Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Mastozoología neotropical
versión impresa ISSN 0327-9383versión On-line ISSN 1666-0536
Mastozool. neotrop. v.15 n.2 Mendoza jul./dic. 2008
Ectoparasites of the common vampire bat (Desmodus rotundus) in Costa Rica: parasitism rates and biogeographic trends
Andrés Rojas1*, Ana Jiménez1, Mario Vargas2, Manuel Zumbado3, and Marco V. Herrero4
1 Department of Parasitology, School of Veterinary Medicine, National University of Costa Rica,
Barreal de Heredia, Heredia, Costa Rica; *<andvet@gmail.com>.
2 Department of Medical Arthropology, School of Microbiology, University of Costa Rica, San Pedro de Montes de Oca, San José, Costa Rica.
3 Department of Entomology, National Institute of Biodiversity, Santo Domingo de Heredia, Heredia, Costa Rica.
4 Department of Entomology, School of Veterinary Medicine, National University of Costa Rica, Barreal de Heredia, Heredia, Costa Rica, Barreal de Heredia, Heredia, Costa Rica.
ABSTRACT: A descriptive study was carried out to determine the ectoparasitic fauna of the common vampire bat Desmodus rotundus (Chiroptera: Phyllostomidae) from twelve locations and eight different life zones in Costa Rica. The biogeographic trends of ectoparasites were described using Geographical Information Systems. A total of sixty seven bats were collected using mist nets and submitted for necropsy. Two species of batflies (Diptera: Streblidae) were identified: Trichobius parasiticus (82.14%) and Strebla wiedemanni (4.05%), as well as two species of mites: Radfordiella desmodi (13.57%) (Acarina: Macronyssidae) and Periglischrus herrerai (0.24%) (Acarina: Spinturnicidae). The highest percentages of infestation were given by T. parasiticus (91.04%) and R. desmodi (19.40%), with an intensity of infestation of 5.65 and 4.38 per bat, respectively. T. parasiticus was the species which was most frequently found infesting vampire bats in their natural habitat; it seems to be the ectoparasite with the widest geographic and ecologic distribution. Additionally new geographical distribution for T. parasiticus and S. wiedemanni are proposed. Humidity, altitude, and average environmental temperature could be factors that influenced the biogeography of the ectoparasitic species found. The finding of Radfordiella desmodi and Periglischrus herrerai are the first reports of these mites for Costa Rica.
RESUMEN: Ectoparásitos del vampiro común (Desmodus rotundus) en Costa Rica: tasas de parasitismo y tendencias biogeográficas. Se realizó un estudio descriptivo sobre la fauna ectoparasitaria del murciélago vampiro Desmodus rotundus (Chiroptera: Phyllostomidae) en Costa Rica. Asimismo, se describieron las tendencias ecogeográficas de los ectoparásitos obtenidos, utilizando Sistemas de Información Geográfica. Un total de 67 animales fueron capturados, lográndose obtener un total de 420 ectoparásitos de los cuales el 82.14% correspondieron a dípteros de la especie Trichobius parasiticus, y el 4.05% a la especie Strebla wiedemanni; mientras que el 13.57% estuvo representado por el ácaro Radfordiella desmodi; y Periglischrus herrerai correspondió a un 1.5%. Los mayores porcentajes de infestación estuvieron dados por T. parasiticus (91.04%) y R. desmodi (19.40%), con una intensidad de infestación de 5.65 y 4.38 por murciélago, respectivamente. Trichobius parasiticus fue la especie más frecuentemente hallada infestando al murciélago vampiro en su hábitat natural, y al mismo tiempo parece ser el ectoparásito con la mayor distribución geográfica y ecológica en Costa Rica. Adicionalmente se proponen nuevos ámbitos geográficos para T. parasiticus y S. wiedemanni. La humedad, la altura y la temperatura ambiental promedio, parecen ser factores que influyen en la ecogeografía de los especimenes hallados. Los hallazgos de Radfordiella desmodi y Periglischrus herrerai, son los primeros registros para Costa Rica de ambas especies.
Key words. Costa Rica; Ecological patterns; Ectoparasites; Vampire bats.
Palabras clave. Costa Rica; Ectoparásitos; Murciélagos hematófagos; Tendencias ecológicas.
INTRODUCTION
Costa Rica has 9 families and 110 species of bats which represents more than 11% of all species of chiropterans currently known. Desmodus rotundus, Diaemus youngi and Diphylla ecaudata are the haematophagous species which have been reported in this country (La Val and Rodríguez, 2002).
