Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Mastozoología neotropical
versión impresa ISSN 0327-9383versión On-line ISSN 1666-0536
Mastozool. neotrop. v.14 n.1 Mendoza ene./jun. 2007
Survival and reproductive potential of different cohorts of Calomys venustus
María D. Gomez1, 2, María C. Provensal2, and Jaime J. Polop2
1 CONICET. 2 Grupo de Investigación en Ecología de Poblaciones (GIEP), Departamento de Ciencias Naturales, Facultad de Ciencias Exactas, Físico-Químicas y Naturales, Universidad Nacional de Río Cuarto, Ruta 36 Km. 608, 5800 Río Cuarto, Córdoba, Argentina. Teléfono: 0358-4676236. <dgomez@exa.unrc.edu.ar>
ABSTRACT: In order to determine winter survival and reproductive potential in cohort 2 (C2) and cohort 3 (C3) of Calomys venustus we compared these parameters between individuals of these cohorts and their offspring. Forty six males (13 C2 and 33 C3) and 29 females (21 C2 and 8 C3) were observed from June to September to determine winter survival. There were no significant differences in male and female survival between cohorts. Twenty three male-female pairs of C2 and 14 of C3 were placed in different shelters from October to January to determine the reproductive potential, which was measured as litter size, number of litters, post-partum pregnacies utilization, offspring survival and growth at weaning. There were not any significant differences in the considered reproductive parameters between the two cohorts, except for post-partum pregnancies. C2 and C3 individuals as overwintering cohort would not contribute differently to cohort 1 abundance.
RESUMEN: Sobrevida y potencial reproductivo en diferentes cohortes de Calomys venustus. Con el objetivo de de determinar la sobrevida invernal y el potencial reproductivo de las cohortes 2 (C2) y 3 (C3) de C. venustus, dichos parámetros fueron comparados entre individuos de ambas cohortes y su progenie. Para determinar sobrevida invernal, 46 machos (13 C2 y 33 C3) y 29 hembras (21 C2 y 8 C3) fueron monitoreados desde junio a septiembre. No se encontraron diferencias significativas en la sobrevida de machos y hembras entre las cohortes. Veintitrés parejas C2 y 14 C3 fueron ubicadas en refugios desde octubre a enero para determinar potencial reproductivo medido como: tamaño de camada, número de camadas, utilización de celo post-parto y sobrevida y crecimiento de la progenie hasta el momento del destete. No se encontraron diferencias significativas en los parámetros reproductivos considerados, excepto para la utilización del celo post-parto. Los individuos de C2 y C3 no contribuirían de forma diferente a la abundancia de la cohorte 1.
Key words. Cohort 2. Cohort 3. Litter. Post-partum estrus. Survival.
Palabras clave. Camada. Celo post-parto. Cohorte 2. Cohorte 3. Sobrevida.
INTRODUCTION
Calomys venustus is one of the most abundant species in the small-rodent communities inhabiting linear and less disturbed border habitats including crop-field edges, railway banks and roadsides of agrarian ecosystem of southern Córdoba province, Argentina (Kravetz and Polop, 1983; Polop and Sabattini, 1993; Priotto and Polop, 1997). This species has specific habitat requirements (Castellarini and Polop, 2002; Castellarini et al., 2002), a partitioned space use and is characterized by a promyscuous-polygynous mating system with females exhibiting territoriality throughout the year (Priotto and Polop, 1997; Priotto et al., 2002). Population densities of C. venustus fluctuate with different magnitudes within and among years (Provensal, 2001; Castellarini and Polop, 2002). Reproduction also varies within and among years in relation to the differential contribution of the cohorts (Polop et al., 2005). According to the season of birth we consider three cohorts in the C. venustus population, cohort 1 (C1, animals born in September-December), cohort 2 (C2, animals born in January-March) and cohort 3 (C3, animals born in April-May) (Polop, 1996; Polop et al., 2005). As Wiger (1979) in Clethrionomys glareolus and Zuleta et al. (1988) in Akodon azarae, Polop et al. (2005) also define an over-wintering cohort as a functional cohort. Individuals of this cohort belong to C2 and C3; they were born at the previous breeding period and survived until the following period, when they produce the first offspring litters (C1). Reproduction starts with a first peak in pregnancy rates of the over-wintering population in early spring. After this, there are two peaks in pregnancy rates, the highest one produced by C1 in early summer and the second one produced by C2 in late summer. In the middle of summer there are not captured over-wintering animals, cohort 1 born in spring becomes resident and an increase in the recruitment of C2 and C3 individuals at the end of the autumn causes a peak in annual population density. In winter, after the breeding period, populations decline sharply probably because of breeding interruption and mortality increase (Polop et al., 2005).
