Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Mastozoología neotropical
versión impresa ISSN 0327-9383versión On-line ISSN 1666-0536
Mastozool. neotrop. v.13 n.2 Mendoza dic. 2006
The flexor tendons in the didelphid manus
Virginia Abdala1, 2, 3, Silvia Moro1, and David A. Flores2, 4
1Instituto de Herpetología-Fundación Miguel Lillo. 2CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas). 3Facultad de Ciencias Naturales Universidad Nacional de Tucumán. Miguel Lillo 205, 4000 San Miguel de Tucumán, Argentina <virginia@webmail.unt.edu.ar>. 4PIDBA (Programa de Investigaciones de Biodiversidad Argentina), Facultad de Ciencias Naturales Universidad Nacional de Tucumán. Miguel Lillo 205, 4000 San Miguel de Tucumán, Argentina, <davflor@gmail.com>.
ABSTRACT. Tetrapods have flexor tendons in the palmar surface of the manus that transmit forces generated by superficial and deep forearm muscles to the digits. Two patterns ("P" and "L") of tendinous connections among the deep layer of the forearm muscles and the digits have been described, one of which (pattern P) shows an empirical correlation with some grade of arboreality. In this article, we focused on the anatomical design of the tendons of the deep layer of the palmar surface of the didelphid manus, and associated muscles. Our objectives are: (1) to describe the pattern found in all taxa of the great didelphid clade, (2) to assign the designs that we found to the L or P pattern, and (3) to discuss our results in the context of the latest available phylogenetic hypotheses proposed for those taxa. All of the didelphids we dissected possess the P pattern. As we compared the tendinous and myological structures, we found that some of the myological differences we describe could have important phylogenetic implications. We selected 10 characters, mapped them on marsupial phylogenies, and discovered six more synapomorphies supporting clades already proposed by other researchers.
RESUMEN. Tendones flexores en didelfidos. Los tetrápodos tienen tendones flexores en la superficie palmar de la mano, que transmiten a los dígitos las fuerzas generadas por los músculos superficiales y profundos del antebrazo. Se han descripto dos patrones (P y L) de conexión tendinosa entre la capa profunda de los músculos del antebrazo y los dedos. De éstos, el patrón P muestra correlación empírica con algún grado de arborealidad. En este artículo, nos enfocamos en el diseño anatómico de los tendones de la capa profunda de la superficie palmar de las manos de los didelfidos y músculos asociados. Nuestros objetivos son: (1) describir el patrón encontrado en todos los taxa del gran clado de los didelfidos, (2) asignar el diseño encontrado al patrón P o L, y (3) discutir nuestros resultados en el contexto de las filogenias más recientes para los grupos considerados. Todos los didelfidos disecados exhiben un patrón P. Cuando comparamos las estructuras miológicas y tendinosas entre los grupos, encontramos algunos que parecían tener importantes implicancias filogenéticas. Así, seleccionamos 10 caracteres, los mapeamos en las filogenias disponibles para marsupiales y encontramos 6 sinapomorfías que soportan clados propuestos previamente por otros autores.
Key words. Didelphidae. Flexor tendons. Manus anatomy.
Palabras clave. Anatomía de la mano. Didelfidos. Tendones flexores.
INTRODUCTION
Tetrapods have flexor tendons in the palmar surface of the mAppèndixanus that transmit forces generated by forearm muscles to the digits. These tendons are coming from both the superficial muscles of the forearm (e.g. m. flexor carpi ulnaris; m. flexor digitorum superficialis) and deeper muscles (e.g. m. flexor digitorum profundus). Each layer (superficial and deep) of these skeletal muscles exerts forces on bones via independent sets of tendons. The m. flexor digitorum profundus flexes all the digits, especially the terminal phalanx (Davis, 1964), via five strong tendons that insert into the base of the terminal phalanges.
