Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Mastozoología neotropical
Print version ISSN 0327-9383On-line version ISSN 1666-0536
Mastozool. neotrop. vol.16 no.1 Mendoza Jan./June 2009
ARTÍCULOS Y NOTAS
Diet variation of the marsupials Didelphys aurita and Philander frenatus (Didelphimorphia, Didelphidae) in a rural area of Rio de Janeiro state, Brazil
Paula Ceotto1, 2, Ricardo Finotti1*, Ricardo Santori3, and Rui Cerqueira1
1 Departamento de Ecologia, Instituto de Biologia - UFRJ, Ilha do Fundão, RJ. Caixa Postal 68044, CEP 21944-970, Brazil
2 Present address: Laboratoire d´entomologie, MNHN/ESA 8043/CNRS, 45, rue Buffon, 75005 Paris, France.
3 Departamento de Ciências, Faculdade de Formação de Professores - UERJ. Rua Dr. Francisco Portela, 794. Paraíso, São Gonçalo, RJ. CEP 24435-000, Brazil.
*[Corresponding author: <finotti@biologia.ufrj.br>]
ABSTRACT: We studied the natural diet of two didelphid marsupial species, Didelphis aurita and Philander frenatus, at a rural area on southeastern Brazil, through fecal sample analysis. Data analysis included intraspecific comparisons (age, gender, and climatic seasons) and interespecific comparisons. Frequencies of food items were compared with other studies to analyze diet differences in a spatial scale. Invertebrates were the most frequent food category found in samples of both species, followed by fruits and vertebrates. There were no differences in the consumption of invertebrates, vertebrates and fruits between sexes in the two species, but both consumed more fruits in more humid months. Juveniles of P. frenatus consumed less vertebrates than adults or subadults. P. frenatus consumed more vertebrates than D. aurita, which exhibited a higher diversity of fruits in its diets. These differences represent an important factor in diminishing intraspecific competition. Among different localities, invertebrates are the more common items in the diet of both species, being a fundamental alimentary resource, whereas fruit consumption seems to be smaller in the areas where water is less available (areas with longer periods or higher hidric deficits), probably reflecting availability of this food resource.
RESUMEN: Variación en la dieta de los marsupiales Didelphis aurita y Philander frenatus (Didelphimorphia, Didelphidae) en un área rural del estado de Río de Janeiro, Brasil. Estudiamos la dieta natural de dos especies de marsupiales Didelfideos, Didelphis aurita y Philander frenatus, en un área rural del sudeste brasilero, a través del análisis de muestras fecales. El análisis de los datos incluyó comparaciones intraespecíficas (edad, género, estaciones climáticas) y comparaciones interespecíficas. Las frecuencias de los ítems alimenticios fueron comparadas con otros estudios para identificar diferencias en la escala espacial. Los invertebrados fueron la categoría más encontrada en las muestras de las dos especies, seguidos por frutos y vertebrados. No hubo diferencias en el consumo de invertebrados, vertebrados y frutos entre los sexos para las dos especies, pero ambas consumieron más frutas en los meses más húmedos. Los juveniles de P. frenatus consumieron menos vertebrados que los adultos o subadultos. Philander frenatus consumió más vertebrados que D. aurita, quien exhibió una mayor diversidad de frutos que P. frenatus en su dieta. Estas diferencias representan un factor importante en la disminución de las competencias interespecífica e intraespecífica. En las diferentes localidades los invertebrados son el ítem más común en las dietas de las dos especies, siendo un recurso fundamental, mientras que el consumo de frutos parece ser menor en áreas con una menor disponibilidad de agua (áreas con periodos de sequía mayores o mayor déficit hídrico), posiblemente reflejando la disponibilidad de este recurso.
Key words. Didelphimorphia; Food habits; Mammal ecology; Neotropics.
Palabras clave. Didelfimorphia; Ecología de mamíferos; Hábitos alimenticios; Neotropico.