A "cohort effect" is a phenomenon where cohorts of a population differ from each other in some average property, such as reproduction and survival (Lindstrom and Kokko, 2002). In natural populations, variability in climatic stochasticity during early development is the most common factor that produces differences between cohorts (Lindstrom, 1999; Rodel et al., 2004) but an increased population density may also have an effect (Lindstrom and Kokko, 2002). Both population density and abiotic variables may interact in determining the strength of the effect of poor environmental conditions on early development (Lindstrom, 1999, Lundberg et al., 2000). Likewise, survival and reproduction may be limited not only by environmental stochasticity but can also be modified by individual characteristics (Lindstrom and Kokko, 2002; Rodel et al., 2004).
Polop et al. (2005) suggested that the mean factor responsible for the differences in abundance among years is the survival and fecundity of cohort 1. They also suggested that the proportion of C2 and C3 individuals in the over-wintering population would be different among years with different abundance, being C2 individuals numerically dominant after a high-density year and C3 dominant at the beginning of increase years. In this way, any difference in survival and reproductive potential between the different components of the over-wintering cohort could then influence C1 abundance.
The aim of this study was to examine winter survival and reproductive potential in cohorts 2 and 3. The reproductive potential was measured as litter size, number of litters, post-partum pregnancies, offspring survival, and growth at weaning. It is important to point out that this study only tested whether innate differences exist among individuals belonging to the two cohorts.
MATERIALS AND METHODS
Experimental design
One hundred and seven rodents, 62 males and 45 females, were captured alive between April and June 2001, in railway bank habitats in Chucul (64º 20' 09" W, 32º 21' 06" S), Río Cuarto Department, Córdoba Province, Argentina. Captured animals were measured and weighed and assigned to cohort 2 or 3 applying classification functions (Provensal, 2001). These classification functions were performed with animals of each cohort in a semi-captivity study, where the majority of individuals could be marked and assigned to their cohort when they were just weaned. The classification functions for each cohort and sex and for each month were expressed by: Y= a x1+ b x2- k; where Y is a value associated with a defined cohort for each month, x1 and x2 were the values for mass and body length respectively; a and b, their respective coefficients, and k the constant for each function (Provensal, 2001). All the analyses were done considering sex, because of the observed sexual dimorphism in the morphological characters considered (Polop and Provensal, 1999, 2000).
Animals uncertainly classified to a cohort were not included in the analysis. Twenty three males and 31 females were assigned to C2 and 39 males and 14 females to C3. Seventy five animals, 46 males (13 C2 and 33 C3) and 29 females (21 C2 and 8 C3), were carried to shelters situated in the Espinal Reservation in the Universidad Nacional de Rio Cuarto campus. Each shelter was enclosed by a concrete circle of 1 m of diameter and 0.7 m of height, and covered by an iron mesh. Ad libitum quantities of commercial trout pellets and water were added weekly during the experiment in order to homogenize food and water availability. On the other hand, thirty two animals were kept under laboratory conditions during winter.
The animals in shelters were observed weekly from June (beginning of winter) to September (beginning of spring) to determine winter survival.
Twenty three male-female pairs of C2 and 14 of C3 were placed in different shelters from October to January to determine reproductive potential. These pairs were formed with the same individuals used to determine survival (13 pairs of C2 and 8 of C3) and with individuals kept under laboratory conditions during winter (10 pairs of C2 and 6 of C3). In order to consider as a unique sample the pairs of the same cohort coming from laboratory or shelters, we compared their reproductive parameters by a Mann-Whitney test.
Litter size was measured as the number of offspring found after parturition. The number of litters was measured as the number of female parturitions until the end of December. To determine post-partum pregnancies we estimated the time elapsed between parturitions. We considered post-partum pregnancies when this period was smaller than 26 days (Polop, 1996).
Pre-weaning survival of the first two litters of C2 and C3 females was estimated weekly. Offspring growth of the first two litters of C2 and C3 females was determined recording mass and body length at weekly intervals, beginning one week after birth. Mass was recorded to 0.5 g and body length to 1 mm.