Prior studies on the ectoparasitic fauna of D. rotundus, which have been carried out in South America (Argentina, Bolivia, Brazil, Colombia, Ecuador, Paraguay, Peru, Surinam and Venezuela), Central America (Costa Rica, El Salvador, Guatemala, Honduras, Nicaragua and Panama) and North America (Mexico), report a great diversity of species (Wenzel et al., 1966; Guerrero, 1997; Autino and Claps, 2001; Graciolli et al., 2001; Dick and Gettinger, 2005). Among the ectoparasites identified in these countries are batflies from the Streblidae and Nycteribiidae families (Guerrero, 1996). Additionally, mites belonging the Trombiculidae, Myobiidae, Sarcoptidae, Spinturnicidae and Macronyssidae families have also been reported (Webb and Loomis, 1977). Finally, ticks of the genera Ornithodorus spp. (Webb and Loomis, 1977) and fleas of the species Hormopsylla fosteri have also been cited (Rodríguez et al., 1999).
In Costa Rica there are few reports concerning the parasites of vampire bats (Zeledón and Vieto, 1957; Tonn and Arnold, 1963; Ubelaker et al., 1977; Webb and Loomis, 1977; Fritz, 1983; Goff, 1988; Rojas and Guerrero, 2007). More studies are needed due to the importance of the ectoparasites in the ecology, maintenance and dissemination as biological agents among the population of those mammals (Fritz, 1983; Fonseca et al., 2005).
The aim of the present study was to further clarify the species of ectoparasites on vampire bats, their parasitism rates and biogeographic trends in Costa Rica.
MATERIALS AND METHODS
Study area
Vampire bats were collected in twelve different areas and eight life zones around Costa Rica. The geographic distribution of the sampling areas were: 1) Guanacaste, Nicoya, Quebrada Honda (10.21502 N, 85.29914 O). 2) Guanacaste, Nandayure, Bejuco (9.77530 N, 85.26066 O). 3) Guanacaste, La Cruz, La Cruz (11.15665 N, 85.63048 O). 4) Guanacaste, Tilarán, Santa Rosa (10.49166 N, 84.98541 O). 5) Guanacaste, Abangares, San Juan (10.19668 N, 84.89324 O). 6) Alajuela, San Ramón, Los Angeles (10.14582 N, 84.49910 O). 7) Alajuela, San Carlos, Venado (10.52183 N, 84.81839 O). 8) Alajuela, San Ramón, San Rafael (9.98291 N, 84.49171 O). 9) Alajuela, San Mateo, Jesús María (9.96495 N, 84.57589 O). 10) Cartago, Turrialba, Tres Equis (9.98221 N, 83.60420 O). 11) Puntarenas, Puntarenas, Cóbano (9.59506 N, 85.14223 O). 12) Puntarenas, Puntarenas, Lepanto (9.89690 N, 85.21064 O). The bioclimatic characteristics of sampling areas were taken using the Geographic Position System (Table 1).
Table 1
Bioclimatics characteristics of the sampling sites from D. rotundus in Costa Rica. Bh-T10 = Base Moist Forest, Transition to Base Dry Forest; Bh-P6 = Premontane Wet Forest, Transition to Base Wet Forest; Bh- T = Base Moist Forest; Bh-T12 = Base Moist Forest, Transition to Premontane Moist Forest; Bmh-P = Premontane Wet Forest; Bp-P = Premontane Rain Forest; Bh-T2 = Base Moist Forest, Transition to Base Rain Forest; Bmh-T12 = Base Wet Forest, Transition to Premontane Wet Forest; *According to Holdridge

Collection
Monthly samplings were realized from October 2003 to July 2005. The vampire bats were collected using mist nets placed in caves, tunnels or abandoned mines. The bats caught were manipulated with leather gloves and placed in cages of galvanized steel, in order to be transferred to the Pathology Laboratory at the School of Veterinary Medicine, National University of Costa Rica to be euthanatized. The animals were euthanized inside a glass container that had a paper towel impregnated with an inhalant anesthetic (chloroform) according to ACUC (1998), and maintained inside for five minutes until they died. Once the animals were dead, the collection of the ectoparasites was realized with the aid of fine tweezers.