INTRODUCTION
The diet of a species can significantly influence many aspects of its ecology, such as habitat selection (Freitas et al., 1997; Allison et al., 2006), geographical distribution (Redford, 1984; Silva, 2005), life history (Maher et al., 2001) and niche separation (Périssé et al., 1988; Leite et al., 1994; Virgos et al., 1999; Carvalho et al., 2005). The importance of the diet in niche partitioning among species of didelphid marsupials has been revealed as an important factor in understanding their coexistence and distribution in several biomes (Leite et al., 1994; Freitas et al., 1997; Vieira and Izar, 1999; Cáceres, 2004)
Didelphis aurita and Philander frenatus (Didelphimorphia, Didelphidae) occur simpatrically in the Atlantic Forest (Gentile et al., 2000), and belong to sister genera (Kirsch, 1997; Voss and Jansa, 2003; Jansa and Voss, 2005). Both species are predominantly terrestrial, but are able to climb trees efficiently (Cunha and Vieira, 2002; Antunes, 2003; Delciellos and Vieira, 2006; Delciellos and Vieira, 2007). However, D. aurita is more arboreal than P. frenatus (Fonseca and Kierulff, 1989; Cunha and Vieira, op. cit.). Previous laboratory studies on their diets showed that they use animal and plant matter (fruits) in a similar way (Perissé et al., 1988; Freitas et al., 1997). Leite et al. (1994), in a field study, found no differences in the feeding habits of these species, regarding the proportions of invertebrates, fruits, and small vertebrates. However, laboratory experiments and digestive morphophisiological studies indicated that P. frenatus is more carnivorous than D. aurita-considered as generalist (Périssé et al., 1988; Périssé et al., 1989; Santori et al., 1995b; Astúa de Moraes et al., 2003).
Previous results concerning age-related differences are not conclusive. Some age-related variation in diet was found for P. frenatus (Santori et al., 1997), and for Didelphis marsupialis (Cordero and Nicolas, 1987). In these studies, the food items rank used by young individuals tended to be narrower than those used by adults. However, other field studies on Didelphis aurita did not find any difference among age classes (Cáceres and Monteiro-Filho, 2001; Cáceres, 2002).
Didelphis aurita and Philander frenatus also exhibited seasonal and spatial differences in the use of food items, possibly related to differences of resource availability in time and space (Leite et al., 1994; Freitas et al., 1997; Santori et al., 1997; Vieira and Izar, 1999; Cáceres and Monteiro-Filho, 2001). Food generalist animals may modify their diet according to variations in resource availability (Alves- Costa et al., 2004). Resources are more scarce in dry seasons and abundant in rainy periods in tropical forests (Wolda, 1988; Van Schaik et al., 1993). This variation in food offer may produce seasonal changes in the diet of D. aurita and P. frenatus (Cordero and Nicolas, 1987; Santori et al., 1997; Cáceres and Monteiro-Filho, 2001). Thus, an important question is to what extent the use of food items can vary geographically in widely distributed species such as D. aurita and P. frenatus. The above-mentioned studies showed that the diet of both species can be classified in three main food categories: invertebrates, small vertebrates and fruits. Astúa de Moraes et al. (2003) demonstrated that the laboratory diets chosen by these species reflect, in quantity and nutritional contents, the same food categories chosen in nature. Therefore, food items can vary geographically and spatially, the proportion of food categories being somewhat fixed, as result of nutritional needs of these species and interespecific differences in digestive and physiological systems.
Most studies on diet of opossums were done in preserved areas (Cáceres et al., 1999; Cáceres et al., 2002; Martins and Bonato, 2004; Carvalho et al., 2005; Allison et al., 2006), while disturbed sites are in general not investigated. These approaches could ignore how plastic is the diet of a species, and do not allow to determine if it would be capable of survive in areas disturbed by human activities. Here, we report the food habits of P. frenatus and D. aurita in a rural area of southeastern Brazil. Faecal samples were examined in order to analyse interspecific differences and intraspecific variation in food consumption concerning sex, age and seasonality. We also investigated the influence of humidity on the frequency of fruit choice in the diet of D. aurita and P. frenatus. The proportions of food categories obtained from other studies applying similar methods and species were also compared, in order to investigate the spatial differences in food use by both species.
MATERIAL AND METHODS
Study area
Samples were collected monthly from July 1992 to November 1995, in the Pamparrão valley (22°02'46" S, 41°41'21" W), Sumidouro municipality, Rio de Janeiro state, Brazil. The region has mainly garden crops and some pasture lands with few small Atlantic Forest fragments. Streams, irrigation channels, and swamp areas are also frequent in the study area. The weather is humid-mesotermic (Nimer, 1989), wet season occurs from November to March and a dry season from May to August (Fig. 1). Meteorological data for the study period were obtained from the National Metereological Institute (Instituto Nacional de Metereologia - INMET). The highest mean monthly temperature occurred in February 1996 (28.8ºC) and the lowest occurred in July 1991 (20.2ºC), the highest precipitation occurred in January 1992 (562.2 mm) and the lowest occurred in July 1993 (0.6 mm).