Working on the muscular and tendinous structures of the lizard forearm and manus, Moro and Abdala (2004) described two patterns of tendon connections among the deep layer of the forearm muscles and the digits, naming them as "L" and "P". In the L pattern (first observed in lizards of the genus Liolaemus) there is an aponeurotic extension between the m. flexor digitorum profundus and the tendons that inserts onto the digits, with one or two palmar sesamoids embedded in it (this extension has been called the flexor plate by Haines, 1950). The L pattern is found both in arboreal and terrestrial vertebrates (e.g., Liolaemus and Ailuropoda). In the P pattern (first observed in lizards of the genus Polychrus), the flexor plate is reduced or absent, and tendons pass directly to the terminal phalanges. Among lizards, the P pattern is only found in strictly arboreal taxa such as Polychrus, Anolis, and Chamaleo. Among other vertebrates, a relationship between the P pattern and some grade of arboreality is once again found: it is present in arboreal anurans as Phyllomedusa sauvagii, Ph. boliviana, and Hyla pulchella (Manzano, 1996); in arboreal mammals as Ptilocercus and Tupaia (Le Gros Clark, 1924, 1926); in Macaca mulata (Howell and Strauss, 1933); in ateline monkeys (Youlatus, 2000); and in tree squirrels (Thorington, 1997).
This empirical correlation between the P pattern of flexor tendon morphology and arboreality led us to analyze this character in the marsupial groups Didelphidae, Phalangeridae, and Dasyuridae, which include fully arboreal forms like Phalanger, Thylamys, Micoureus, and Caluromys. scansorial forms such as Didelphis and Myoictis. and strongly terrestrial forms like Metachirus and Monodelphis.
Since comparative anatomy provides data for initial hypothesis about the functional differences between animals, in this article we focused on the anatomical design of the tendons of the deep layer of the palmar surface of the didelphid manus and their associated muscles. Our objectives are: (1) to describe the pattern found among representatives of the great didelphid radiation (Jansa and Voss, 2000; Voss and Jansa, 2003), (2) to assign the designs that we found to the L or P patterns, and (3) to discuss our results in the context of the latest available marsupial phylogenies (Cardillo et al., 2004; Voss et al., 2005).
MATERIAL AND METHODS
Specimens from the following museum collections (acronyms given in parenthesis) were examined: The American Museum of Natural History, New York, USA (AMNH), and Colección Mamíferos Lillo, Universidad Nacional de Tucumán, Argentina (CML).
Basic details of the aponeurotic complex of the didelphid manus were obtained by dissecting the following specimens: Caluromys derbianus AMNH 170646, 48190; Chironectes minimus AMNH 97335, 169949; Didelphis albiventris CML 3173, 3174, 4119, 4221, 5971; Cryptonanus chacoensis CML w/n, 5910, 5915, 5916, 5918, 5920, 5921, 5926; Lutreolina crassicaudata CML 4114, 4115, 4116, 4117, 6701; Marmosa robinsoni AMNH 10290, 149501, 244887, 257208; Marmosops fuscatus AMNH 144871, 234952, 234954; Metachirus nudicaudatus AMNH 2027, 2143, 263127, 263133; Micoureus constantiae CML 5688; Monodelphis dimidiata CML 4118, 4120; Philander opossum AMNH 137158, 190447, 202705; Thylamys venustus CML 4148, 5586; T. pallidior CML 3190, 4463; T. pusillus CML 3946; Phalanger orientalis AMNH 79750; Myoictis melas AMNH 194403; Phascogale tapoatafa AMNH 160070, 202047, 244882; Neophascogale lorentzii AMNH 152738.
Our descriptions are based on the scansorial didelphid Didelphis albiventris, which is subsequently compared with the other aforementioned taxa. We decided to include australidelphians for the sake of a better comparison of the pattern of flexor tendon in a broader marsupial group.
As we compared the tendinous and myological structures we realized that some of the myological differences we encountered could have important phylogenetic implications. To test this, we defined 10 characters and mapped them on the marsupial phylogenies recently proposed by Cardillo et al. (2004; CEA henceforth) and Voss et al. (2005; VLJ henceforth). In the case of some species that VLJ did not include in their phylogenetic analysis (e.g., Marmosops fuscatus, Micoureus constantiae, Monodelphis dimidiata, and Caluromys derbianus), we inferred that they belong to the same clades as other congeneric species. In no case did we find intraspecific variation of the proposed characters.
Cladistic mapping was accomplished using the computer program "Tree analysis using New Technology" (TNT; Goloboff, Farris, and Nixon 2004). All relevant methodological aspects of this application are explained under the subheading Character Mapping (below).
RESULTS
Because of the complex anatomical relationships among the ventral muscles and tendons of the manus among the species studied, a brief description of these structures is given for Didelphis albiventris, with divergent conditions in other dissected taxa noted subsequently. Our results are summarized as character data in Table 1.