Preservation, mounting and species identification
The ectoparasites were preserved in individual containers filled with alcohol 70%-glycerol and mounted using Hoyer's solution. The ectoparasites were identified using specific morphological keys (Machado-Allison, 1965; Wenzel et al., 1966; Radovsky, 1967; Guerrero, 1994; Guerrero, 1996). The batflies were deposited in the entomology collections of the National Institute of Biodiversity (deposit numbers 4015662-4016997), Museum of Insects at the University of Costa Rica and the Laboratory of Parasitology of the Veterinary Medicine School at the National University of Costa Rica (deposit numbers not applicable). The mites found were deposited in the entomology collection of the Museum of Insects at the University of Costa Rica (deposit numbers not applicable).
Parasitism rates
The percentage of infestation and the intensity of infestation were determined. The percentage of infestation was defined as the total number of positive bats to ectoparasite between the total bats captured, whereas the intensity of infestation was defined as total number of ectoparasites by bat, between the total of positive bats to each ectoparasites (Muñoz et al., 2003).
Biogeographic trends
Data base in Arc View (Arc View 3,3 ESRI. Inc., 2001), was created using the Lambert comformal Conic projection, in which the following information was included: province, county and district, latitude, longitude, altitude, species of ectoparasites and life zone; in order to classify the geography and ecology of each locality sampled using the Holdridge's life zones system (Holdridge, 1987).
RESULTS
A total of 67 bats were captured (15 males and 52 females) and 420 ectoparasites were obtained. Three hundred and sixty two (86.20%) were batflies of the species Trichobius parasiticus and Strebla wiedemanni; and 58 (13.80%) corresponds to mites R. desmodi and P. herrerai (Table 2).
Table 2
Ectoparasites collected from D. rotundus in Costa Rica. * Protonymph

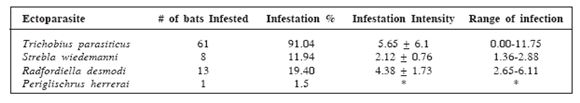
The percentage of infestation of T. parasiticus and S. wiedemanni on bats was 91.04% (61) and 11.94% (8) respectively. Additionally 13 (19.40%) bats were found positively infested by the mite R. desmodi. The spinturnicid mite P. herrerai could be only found in 1 (1.50%) bat. The infestation intensity average by ectoparasitic species was 6.27ectoparasites/bat. The intensity average by species was 5.65 T. parasiticus/bat, 2.12 S. wiedemanni/bat, 4.38 R. desmodi/bat and 1.00 P. herrerai/bat, respectively (Table 3).
Table 3
Infestation percentages and intensity averages of the ectoparasites species found. (*Not calculated as there was only one specimen).
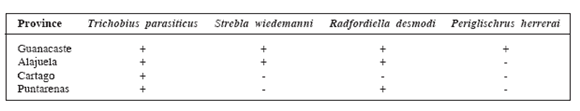
Most of the ectoparasites collected were located in the provinces of Alajuela and Guanacaste (Table 4).
Table 4
Geographic distribution of the ectoparasites collected from D. rotundus (+Positive to ectoparasites, - Negative to ectoparasites).
The ecological distribution varied for each species. Of a total of 345 specimens of T. parasiticus collected, 27.53% (95) were found in the life zone corresponding to premontane wet forest; 22.60% (78) in base moist forest, transition to premontane moist forest; 22.00% (61) in base moist forest; 11,88% (41) in premontane rain forest, and the remain 15.28% (54) were distributed in life zones corresponding to premontane moist forest, transition to base moist forest; base moist forest, transition to base dry forest; base moist forest, transition to premontane moist forest and base moist forest, transition to base wet forest. A total of 17 specimens of S. wiedemanni were collected, 52.93% (9) could be found in base moist forest; 29.41% (5) in base moist forest, transition to base dry forest; 11.76% (2) was found in base moist forest, transition to premontane moist forest, and 5.88% (1) in zones corresponding to premontane wet forest. A total of 57 specimens of R. desmodi were obtained, of which 49.12% (28) were located in premontane rain forest; the 43.85% (25) in base moist forest; 5.26% (3) in life zones corresponding to premontane wet forest, and 1.75% (1) in base moist forest, transition to base dry forest. The only specimen of P. herrerai corresponds to one protonymph and was found in the life zone corresponding to base moist forest (Table 5).
Table 5
Ecological distribution of the ectoparasites obtained from D. rotundus in Costa Rica (+Positive to ectoparasites, -Negative to ectoparasites). *The simbology of the life zones are described in Table 1.