Statistical analysis
We compared male winter survival data obtained from each cohort employing LR test (Pyke y Thompson, 1986). Female winter survival was tested by a non-parametric test (Mann-Whitney). In order to obtain a reliable result and considering that the number of individuals was almost three times greater in one cohort than in the other, different comparisons were made considering the same number of animals of C2 and C3. We selected a random sample of animals of the most abundant group of equal size of the group with fewer individuals.
Comparisons between cohorts (C2 and C3 females) of the litter size of the first and second litter, the number of litters and the pre-weaning survival of the offspring were made with the Mann-Whitney non-parametric test.
We used a 2 x 2 contingency table to determine if there was an association between cohorts and post-partum pregnancies.
The offspring growth curves of C2 and C3 females were compared using the equal slopes of regression line test (Sokal y Rohlf, 1979) for each litter and sex. Assumptions of normality were tested by Kolmogorov-Smirnov test, and homogeneity of variance was tested using Bartlett 's test.
RESULTS
Winter survival did not vary significantly between the two cohorts for both males (p>0.05) and females (p>0.05). Survivorship curves for each cohort and sex are shown in Fig. 1. Cohort 3 males declined in week 5 and 9 but 90% of males lived up to the end of winter. Cohort 2 males declined in weeks 3, 6 and 9, but 75% of males lived up to the end of winter. Six of 21 C2 females did not survive to winter whereas all C3 females survived.

Fig. 1. Survivorship curves of Calomys venustus males and females of cohorts 2 and 3.
Mates of laboratory and sheltered animals did not differ significantly in reproductive parameters. The number of parturitions, the means and ranges of litter sizes for the two female cohorts are shown in Table 1. First and second litter sizes did not differ significantly between C2 and C3 females (U= 102.5; p>0.05 and U= 112.5; p>0.05 respectively). The number of litters was also not different between C2 and C3 females (U= 69; p>0.05). The number of litters for both cohorts ranged between 0 and 3.

Post partum pregnancies were significantly greater in C3 than in C2 females (p= 0,002; X 2 = 14,08, 1 df). Throughout the study, 73% of females belonging to C3 had post partum pregnancies, whereas less than a half of C2 females had post partum oestrous.
The first litter had a high survival rate for both cohorts, reaching values higher than 90%. The difference in the survival rate of C2 and C3 offspring was not significant (U= 73; p>0.05) (Fig. 2). In reference to the second litter, C3 offspring had a high survival rate (85%), whereas C2 offspring survival decreased progressively until reaching 65% at the end of the study. Nevertheless, the difference between them was not significant (U= 20; p>0.05) (Fig. 2).

Fig. 2. Survivorship curves of Calomys venustus first and second litters of cohort 2 and 3.
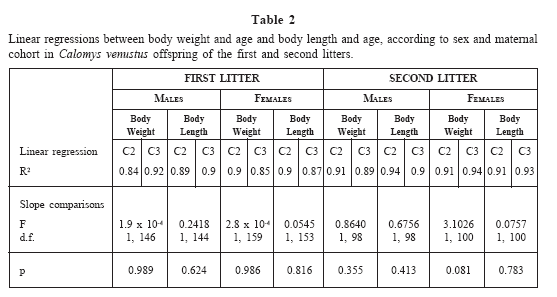
There was a good fit between mass and age; and body length and age for each sex of the first and second litter (Table 2). Both litters had similar growth curves, with individuals reaching similar mass and body length. Male and female body growth curves of the first litter are shown in Fig. 3. The curves showed sexual dimorphism, with males achieving higher mass and length than females. Mass and lengths of both male and female offspring from C2 and C3 mothers were not significantly different (Table 2).

Fig. 3. Growth curves of male and female offspring of the first litter for cohorts 2 and 3. a) Body length; b) body mass.
DISCUSSION
Our results showed a high rate of winter survival. This suggests that in C. venustus mortality would not explain the high winter disappearance rate observed by Castellarini and Polop (2002) and Polop et al. (2005) in field studies. The optimal experimental conditions in our study such as abundant food, presence of shelters, absence of competitors, safety from predation and the impossibility of animals to disperse may be the cause of the high survival of the cohorts, as it was observed in other species (Beacham, 1979; Crespin et al., 2002). These manipulations of the experimental conditions may reduce the possibilities of extrapolation of the results to natural conditions. Nevertheless, due to the aim of this study, the requirement of isolating the effect of cohorts from the effect of environmental conditions was given priority.