Fig. 1. Ombrothermic climate diagram of the study period, from July 1992 to November 1995, used to classify months according to humidity. Dashed line shows precipitation, while continuous lines show mean monthly temperature. When the average rainfall line is above temperature line there is surplus water in the system and the month is a humid one. When rainfall is below temperature there is water deficit and the month is considered dry. Rainfall above 100 mm in a month means that such month is superhumid.
Small mammals trapping sessions were done at seven linear transects along the valley. All transects were placed along small streams and had different vegetational cover along them (see details in D'Andrea et al., 1999).
In order to avoid contamination of the samples with exogenous material, faeces of both species were collected carefully from the bottom of the live traps where the animals were captured.
Faeces were stored in ethanol 70%. The samples were filtered in a 1 mm mesh screen sieve under running tap water. The undigested material retained on the sieve was air-dried, for analysis under a stereoscopic microscope, and classified in three food categories: fruits (seeds), vertebrates and invertebrates (Santori et al., 1997; Southwood and Hendersen, 2000; Cáceres and Monteiro-Filho, 2001). Taxonomic classification of invertebrates was determined by specialists and the seeds were separated as morphospecies. Bones, feathers and scales were used as an evidence of vertebrates consumption although it was not possible to identify them. Hairs were identified by observation of scale patterns on a layer of 10% of colourless jelly set on a plate (Day, 1966). Additionally, a reference collection of mammal hairs found in the study area was established to compare with those found in faecal contents (Santori et al., 1997).
Data analysis
The relative monthly frequency of vertebrates, invertebrates and fruits in the field diet was calculated by dividing the number of faeces samples where a given food category occurred by the total number of samples collected in that month (Southwood, 2002). Thus, the relative frequency for each food item was obtained for each month (monthly relative frequency). For intraspecific comparisons, samples were divided into categories (sex, age and climatic seasons), and relative monthly frequencies were calculated for each sample (means and standard errors [SE] are presented for the variables). As this study is part of a capture-markrecapture program, only samples collected in the first day of each trapping session were analysed, which were used to calculate the relative frequencies. This procedure avoids the overestimation of a food item gradually eliminated in several faecal samples of an individual, as the time needed for the digestion of a given item and its complete defecation can be longer than one day (Santori et al., 1995b).
Individuals were classified in three age classes by sequence of teeth eruption following Gentile et al. (1995) and Macedo et al. (2006): juveniles, which had third pre-molar deciduous; sub-adults, with definitive pre-molars and incomplete dentition (M3); and adults (M4), with complete dentition.
Water availability was estimated following the Bagnouls and Gaussen (1957) method. This method indicates when there is an excess or deficit of water in the system in a month. Thus; when the total monthly rainfall line is above the double mean monthly temperature line (Fig. 1), the month can be considered as humid; when the total monthly rainfall line lies below the double mean temperature line, the month is classified as dry; super humid months are those with rainfall above 100 mm (Bagnouls and Gaussen, 1957; Santori et al., 1995a).
An empirical formula of Setzer (1954) was also used to obtain a humidity index:
Π= P/ 1.07t
where P is the total monthly rainfall (millimeters), t= the mean monthly temperature in Celsius degrees, and p is the monthly index of humidity.
The Mann-Whitney (U) test was used to compare the relative monthly frequencies of each food category between species and sex in each species. Kruskal-Wallis (H) test was used to compare relative frequencies between age and between months classified according to humidity and a Cochran ttest (Q) was used as a post-hoc test (Zar, 1996). Spearman rank correlation was used between the relative frequencies of fruits in each month and months classified by Setzer humidity indices (Setzer, 1954) for both species, in order to analyse the influence of environmental humidity on fruit consumption; Spearman rank correlations coefficients (rs) and significance level (p) were presented. The diversity of fruits and invertebrates present in the faeces of both species was calculated with the Shannon-Weiner index (H') and compared using Hutcheson´s t-test (Zar, 1996).