DISCUSSION AND CONCLUSIONS
In our study only four species of ectoparasites could be found. These species correspond to T. parasiticus and S. wiedemanni (Diptera: Streblidae), R. desmodi (Acarina: Macronyssidae) and P. herrerai (Acarina: Spinturnicidae). Only T. parasiticus and S. wiedemanni were the streblids collected on D. rotundus in this work, findings similar to others studies carried out in Costa Rica (Tonn and Arnold, 1963; Guerrero, 1997). Wenzel et al. (1966) also reported S. hertigi infesting D. rotundus in Costa Rica; however this streblid was not found in this research which could indicate that the common vampire bat is an accidental host. The total number of ectoparasitic species found per vampire bat in this study is lowest than other previous reports (Machado-Allison, 1965). This difference could be explain by the natural history of the ectoparasite, in addition to a small sample size and ecological factors such as competence between species of parasites (Linhares and Komeno, 2000), reproductive behavior (Ter Hofstede et al., 2004; Berlota et al., 2005) and its life cycle (Whitaker et al., 2000).
Trichobius parasiticus and R. desmodi had the highest parasitism rates. Therefore it can be hypothesized that both species are frequent ectoparasites of D. rotundus in its natural habitat. Our results agree with the reported for this streblids in Paraguay (Dick and Gettinger, 2005), Brazil, Mexico, Peru and Trinidad (Wenzel et al., 1966). However, our results are not in accordance with other studies that propose the same geographic distribution for S. wiedemanni and D. rotundus (Guerrero, 1996). Additionally, P. herrerai and R. desmodi have been reported as common ectoparasites of vampire bats in several countries of Latin America (Marinkelle and Groose, 1981; Whitaker and Abrell, 1987; Azevedo et al., 2002; Mendoza-Uribe and Chavez, 2003), but it is the first record of these mites infesting D. rotundus in Costa Rica. Periglischrus herrerai was distributed in few areas which may signify that the occurrence of these mites in the populations of vampire bats is low. In general, the infestations with Spinturnicidae mites are found in very few individuals (Wohland, 2000).
The finding of T. parasiticus in the Guanacaste, Puntarenas, Alajuela and Cartago provinces, as well as S. wiedemanni in the Alajuela and Guanacaste provinces, suggests new geographic ranges for them inside Costa Rica.
Previous studies about the ectoparasitic fauna of D. rotundus in Costa Rica are focused mainly in a geographic description in which these ectoparasites were found; without giving reference to their ecology. The results obtained according to the ecology indicate that T. parasiticus and R. desmodi seem to prefer relatively cold habitats and low humidity, where the precipitation indices are between the 2000-8000 millimeters per year, with a mean potential evapotranspiration ratio between 0.125-0.5, and environmental temperature average oscillating in the rank of 12-24°C. Due to the fact that the life zones where these two species were found corresponded mainly to altitudinal belts of premontane character, it is possible that both species might be found only in geographic areas with elevations between 500-2000 meters above sea level; results that are similar to the altitudinal ranks near to 1700 meters above sea level, that have been proposed for some streblid species (Wenzel et al., 1966). In contrast, S. wiedemanni and P. herrerai seem to prefer warmer zones of life and humidity, with an annual precipitation between 2000-4000 millimeters per year, a potential evapotranspiration ratio of 0.25-0.5, and environmental temperatures over 24°C, being frequently found in basal altitudinal belts in which the elevation average is 0-500 meters above sea level (Holdridge, 1987). These results suggest that the altitude, temperature and environmental humidity average are important factors in the ecology of these species, in accordance with previous studies which propose that seasonal temperatures may affect the reproductive and mortality rates of some bat ectoparasites (Rui and Graciolli, 2005).
Additionally, our results suggest that the ecological distribution of the ectoparasitic species found might be the same in other geographic areas of our country, as well as in other Latin American countries with similar life zones. Nevertheless more studies are needed in order to clarify more about their ecology.
ACKNOWLEDGMENTS
The authors would like to thank all those individuals who have provided samples, assistance and bibliography for this research, including Dr. Frank Radovsky from Department of Parasitology, Oregon State University; Dr. Ricardo Guerrero from Department of Tropical Zoology, Central University of Venezuela; Dr. Victor H. Sancho and Dr. Luis Matamoros from Department of Agriculture of Costa Rica and M.Sc. Hannah Kurtis from Department of Epidemiology and Public Health, Yale University. This research was displayed at the annual conference of the American Association of Zoo Veterinarians, September 2006, Tampa, Florida.