ANATOMICAL MUSCLE DESCRIPTIONS AND COMPARISONS
Flexor group of the forearm
M. palmaris longus (Fig. 1) arises from the distal portion of the ventral surface of the humerus. It is piriform, elongated, and not very bulky. It is divided into two bellies, each with its own tendon. The external tendon inserts onto digit I. The internal tendon, which is thicker than the external tendon, is fused with the superficial aspect of the main tendon of m. flexor digitorum profundus at the wrist. The proximal portion of m. palmaris longus joins the proximal portion of m. flexor digitorum superficialis. The origin of this muscle is on the radial side of the forearm.
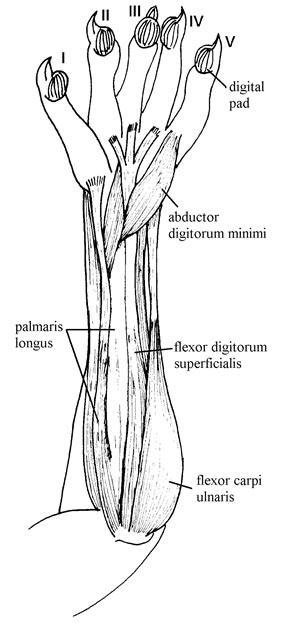
Fig. 1. Forearm flexor muscles of Didelphis albiventris CML 3174. Superficial view.
Comparative survey: No differences were observed in Micoureus constantiae, Philander opossum and Marmosa robinsoni. In the other taxa analyzed this muscle has only one belly, which is also piriform, elongated and flattened. In Thylamys spp. and Marmosops fuscatus, the origin is displaced to the ulnar side of the forearm. In Caluromys derbianus and Cryptonanus chacoensis, the muscle is very small and flat.
M. flexor carpi ulnaris (Fig. 1) arises from the medial surface of the humerus, close to the origin of m. flexor digitorum longus superficialis. Its muscular body is flat and piriform but with a wide surface. The muscular body is undivided and occupies about one-third the length of the forearm. It inserts tendinously onto the pisiform.
Comparative survey: In Monodelphis dimidiata, Marmosops fuscatus, Marmosa robinsoni, Cryptonanus chacoensis, and some Australasian groups such as Phalanger orientalis and Phascogale tapoatafa, this is a very bulky and well-developed muscle. In M. constantiae and C. chacoensis, the muscle has a fleshy origin from the ulna, and its tendon of insertion joins the common tendon of m. flexor digitorum profundus.
M. flexor carpi radialis (not illustrated) originates tendinously from the ventral radial surface of the distal end of the humerus. It is bulky, especially at the origin, and flat and wide in the medial distal portion. It inserts tendinously onto the scaphoid.
Comparative survey: No differences were observed in Thylamys spp., C. derbianus, C. minimus, P. opossum, M. nudicaudatus, M. constantiae, M. robinsoni, nor in any of the Australasian groups. In M. dimidiata, C. chacoensis, and L. crassicaudata, however, this muscle has two parts: a superficial belly that originates from the humerus and inserts by a well-developed tendon onto the scaphoid, and a deep belly that also originates from the humerus but inserts fleshily onto the proximal half of the radius. Note that our observation that this muscle inserts onto the scaphoid in Didelphis and Thylamys is at odds with previous descriptions of the myology of those taxa (Coues, 1869; Mann Fisher, 1956), wherein the muscle is said to insert onto the metacarpals.
Extrinsic digital flexors
M. flexor digitorum superficialis (Figs. 1, 2) originates from the medial condyle of the humerus. The muscle is superficially covered by m. palmaris longus and m. flexor carpi ulnaris. M. flexor digitorum superficialis is narrow and divided into two branches (superficial and deep), each with its own tendon. The superficial tendon is longer and arises in the carpal region, where it divides into three parts that insert onto digits II, III, and IV. These tendons have interwoven muscular fibers and are resting over the common tendon of m. flexor digitorum profundus. The deep tendon inserts onto the principal portion of m. flexor digitorum profundus.
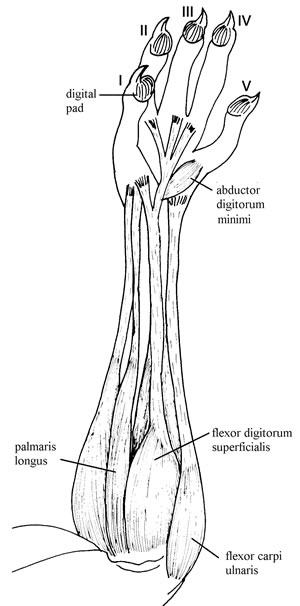
Fig. 2. Forearm muscles of Thylamys venustus CML 5586, showing their fibers reaching the middle of the forearm.