The proportions of each food category for each species were compared with field data collected by several authors in studies on other Atlantic Forest sites (Leite et al., 1994; Santori et al., 1995a; Santori et al., 1997; Carvalho et al., 1999; Cáceres and Monteiro-Filho, 2001; Carvalho, 2003). We chose these studies because the authors used similar faecal sample methodologies. They were compared with our study and between them, a Z value was calculated for each comparison (two studies data) (Rodrigues, 1993), as follows:
Z= p1-p2 / √p1q1/n1 + p2q2/n2
where p1 and p2 are the relative frequencies of two samples, n1 and n2 are the number of cases, q1= 1-p1 and q2= 1-p2. The level of significance of Z value is obtained on a normal curve table of proportions (Rodrigues, 1993).
RESULTS
Fifty-four individuals of D. aurita were adults, 37 were sub-adults, and 43 were juveniles. Fifty-four individuals of P. frenatus were adults, 13 were sub-adults, and 4 were juveniles. D. aurita had 21 males (51 samples) and 31 female individuals (83 samples). P. frenatus had 28 males (46 samples) and 15 females (28 samples).
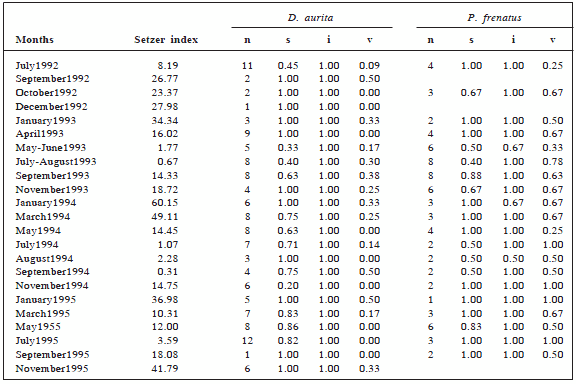
There were collected 134 faecal samples of D. aurita and 74 of P. frenatus. Invertebrates, followed by fruits, and then by vertebrates were the most frequent food items for both species (Table 1).
Table 1. Temporal variation in relative frequencies of the three alimentary categories found in faecal contents of Didelphis aurita and Philander frenatus in each month collected. n = number of samples, s=seeds, i=invertebrates and v=vertebrates.
Intraespecific diet variation
There were no differences between sexes in both species. For D. aurita, consumption of the three food categories was not age-related. For P. frenatus, the relative frequency of vertebrates in faeces of juvenile individuals (n=4, mean=0.14, SE=0.04) was smaller than in those of adults (n=17, mean=0.47, SE=0.04) and subadults (n=13, mean=0.65, SE=0.03) (H=7.37, p=0.03; sum of ranks: adults= 285.0; subadults= 282.5 and young= 27.5, between adults and juveniles Q=2.84, p<0.05 and between sub-adults and juveniles Q=2.4, p<0.05). No differences in the relative frequency of the remaining food categories among age classes were found for P. frenatus.
Seasonal diet variation
All samples of D. aurita contained invertebrates, mainly insects; hence we found no variation in relative frequency of this food category. The relative frequency of seeds in the diet of D. aurita was significantly higher in the super-humid months (n=8, mean= 0.96, SE=0.03) than in humid (n=6, mean= 0.69, SE=0.11) and dry months (n=7, mean=0.75, SE=0.09) (H=9.04, p=0.01, KW sum of ranks: super-humid= 127.5, humid= 52.0 and dry= 51.5; between super-humid and humid Q=2.76, p<0.05, and between super-humid and dry Q=2.42, p<0.05). No differences in the relative frequency of the three food categories eaten by considering the months classified according to humidity were found. However, relative frequency of seeds for this species presented a low p value (H=5.76, p=0.06). There were significant positive correlations between the Setzer index of humidity and the relative frequency of fruits in faeces of both species (D. aurita: rs=0.616, p=0.002, n= 23; P. frenatus: rs =0.630, p=0.003, n= 20).
Interspecific diet variation
Samples of P. frenatus showed a significantly higher relative frequency of vertebrates (mean=0.64, SE=0.05) than those of D. aurita (mean=0.22, SE=0.04) (U=53.5, p=0.01). There were no differences in the relative frequency of invertebrates (P. frenatus- mean=0.94, SE=0.03; D. aurita - mean=1.0, SE=0)(U=218.0, p=0.26) and fruits (P. frenatus - mean=0.82, SE=0.05; D. aurita- mean=0.8, SE=0.05) (U=157.0, p=0.39) between species.