LITERATURE CITED
1. ANIMAL CARE AND USE COMMITTEE (ACUC). 1998. Guidelines for the capture, handling, and care of mammals as approved by The American Society of Mammalogists. Journal of Mammology 79:1416- 1431. [ Links ]
2. AUTINO AG and GL CLAPS. 2001. Catalogue of the ectoparasitic insects of the bats of Argentina. Insecta Mundi 14:193-209. [ Links ]
3. AZEVEDO AA, MP LINARDI and M T COUTINHO. 2002. Acari Ectoparasites of Bats from Minas Gerais, Brazil. Journal of Medical Entomology 39:553-555. [ Links ]
4. BERLOTA PB, C COTRIM, S FAVORITO, G GRACIOLLI, M AMAKU and R PINTO DA ROSA. 2005. Batflies (Diptera: Streblidae, Nycteribiidae) parasitic on bats (Mammalia: Chiroptera) at Parque Estadual de Cantareira, São Paulo, Brazil: parasitism rates and host-parasite associations. Memorias do Instituto Oswald Cruz 100:25-32. [ Links ]
5. DICK C W and D GETTINGER. 2005. A faunal survey of Streblid flies (Diptera: Streblidae) associated with bats in Paraguay. Journal of Parasitology 91:1015- 1024. [ Links ]
6. FONSECA M, M VALIM, R BOTÃO-MIRANDA, C GITTI, M AMORIN and N SERRA-FREIRE. 2005. Ocorrência de Dentocarpus silvai silvai Dusbabek & Cruz, 1966 (Acari: Chirodiscidae) em duas espécies de molossídeos (Mammalia: Chiroptera) no estado do Rio de Janeiro, Brasil. Entomology Vectors 12:117-121. [ Links ]
7. FRITZ G. 1983. Biology and ecology of bat flies (Diptera: Streblidae) on bats in the genus Carollia. Journal of Medical Entomology 20:1-10. [ Links ]
8. GOFF ML. 1988. A new species of chigger (Acari: Trombiculidae) from a vampire bat (Chiroptera: Desmontodidae) collected in Costa Rica. International Journal of Acarology 14:5-7. [ Links ]
9. GRACIOLLI G, NC CÁCERES and MR BORNSCHEIN. 2001. Novos registros de moscas ectoparasitas (Dipetra, Streblidad e Nycteribiidae) de morcegos (Mammalia, Chiroptera) em àreas de transição cerrado-floresta no Mato Grosso do Sul, Brasil. Biota Neotropica 6:1-4. [ Links ]
10. GUERRERO R. 1994. Catálogo de los Streblidae (Diptera: Pupípara) parásitos de murciélagos (Mammalia: Chiroptera) del nuevo mundo II. Los grupos: Pallidus, Caecus, Major, Uniformis y Longipes del género Trichobius Gervalis, 1844. Acta Biológica Venezuélica 15:1-18. [ Links ]
11. GUERRERO R. 1996. Catálogo de los Streblidae (Diptera: Pupípara) parásitos de murciélagos (Mammalia: Chiroptera) del nuevo mundo VI. Streblidae. Acta Biológica Venezuélica 16:1-25. [ Links ]
12. GUERRERO R. 1997. Catálogo de los Streblidae (Diptera: Pupípara) parásitos de murciélagos (Mammalia: Chiroptera) del nuevo mundo VII. Lista de especies, hospedadores y países. Acta Biológica Venezuélica 17:9-24. [ Links ]
13. HOLDRIDGE LR. 1987. Life Zone Ecology. IICA, San José, Costa Rica. [ Links ]
14. LA VAL R and RB RODRÍGUEZ. 2002. Murciélagos de Costa Rica. Editorial Instituto Nacional de Biodiversidad, Santo Domingo de Heredia, Costa Rica. [ Links ]
15. LINHARES A X and C A KOMENO. 2000. Trichobius joblingi, Asidoptera falcata and Megistopoda proxima (Diptera: Streblidae) parasitic on Carollia perspicillata and Sturnira lilium (Chiroptera: Phyllostomidae) in southeastern Brazil: sex rations, seasonality, host site preference, and effect of parasitism on the host. Journal of Parasitology 86:167-170. [ Links ]