Comparative survey: In T. venustus, M. dimidiata, M. fuscatus, M. robinsoni, C. chacoensis, and L. crassicaudata the origin of m. flexor digitorum superficialis has a more radial location, and it has a small, flat, piriform shape. In C. derbianus, P. opossum, M. nudicaudatus, M. dimidiata, M. constantiae, M. robinsoni, and some Australasian taxa (e.g., Phascogale tapoatafa, Neophascogale lorentaii), the muscle has only one insertion via a strong tendon onto the pisiform. Lutreolina crassicaudata has the same pattern as Didelphis albiventris. In M. fuscatus, T. venustus, C. minimus, and C. chacoensis, the tendons of insertion join the common tendon of m. flexor digitorum profundus. The latter condition was also observed in Australasian groups such as Phalanger orientalis and Myoictis melas. In Cryptonanus chacoensis this muscle has a very broad origin that includes the distal portion if the humerus and the proximal portion of the ulna.
M. flexor digitorum profundus (Fig. 3) is a large mass of muscle that originates from different parts of the forearm. At least three bellies could be distinguished. The main mass originates tendinously from the ventral surface of the central portion of the distal humerus. The second belly originates fleshily from the proximal two-thirds of the ventral mesial surface of the radius. The third belly originates fleshily from the proximal two-thirds of the ventral mesial surface of the ulna. Distally, all three bellies converge to form a single muscular body that gives rise to a single common tendon just proximal to the wrist. This stout tendon (tendon common of m. flexor digitorum longus of Davis, 1964; flexor tendon of Evans, 1993; common tendon of flexor digitorum profundus muscle of Stein 1981) occupies the carpal tunnel, which is very deep and enclosed by the annular ligament. After emerging from the carpal tunnel, the tendon divides into branches that pass separately across the palmar surface directly to the terminal phalanges. Thus, there is no aponeurotic sheet (flexor plate of Haines, 1950) on the palmar surface.
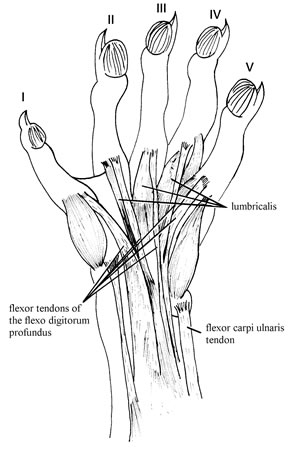
Fig. 3. Manus musculature of Didelphis albiventris CML 3174 showing the flexor tendons.
Comparative survey. In Thylamys spp., M. constantiae, M. robinsoni, M. fuscatus, and C. chacoensis the common tendon does not includes any muscular fibers. In Monodelphis dimidiata the main mass of the muscle has fibers reaching the middle part, which are joined to those of m. flexor carpi ulnaris. Muscular fibers are reaching also the distal part of the common tendon. In the proximal to the middle of the muscle, the main belly is joined to m. flexor carpi radialis.
Unlike didelphids and Phalanger (with the P pattern as described above), all of the dasyurids that we dissected conform to the L pattern, having an aponeurotic sheet to which the tendons of m. flexor digitorum longus attach on the palmar surface.
The described muscles of the forearm have their bellies so much blended that they cannot be easily separated, forming a great muscular mass. Detailed descriptions of the forearm muscular masses in some didelphids can be found in Coues (1869), Stein (1981), and Brandell (1963).
CHARACTER MAPPING
Mapping characters of the palmar flexor tendons and associated musculature (Table 1) on marsupial phylogenies resulted in the discovery of several novel synapomorphies. Character 10 codes the different tendinous patterns P and L, which we optimized on the topology of the CEA supertree (Fig. 4). In this reconstruction, state 1 (L pattern present) supports the monophily of dasyurids, whereas Phalanger exhibits the P pattern (the plesiomorphic condition that it shares with all didelphids). Thorington et al. (1997) described the tendinous pattern of the m. flexor digitorum profundus for squirrels, which conforms to our P pattern, and stressed that arboreality is presumed to be primitive for the Sciuridae; accordingly, this forelimb anatomy was assumed to be primitive for the family. The same condition is recovered by our analysis: recovering of pattern P as a basal character state suggests that arboreal-grasping is the plesiomorphic marsupial condition.