Twelve orders of invertebrates were present in the diets of D. aurita and P. frenatus, but they presented a different composition in invertebrate orders consumed. Crustacea and Mollusca were included amongst invertebrates consumed by P. frenatus, whereas D. aurita consumed mainly arthropods found in the litter (Table 2). However, there was no significant difference in the diversity of invertebrates consumed by these species (for D. aurita: H'=1.88 and for P. frenatus: H'=1.81; t = 0.657, p =0.2). Fruit diversity consumed by D. aurita (H'=3.12) was significantly higher than those of P. frenatus (H'= 2.38; t = 4.176, p < 0.001).
Table 2. Relative frequency of invertebrates identified in faecal contents of Didelphis aurita and Philander frenatus (n.i. refers to not identified items).

Comparison between localities
For Didelphis aurita, the smallest relative frequency of invertebrates amongst all studies examined occurred at one of the lowland forests (Leite et al., 1994) (Z=2.29, p< 0.01 for all comparisons). The relative frequency of seeds in the restinga shrubland (Santori et al., 1995a) was significantly smaller than the others (lowland forest [Leite et al., 1994]: Z= 2.47, p<0.01; secondary ombrophilous forest [Cáceres and Monteiro-Filho, 2001]: Z= 3.2, p<0.01; lowland forest [Carvalho, 2003]: 2.47, p<0.01; This study: Z=4.8, p<0.01) (Table 3). Frequency of vertebrates was significantly higher in the secondary ombrophilous forest (Cáceres and Monteiro-Filho, 2001) than in the others (restinga shrubland [Santori et al., 1995a]: Z=4.68, p<0.01; lowland forest [Leite et al., 1994]: Z= 2.47, p<0.01; This study: Z=8.08, p<0.01) (Table 3).
Table 3. Relative frequency of vertebrates (v), invertebrates (i) and fruits (f) found in the same studies on D. aurita and P. frenatus in different localities of Atlantic Forest biome. n = number of samples.

For Philander frenatus, no differences were found in the frequency of invertebrates amongst the studies analysed. The frequency of fruits was significantly smaller in the restinga shrubland (Santori et al., 1997) comparing with other studies (Rural area [Carvalho et al., 1999]: Z=1.72, p<0.01, This study: Z=7.55, p<0.01). The vertebrate frequency was significantly higher in our study area comparing with restinga shrubland (Santori et al., 1997) (Z=6.64, p<0.01) but did not present significative differences from the frequencies encountered for the other rural area (Carvalho et al., 1999).
DISCUSSION
All didelphids studied so far are omnivorous to some extent, as all species use animal matter (invertebrates or small vertebrates), seed and fruits. However, they show a gradient of omnivory (Astúa de Moraes et al., 2003). In a food preference study carried out in our laboratory, P. frenatus consumed higher proportion of proteins than D. aurita (Astúa de Moraes et al., 2003). According to Santori et al. (1995b), the digestive morphology and the high assimilation rate of a highly proteic diet by P. frenatus is related to a more carnivorous habit in this species than in D. aurita, which assimilates both diets composed only by fruits or only by meat as well. P. frenatus is able to survive under a diet with high protein levels, composed only of meat and eggs, for at least seven days, as long as there is water available (Fonseca and Cerqueira, 1991). Interspecific comparisons of vertebrate relative frequency in the faeces between these two marsupials also support the assertion that P. frenatus consumes a larger proportion of animal matter and uses less fruit items than D. aurita, which seems to have a larger diet width than P. frenatus (Perissé et al., 1988)
A recent study on the interaction between D. aurita and other species (Moura, 2004) showed that this opossum does not compete for resources with P. frenatus causing no influence on the population levels of the latter species. The differences in feeding habits reported here could help to explain this.
