links ]
VELAZCO PM. 2005. Morphological phylogeny of the bat genus Platyrrhinus Saussure, 1860 (Chiroptera: Phyllostomidae) with the description of four new species. Fieldiana: Zoology, new series 105:iv + 1-53. [ Links ]
VIE JC, V VOLOBOUE, JL PATTON, and L GRANJON. 1996. A new species of Isothrix (Rodentia: Echimyidae) from French Guiana. Mammalia 60:393-406. [ Links ]
VOSS RS and R ANGERMANN. 1997. Revisionary notes on Neotropical porcupines (Rodentia: Erethizontidae). I. Type material described by Olfers (1818) and Kuhl (1820) in the Berlin Zoological Museum. American Museum Novitates 3214:1-44. [ Links ]
VOSS RS, DP LUNDE, and NB SIMMONS. 2001. The mammals of Paracou, French Guiana: A Neotropical lowland rainforest fauna. Part 2. Nonvolant species. Bulletin of the American Museum of Natural History 263:1-236. [ Links ]
WAGNER A. 1845. Diagnosen einiger neuen Arten von Nagern und Handflüglern. Wiegmann's Archiv für Naturgesch, Berlin 11:145-149. [ Links ]
WEKSLER M, AR PERCEQUILLO, and RS VOSS. 2006. Ten new genera of oryzomyine rodents (Cricetidae: Sigmodontinae). American Museum Novitates 3537:1-29. [ Links ]
WILSON DE and DM REEDER. 2005. Mammal species of the world: a taxonomic and geographic reference, 3rd ed. Johns Hopkins University Press, Baltimore, MD. [ Links ]
WOODS CA. 1993. Suborder Hystricognathi. Pp. 771-806, in: Mammal Species of the World (DE Wilson and DM Reeder, ed.). Smithsonian Institution Press, Washington, DC. [ Links ]
WOODS CA and EB HOWLAND. 1979. Adaptive radiation of capromyid rodents: Anatomy of the masticatory apparatus. Journal of Mammalogy 60:95-116. [ Links ]
Recibido 12 julio 2006.
Aceptación final 13 noviembre 2006.