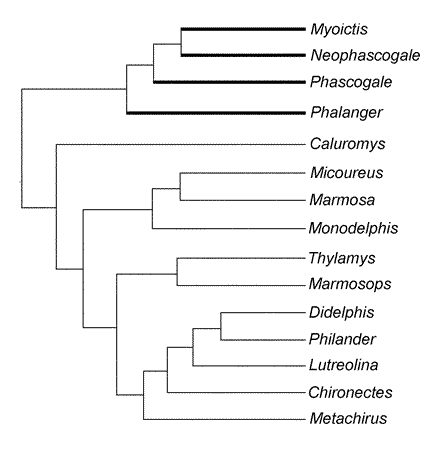
Fig. 4. Mapping of characters on the Cardillo et al. (2004) cladogram. Heavy lines denote the clade supported by our character 10.
Mapping our characters on the VLJ phylogeny (Fig. 5) suggests that 5 characters (0, 3, 4, 5, and 9) are potential synapomorphies of clades recognized by those authors.

Fig. 5. Mapping of characters on the Voss et al. (2005) combined cladogram. Heavy lines denote clades supported by our characters.
Alphabetic labels C and G identify clades proposed by Jansa and Voss (2000).
State 0 of character 0 (m. palmaris longus with two bellies) supports the monophyly of two independent nodes, one including Didelphis and Philander; and the other including Micoureus and Marmosa. Because a divided muscle palmaris longus has been reported by Haines (1950, and cites therein) for some carnivores and insectivores, it seems that a division of this muscle originated many times in different mammalian lineages.
State 1 of character 3 (normal location of the m. flexor digitorum superficialis) appears as a synapomorpy of a clade composed by Cryptonanus, Marmosops, and Thylamys (node C in the phylogeny of Jansa and Voss, 2005; where Cryptonanus was included in Gracilinanus). The same clade is recovered by state 1 of character 4 (insertion tendon joined to the common tendon). We propose that these morphological conditions are new synapomorphies for grouping these three taxa in the same monophyletic group.
In some of the marsupials that we analyzed, the tendons of insertion of m. flexor digitorum superficialis are joined to the common tendon of m. flexor digitorum profundus (states 1 and 2 of character 4). Youlatus (2000) stressed that some monkeys appear to rely more on m. flexor digitorum profundus for powerful grasping of the arboreal support, whereas others rely on m. flexor digitorum superficialis. In the forms that he analyzed, these muscles are independent. Connections between these muscles might contribute to a more forceful manual grip, by combining the forces generated by their contraction. However it might also result in less independent mobility of the digits. Thus, a trade-off between strength and flexibility of movement could be relevant for interpreting the adaptive significance of taxonomic patterns in these muscles.
State 0 of character 5 (m. flexor carpi ulnaris flat and piriform) supports clade G of the phylogeny of Jansa and Voss (2005), which is composed of the large opossums Didelphis, Philander, Lutreolina, Chironectes, and Metachirus. This clade is supported by empirical evidence from many sources (Tate, 1933; Reig et al., 1987; Kirsch and Palma, 1995; Kirsch et al, 1995; Jansa and Voss, 2000, 2005; Voss and Jansa, 2003). We propose this morphological condition as another synapomorphy for this widely recognized group. This trait has apparently also been acquired independently in Thylamys, Micoureus, and Caluromys.
Character 9 is related to the length of muscle fibers of the forearm flexors. State 1 (muscle fibers reaching only the proximal to middle third of the forearm) supports the node composed of Marmosops, Thylamys and Cryptonanus (clade C in the phylogeny of Jansa and Voss, 2005; where Cryptonanus was included in Gracilinanus); however, this character state is also present in Marmosa and Micoureus, providing a new synapomorphy for this highly corroborated clade (Kirsch and Palma, 1995; Patton et al., 1996; Voss and Jansa, 2003). The reduction of contractile tissue volume in both of these clades presumably reduces the metabolic energy required for force development in those muscles (Alexander, 2002). Moreover, relative shortening and velocity will increase if part of the distance between origin and insertion of a muscle is occupied by connective tissue rather than sarcomeres (Gans and de Vree, 1987). This seems to be the case in Marmosa robinsoni, Marmosops fuscatus, Thylamys venustus, Cryptonanus chacoensis, and Micoureus constantiae, all of which have most of the distance between origin and insertion of the muscles of the forearm occupied by tendon. Phylogenetic mapping suggests that this condition could be interpreted as a convergent adaptation linked to some (as yet unknown) ecological requirement.