16. MACHADO-ALLISON CE. 1965. Las especies venezolanas del género Periglischrus Kolenati 1857 (Acarina: Mesostigmata, Spinturnicidae). Acta Biológica Venezuélica 4:259-348. [ Links ]
17. MARINKELLE CJ and SE GROOSE. 1981. A list of ectoparasites in Colombian bats. Revista de Biología Tropical 29:11-20. [ Links ]
18. MENDOZA-URIBE L and J CHAVEZ. 2003. New records of mites (Gamasida: Spinturnicidae) on Peruvian bats (Chiroptera: Phyllostomidae). Revista de Biología Tropical 51:276-277. [ Links ]
19. MUÑOZ L, M AGUILERA and M CASANUEVA. 2003. Prevalencia e intensidad de ectoparásitos asociados a Tadaria brasiliensis (Geoffroy Saint-Hilaire, 1824) (Chiroptera: Molossidae) en Concepción. Gayana 6:1-8. [ Links ]
20. RADOVSKY FJ. 1967. The Macronyssidae and Laelapidae (Acarina: Mesostigmata) parasitic on bats. University of California Publications on Entomology 46:153-159. [ Links ]
21. RODRÍGUEZ Z, EC MOREIRA, PM LINARDI and HA SANTOS. 1999. Notes on the bat flea Hormopsylla fosteri (Siphonaptera: Ischnopsyllidae) infesting Molossus abrasus (Chiroptera). Memorias do Instituto Oswaldo Cruz 94:727-728. [ Links ]
22. ROJAS A and R GUERRERO. 2007. Capillaria (s.l.) sp., isolated from Desmodus rotundus (Chiroptera: Phyllostomidae) in Costa Rica. Mastozoología Neotropical 14:101-102. [ Links ]
23. RUI AM and G GRACIOLLI. 2005. Moscas ectoparasitas (Díptera: Streblidae) de morcegos (Chiroptera, Phyllostomidae) no sul do Brasil: associações hospedeiros-parasitos e taxas de infestação. Revista Brasileira de Zoología 2:438-445. [ Links ]
24. TER HOFSTEDE H, FM BROCK and JR WHITAKER. 2004. Host and host-site specificity of bat flies (Diptera: Streblidae and Nycteribiidae) on Neotropical bats (Chiroptera). Canadian Journal of Zoology 82:616-626. [ Links ]
25. TONN JR and K ARNOLD. 1963. Ectoparásitos de aves y mamíferos de Costa Rica. Revista de Biología Tropical 11:171-176. [ Links ]
26. UBELAKER JE, DS ROBERT and WD DONALD.1977. Endoparasites. Pp 7-56, in: Biology of bats of the New World, Family Phyllostomidae (RJ Baker, JK Jones, DS Carter and J Robert, eds.). Special Publications. The Musseum of Texas Tech University. [ Links ]
27. WEBB PJ and RB LOOMIS. 1977. Ectoparasites. Pp 57-119, in: Biology of bats of the New World, Family Phyllostomidae (RJ Baker, JK Jones, DS Carter and J Robert, eds.). Special Publications, The Musseum of Texas Tech University. [ Links ]
28. WENZEL RL, VJ TIPTON and A KIEWLICZ. 1966. The Streblid batflies of Panama. Pp. 432-661, in: Ectoparasites of Panama (RL Wenzel and VJ Tipton, eds). Field Museum of Natural History, Chicago, USA. [ Links ]
29. WHITAKER JO and DB ABRELL. 1987. Notes on some ectoparasites from mammals of Paraguay. Entomology News 98:198-204. [ Links ]
30. WHITAKER JO, J DEUNFF and J BELWOOD. 2000. Ectoparasites of neonate Indiana bats, Myotis sodalis (Chiroptera: Vespertilionidae), with description of male of Paraspinturnix globosa (Acari: Spinturnicidae). Journal of Medical Entomology 37:445-453. [ Links ]
31. WOHLAND P. 2000. Grooming behavior and parasitic load in the Greater horseshoe bat (Rhinolophus ferrumequinum). Bristol University, UK. [ Links ]
32. ZELEDÓN R and P VIETO. 1957. Hallazgo de Schizotrypanum vespertilionis (Battaglia, 1904) en la sangre de murciélagos de Costa Rica. Revista de Biología Tropical 5:123-128. [ Links ]
Recibido 16 julio 2007.
Aceptación final 8 noviembre 2007.