Cohorts 2 and 3, as the over-wintering cohorts, constitute the breeding population at the beginning of the breeding period. The number of individuals of each cohort at this time might depend on both the number of individuals of each cohort at the end of the previous breeding period and on mortality/dispersal during winter. Provensal (2001) found differences in C. venustus in the proportion of C2 and C3 individuals between the end of the breeding period and the beginning of the next one in field studies. This author suggested that these differences may be the consequence of differences in cohort survival, although in field only apparent survival can be estimated since permanent emigration is not distinguished from death (Crespin et al., 2002). In this study, we tested if the innate differences among the individuals belonging to the two cohorts could influence their survival in semi-captivity conditions. We did not find any differences in survival between the cohorts. We would expect that older individuals (C2 animals) would have less winter survival rates than younger individuals (C3 animals), as it was observed in other species (Wiger, 1979; 1982). The absence of significant differences in winter survival between both cohorts suggests that the physiological differences among individuals of both cohorts, if any, would not be enough to generate differences in survival. Therefore, differences in proportions of C2 and C3 individuals between the end of a breeding period and the beginning of the next one must have alternative explanations, 1) a differential dispersal of C2 and C3 individuals, and in consequence survival differences between cohorts in the field. There was no evidence to prove it in C. venustus; 2) an interaction between the impoverishment of habitats and the cohort might produce differential mortality in field conditions, since the quality of the environment and the quality of the individual might affect the performance of individuals (Lindstrom, 1999; Lambin and Yoccoz, 2001; Lindstrom and Kokko, 2002).
There was only one significant difference in the reproductive potential between the two cohorts. Cohort 3 females had more post-partum pregnancies than C2 females. However, the number of second litters was very similar between the two cohorts. Thus, the shorter interbreeding interval in C3 females is unlikely to have much influence on subsequent dynamics.
Nevertheless, the length of the breeding period in C. venustus is variable (Polop et al., 2005). Therefore, the association found between post-partum pregnancies and cohort might influence the number of offspring produced by each cohort during a shortened breeding period. Under these circumstances, a different number of C2 and C3 individuals in the over-wintering population at the beginning of the breeding season might generate a different number of cohort 1 individuals. During post-partum pregnancies, females must simultaneously meet the energetic demands of lactation and pregnancy (Lambin and Yoccoz, 2001). Therefore, the low number of postpartum pregnancies of C2 females may be caused by the inability of these individuals to undergo two reproductive periods with high energetic cost (gestation and lactation). This decreased reproductive ability may be an evidence of senescence of these eldest females.
In our study, C2 and C3 animals would not contribute differently to cohort 1 abundance, and in consequence the composition of the over-wintering population does not fully explain interannual variations in cohort 1 abundance. However, the lack of significant differences between the cohorts in survival and reproductive potential may be due to the relative small sample sizes used in this study, which probably increased the probability of not detecting significant differences when they occurred.
Previous studies on C. venustus established that females of the same age had different survival and reproductive potential when they came from populations with different densities (Provensal, 2001). These results and the results obtained in the present study may suggest that in C. venustus, density effects would be interacting with cohort effects to determine survival and reproductive potential. However, we could not analyze the two cohorts in different density conditions, because our study was conducted for only one year. To support the conclusion, we suggest that a more controlled long-term study should be conducted using greater sample sizes.
ACKNOWLEDGEMENTS
We thank Douglas Morris and an anonymous reviewer for helpful comments. We thank Marcos Torres for his assistance in fieldwork. This study was supported by grants from Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET) and the Secretaría de Ciencia y Técnica of Universidad Nacional de Río Cuarto (SECyT-UNRC).