DISCUSSION
The didelphid radiation includes forms classified as arboreal, scansorial, and terrestrial on a behavioral basis (Vaughan, 1972). However, all didelphids examined here show flexor pattern P (Moro and Abdala, 2004) in the hand, a pattern that, in other tetrapod groups, is correlated with arboreal habits: they lack a flexor plate, and flexor tendons run separately towards each digit. Therefore, it seems that in didelphids the flexor pattern P has little or no direct association with locomotor behavior. Within the Australasian group analyzed here, only the arboreal Phalanger orientalis (Nowak, 1991) shows the pattern P, similar to didelphids. By contrast, the three dasyurids examined, the scansorial Myoictis melas (Collins, 1973), the arboreal Phascogale tapoatafa, and the also arboreal Neophascogale lorentzii exhibit flexor pattern L. Many vertebrate taxa with the L pattern are arboreal, but their climbing is performed using mainly their claws (e.g. Tropidurus hispidus, Iguana iguana). Interestingly, marsupials with the L pattern have remarkable claw development (indeed, Neophascogale is known as the "long-clawed marsupial mouse"). These marsupials may climb using claws (instead of a prehensile manus) as a response to the rigidity produced by the presence of a flexor plate with a sesamoid (the L pattern), which might preclude versatile palmar mobility (as is the case in many lizards; Virginia Abdala, personal observations).
In ateline monkeys (Youlatus, 2000) and squirrels (Thorington et al. 1997), digital flexor tendons run separately to the fingers, and there is no flexor plate. Although this condition resembles the P pattern, there are differences in details among observed morphologies. In didelphids and squirrels, there is a common tendon of the m. flexor digitorum profundus at the wrist. The presence of this common tendon, which originates from a single head of the m. flexor digitorum profundus, probably prevents independent movement of the digits. Coues (1869) also stressed that tendons of the deep digital flexors do not confer individual digital mobility. Therefore, grasping in didelphids probably consists of convergence of all fingers. A contrasting pattern appears in Primates, which are capable of complex manipulation (e.g., the powerful yet precise human manual grip). Thus, their flexor tendons have different fascicles arising from different heads of m. flexor digitorum profundus (Kaplan, 1953; Tuttle and Basmajian, 1974; Youlatus, 2000). In primates and other vertebrates, arboreal habits are frequently associated with an increased manipulative ability of the forefeet (Hunsaker and Shupe, 1977; Lemelin 1999). Models of primate origins proposed by some authors (Cartmill, 1972, 1974a, b; Sussman, 1991; Lemelin, 1999) have suggested that locomotion on small-diameter supports was an important factor for the development of prehensile extremities in early primates. These authors also stressed that grasping ability is of greater advantage to scansorial or arboreal species than to terrestrial ones (see also Taylor, 1978).
In spite of these differences, both patterns (with one common tendon, or separated bellies) seem to facilitate the manual convergence that permits effective climbing by grasping. In fact, all didelphid specimens analyzed had the fingers bent due to the contraction of the flexor tendons after their death. Since didelphid claws are weaker than those of many arboreal dasyurids (e.g., Neophascogale, Phascogale), climbing would be not possible without a manus that is prehensile to some degree. So, we propose that in marsupials the P flexor pattern is related to grasping abilities which may make climbing possible.
ACKNOWLEDGMENTS
R. Voss, N. Giannini, and an anonymous reviewer helped us to improve the first version of the manuscript. R. M. Barquez (Colección Mamíferos Lillo) and R. Voss (American Museum of Natural History) kindly provided access to the specimens used here. This work was made while DAF was a postdoctoral fellowship at AMNH. Consejo de Investigaciones de la Universidad Nacional de Tucumán (CIUNT) Argentina, and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina partially supported this investigation.
LITERATURE CITED
ALEXANDER RM. 2002. Tendon elasticity and muscle function. Comparative Biochemistry and Physiology Part A 133:1001-1003. [ Links ]
BRANDELL BR. 1963. An investigation of the forearm and hand flexor of Didelphis marsupialis virginiana Kerr. Ph. D. Thesis, University of Michigan. [ Links ]
CABRERA A and J YEPES. 1960. Mamíferos Sudamericanos. Editorial Ediar, Buenos Aires. Vol I y II. 347 pp. [ Links ]
CARDILLO M, ORP BININDA-EMONS, E BOAKES, and A PURVIS. 2004. A species-level phylogenetic supertree of marsupials. Journal of Zoology, London 264:11-31. [ Links ]
CARTMILL M. 1972. Arboreal adaptations and the origin of the Order Primates. Pp. 3-35, in. The functional and evolutionary biology of Primates (RH Tuttle, ed.). Aldine-Atheton, Chicago. [ Links ]
CARTMILL M. 1974a. Pads and claws in arboreal locomotion. Pp. 45-76, in. Primate Locomotion (FA Jenkins, ed.). Academic Press, New York. [ Links ]
CARTMILL M. 1974b. Rethinking primate origins. Science 184:436-443. [ Links ]
COLLINS LR. 1973. Monotremes and Marsupials. A reference for Zoological Institutions. Smithsonian Institute Press. Washington 323 pp. [ Links ]
COUES E. 1869. The osteology and myology of Didelphyidae Didelphis virginiana. Memories of the Boston Society of Natural History Vol. II:41-154. [ Links ]
DAVIS DD. 1964. The Giant Panda. A morphological study of evolutionary mechanisms. Fieldiana Zoology Memoirs 3:1-339. [ Links ]
EVANS HE. 1993. Miller's Anatomy of the dog. 3rd edition. WB Saunders Company, Philadelphia 1113 pp. [ Links ]
FLORES DA. 2003. Estudio taxonómico y zoogeográfico de los marsupiales argentinos. Doctoral Thesis. Facultad de Ciencias Naturales e Instituto Miguel Lillo, Universidad Nacional de Tucumán, Tucumán 324 pp. [ Links ]
GANS C and F De VREE. 1987. Functional bases of fiber length and angulation in muscle. Journal of Morphology 192:63-85. [ Links ]
GOLOBOFF PA and JS FARRIS. 2001. Methods for quick consensus estimation. Cladistics 17:26-34. [ Links ]
GOLOBOFF PA, JS FARRIS, and K NIXON. 2003. T.N.T.: Tree analysis using new technologies. Program and documentation. Available at www.cladistics.org [ Links ]
HAINES RW. 1950. The flexor muscles of the forearm and hand in lizards and mammals. Journal of Anatomy 84:13-29. [ Links ]
HOWELL AB and W STRAUS Jr. 1933. The muscular system. Pp. 89-175, in. The anatomy of the Rhesus Monkey (Macaca mulata) (CG Hartman and WL Straus Jr, eds.). Publishing Co., New York. [ Links ]
HUNSAKER D and D SHUPE. 1977. Behavior of new world marsupials. Pp. 279-347, in. The Biology of marsupials (D Hunsaker, ed.). Academic Press, New York. [ Links ]
JANSA SA and RS VOSS. 2000. Phylogenetic studies on Didelphid Marsupials. I. Introduction and preliminary results from nuclear IRBP gene sequences. Journal of Mammalian Evolution 7:43-77. [ Links ]
JANSA SA and RS VOSS. 2005. Phylogenetic relationships of the marsupial genus Hyladelphys based on nuclear gene secuences and morphology. Journal of Mammalogy 86:853-865. [ Links ]
KAPLAN EB. 1953. Functional and Surgical Anatomy of the Hand. J.P. Lippincot Company 1-288. [ Links ]
KIRSCH JAW and RE PALMA.1995. DNA/DNA hybridization studies of carnivorous marsupials. V. A further estimate of relationships among opossums (Marsupialia, Didelphidae). Mammalia 59:403-425. [ Links ]
KIRSCH JAW, AW DICKERMAN, and OA REIG. 1995. DNA/DNA Hybridization studies of Carnivorous Marsupials IV. Intergeneric relationships of the opossum (Didelphidae). Marmosiana, Acta Teriologica Latinoamericana 1:57-78. [ Links ]
LE GROS CLARK WE. 1924. The myology of the Tree-Srew (Tupaia minor). Proceedings of the Zoological Society of London 31:461-497. [ Links ]
LE GROS CLARK WE. 1926. On the anatomy of the pen-tailed Tree-Srew (Ptilocercus lowii). Proceedings of the Zoological Society of London 1179-1309. [ Links ]