LITERATURE CITED
BEACHAM T. 1979. Survival in fluctuating populations of the vole Microtus townsendii. Canadian Journal of Zoology 57:2375-2384. [ Links ]
CASTELLARINI F and JJ POLOP. 2002. Effects of extra food on population fluctuation patterns of the muroid rodent Calomys venustus. Austral Ecology 27:273-283. [ Links ]
CASTELLARINI F, C PROVENSAL, and J POLOP. 2002. Effect of weather variables on the population fluctuation of muroid Calomys venustus in central Argentina. Acta Oecologica 23:385-391. [ Links ]
CRESPIN L, R VERHAGEN, N STENSETH, N YOCCOZ, AC PREVOT-JULLIARD, and JD LEBRETON. 2002. Survival in fluctuating bank vole populations: seasonal and yearly variations. OIKOS 98:467-497. [ Links ]
KRAVETZ FO and JJ POLOP. 1983. Comunidades de roedores en agroecosistemas del departamento de Río Cuarto, Cordoba. Ecosur 10:1-18. [ Links ]
LAMBIN X and N YOCCOZ. 2001. Adaptative precocial reproduction in voles: reproductive costs and multivoltine life-history strategies in seasonal environments. Journal of Animal Ecology 70:191-200. [ Links ]
LINDSTROM J. 1999. Early development and fitness in birds and mammals. TREE 14:343-348. [ Links ]
LINDSTROM J and H KOKKO. 2002. Cohort effects and population dynamics. Ecology Letters 5:338-344. [ Links ]
LUNDBERG P, E RANTA, J RIPA, and V KAITALA. 2000. Population variability in space and time. TREE 15: 460-464. [ Links ]
PYKE DA and JN THOMSON. 1986. Statistical analysis of survival and removal rate experiments. Ecology 67:240-243. [ Links ]
POLOP JJ. 1996. Análisis de la respuesta adaptativa del género Calomys. PhD Thesis inedit. Facultad de Ciencias Exactas, Físico-Químicas y Naturales. Universidad Nacional de Río Cuarto. Río Cuarto, Argentina. [ Links ]
POLOP JJ and MS SABATINI. 1993. Rodent abundance and distribution in habitats of Agrocenosis in Argentina. Studies in Neotropical Fauna and Environment 28:39-136. [ Links ]
POLOP JJ and MC PROVENSAL. 1999. Contribution to the identification of Calomys venustus (Thomas, 1894) (Rodentia, Muridae) by morphological characters. Facena 15:163-168. [ Links ]
POLOP JJ and MC PROVENSAL. 2000. Morphological variation and age determination in Calomys venustus (Thomas, 1894) (Rodentia, Muridae). Journal of Neotropical Mammalogy 7:101-115. [ Links ]
POLOP JJ, MC PROVENSAL, and P DAURIA. 2005. Reproductive characteristics of free-living Calomys venustus (Rodentia, Muridae). Acta Theriologica 50:357-366. [ Links ]
PRIOTTO JW and JJ POLOP. 1997. Space and time use in syntopic populations of Akodon azarae and Calomys venustus (Rodentia, Muridae). Zeitschrift für Säugetierkunde 62:30-36. [ Links ]
PRIOTTO JW, A STEINMANN, and JJ POLOP. 2002. Factor affecting home range size and overlap in Calomys venustus (Muridae: Sigmodontinae) in Argentine agroecosystems. Mammalian Biology 64:97-104. [ Links ]
PROVENSAL MC. 2001. La edad y la densidad en la regulación de poblaciones de Calomys venustus (Rodentia, Muridae). PhD Thesis inedit. Facultad de Ciencias Exactas Físico-Químicas y Naturales, Universidad Nacional de Río Cuarto. Río Cuarto, Argentina. [ Links ]
RODEL H, A BORA, P KAETZKE, M KHASCHEI, H HUTZELMEYER, and D VON HOLST. 2004. Over-winter survival in subadult European rabbits: weather effects, density dependence, and the impact of individual characteristics. Oecologia 140:566-576. [ Links ]
SOKAL R and F ROHLF. 1979. Biometría: principios y métodos estadísticos en la investigación biológica. Ed. Blume. [ Links ]
WIGER R. 1979. Demography of a cyclic population of the bank vole Clethrionomys glareolus. Oikos 33:373-385. [ Links ]
WIGER R. 1982. Roles of self regulatory mechanism in cyclic populations of Clethrionomys with special reference to C. glareolus: a hypothesis. Oikos 38:60-71. [ Links ]
ZULETA G, F KRAVETZ, M BUSCH, and R PERCICH. 1988. Dinámica poblacional del ratón del pastizal pampeano (Akodon azarae) en ecosistemas agrarios de Argentina. Revista Chilena de Historia Natural 61:231-244. [ Links ]
Recibido 8 marzo 2005.
Aceptación final 13 noviembre 2006.