LEMELIN P. 1999. Morphological correlates of substrate use in didelphid marsupials: implications for primate origins. Journal of Zoology 247:165-175. [ Links ]
MANN FISCHER G. 1956. Filogenia y función en la musculatura de Marmosa elegans (2a parte) (Marsupialia, Didelphidae). Investigaciones Zoológicas Chilenas (III) 1-28. [ Links ]
MANZANO AS. 1996. Análisis de la musculatura de la familia Pseudidae (Amphibia: Anura). Doctoral Thesis, Facultad de Ciencias Naturales e Instituto Miguel Lillo, Universidad Nacional de Tucumán 206 pp. [ Links ]
MARSHALL LG. 1978. Lutreolina crassicaudata. Mammalian Species 91:1-4. [ Links ]
MORO S and V ABDALA. 2004. Análisis descriptivo de la miología flexora y extensora del miembro anterior de Polychrus acutirostris (Squamata, Polychrotidae). Papéis Avulsos de Zoología 44(5):81-89. [ Links ]
NOWAK RM. 1991. Walker 's Mammals of the World. Vol. 1. The Johns Hopkins University Press. Baltimore and London. [ Links ]
PATTON JL; SF DOS REIS, and MNF DA SILVA. 1996. Relationships among Didelphid Marsupials based on Sequence Variation in the Mitochondrial Cytochrome B Gene. Journal of Mammalian Evolution 3:1-29. [ Links ]
REED CA. 1951. Locomotion and Appendicular anatomy in three Soricid insectivores. The American Midland Naturalist 45(3):513-671. [ Links ]
REIG OA, JAW KIRSCH, and LG MARSHALL. 1987. Systematic relationships of the living and Neocenozoic american "opossum-like" marsupials (Suborder Didelphimorphia), with comments on the classification of these and of the cretaceous and paleogene New World and European metatherians. Pp 1-89, in. Possums and opossums: studies in evolution (M Archer, ed.). Sydney, Australia. [ Links ]
SMITH LK, EL WEISS, and LD LEHMKUHL. 1996. Brunnstrom's clinical kinesiology. 5th ed. Philadelphia. FA Davis. [ Links ]
STEIN BR. 1981. Comparative limb myology of two opossums, Didelphis and Chironectes. Journal of Morphology 169:113-140. [ Links ]
STEIN BR. 1986. Comparative limb myology of four Arvicolid rodent genera (Mammalia, Rodentia). Journal of Morphology 187:321-342. [ Links ]
SUSSMAN RW. 1991. Primate origins and the evolution of the angiosperms. American Journal of Primatology 23:209-223. [ Links ]
TATE GHH. 1933. A systematic revision of the marsupial genus Marmosa, with discussion of the adaptive radiation of the murine opossum. Bulletin of the American Museum of Natural History 46:1-250. [ Links ]
TAYLOR BK. 1978. The anatomy of the forelimb in the anteater (Tamandua) and its functional implications. Journal of Morphology 157:347-368. [ Links ]
THORINGTON FW Jr., K DARROW, and ADK BETTS. 1997. Comparative myology of the forelimb of the Squirrels (Sciuridae). Journal of Morphology 234:155-182. [ Links ]
TUTTLE RH and JV BASMAJIAN. Electromyography of forearm musculature in Gorilla and Problems related to knuckle-walking. Pp. 293-347, in. Primate Locomotion (F Jenkins, ed.). Academic Press, New York. [ Links ]
VAUGHAN TA 1972. Mammalogy. W.B. Saunders Company. Philadelphia, London, Toronto. 463pp. [ Links ]
VOSS RS and SA JANSA. 2003. Phylogenetic studies on Didelphid marsupials II. Nonmolecular data and new IRBP sequences: separate and combined analyses of Didelphine relationships with denser taxon sampling. Bulletin of the American Museum of Natural History 276:1-82. [ Links ]
VOSS RS, DP LUNDE, and SA JANSA. 2005. On the contents of Gracilinanus Gardner and Creighton, 1989, with the description of a previously unrecognized clade of small didelphid marsupials. American Museum Novitates 3482:1-34. [ Links ]
YOULATUS D. 2000. Functional analysis of forelimb muscles in Guianan Atelines (Platyrrhini: Primates). Annales des Sciences Naturelles 21(4):137-151. [ Links ]
ZIEGLER AC. 1972. Additional specimens of Planigale novaeguinae (Dasyuridae: Marsupialia) from territory of Papua. Australian Mammalogy 1:43-45. [ Links ]
Recibido 10 junio 2005.
Aceptación final 15 mayo 2006.