Although we did not perform any evaluation of temporal resource availability it is reasonable to suppose that different factors are influencing fruit consumption at different localities. In this study, the highest fruits frequency in the diet of D. aurita and P. frenatus in more humid months may be a consequence of the increase in fruit availability during the rainy season, as in this period occurs the maturation of fleshy fruits in tropical forests (Costa et al., 1997). It also corroborates other findings on didelphid feeding ecology in the Atlantic Forest. These also found a higher frequency of seeds in faeces collected in wet months (Cáceres et al., 1999; Cáceres and Monteiro-Filho, 2001). At a Restinga forest in Rio de Janeiro State, the faecal samples of D. aurita and P. frenatus had greater relative frequency of fruits in dry months (Santori et al., 1997). At this study site, the dry period is more severe than Sumidouro, because in the Restinga sandy soil the water flows through faster, which leads to low water retention and availability. It is probable that D. aurita and P. frenatus increase the amount of fruit intake to increase water acquisition from it (Santori et al., 1995b; 1997). This fact is supported by the results of Fonseca and Cerqueira (1991) on water balance of P. opossum (=P. frenatus) from Restinga of Maricá, RJ (Brazil). At Sumidouro, where there is no such water limitation during dry months, and natural sources of free water are abundant, fruit consumption does not seem to reflect water need per se. In our study area, fruit consumption is probably reflecting an optimal foraging behaviour, eating most abundant items when they are available (Stephens and Krebs, 1986). Similar results were found for this species (Cáceres et al., 1999), and for other marsupials with invertebrate (Chen et al., 2004; Allison et al., 2006) and foliage (Cork, 1996) consumption.
The relative frequencies of the three food categories considered here (invertebrates, vertebrates and seeds) show interesting differences between study sites. Data comparison on diet analysis among several studies is difficult because of their different sample size and methodologies (such as faecal analysis, gut contents, or laboratory food preference tests). However, after comparing our data with other studies on the same species, we can suggest that, geographically, reported changes in their diet can reflect minor adjustments in response to local availability of food items. They can be important to reduce interspecific competition and can be an additional evidence to support their opportunistic behaviour. The high frequency of invertebrates and the significant lower fruit consumption in the Restinga vegetation for both species suggest that resource availability is a very important factor in food item choice. Invertebrates seem to be the major food item for these species, and not scarce, as they have been found with high frequencies in all study sites. Differences found in vertebrate consumption are less clear and the lack of this food category in some studies make generalisations difficult.
Differences in the consumption of vertebrates among individuals of P. frenatus from different dental age classes may be related to their small body size, fewer tooth and lower cusps height of the juveniles, compared to sub-adult and adult individuals of this species (Charles- Dominique et al., 1981; Streilein, 1982; Santori et al., 1997). In didelphids, pre-molars and molars form the main masticatory surface. It is therefore probable that, in spite of body size, juvenile P. frenatus are not as able to catch vertebrate prey as the sub-adults and adults due to the smaller chewing area. Similar results were found by Cordero and Nicolas (1987) for D. marsupialis in Venezuela and by Santori et al. (1997) for P. frenatus in a Restinga Forest in Brazil. Small vertebrates could have been preyed alive or eaten as carrion, a distinction hard to make when dealing with faecal content. Carrion consumption by Didelphis and Philander was already documented by some authors (Charles-Dominique, 1981; Streilen, 1982; Cordero and Nicolas, 1987). However, in the present study, we did not find dipteran pupae residues from fleshflies, which would indicate carrion feeding as found in the Restinga study (Santori et al., 1997). Thus, as all individuals, in spite of their age classes, have the same possibility to eat carrion, it seems that vertebrates were preyed alive, with body and tooth size of juvenile being the main factor making difficult vertebrate predation (Santori et al., 1997). Availability of carrion in an agricultural area can be lowered by competition with domestic dogs (Butler & DuToit, 2002) and the absence of carrion in the diet of these opossums may be due to the large dog population common in Brazilian rural areas.
In the rural environment studied, major prey category used as food are the same found in natural areas, which does not represent a nutritional constraint for D. aurita and P. frenatus, because they supply their needs with a variable quantity of invertebrates, fruits and meat. This shows how generalist are these species (mainly D. aurita), a quality that contributes to the high survival capacity in diverse habitats. Thus, our study shows a great ability of D. aurita and P. frenatus in adapting to habitat modifications. Even when choosing different prey, they tend to balance their overall intake to satisfy their nutritional needs.
ACKNOWLEDGEMENTS
We thank all the staff of the Laboratório de Biologia e Controle da Esquistossomose at FIOCRUZ, especially C. Horta, Dr. P. S. D´Andrea and L. Maroja, for sample collections; Dr. J. Becker, from the Museu Nacional- UFRJ, for the identification of arthropods; N. Pereira de Barros and A. M. Marcondes from the Laboratório de Vertebrados - UFRJ, for their technical and clerical support. We thank also German Forero, Diogo Loretto, Dr. Diego Astúa de Moraes, Dr. Carlos Eduardo Grelle, Dr. David Flores and two anonymous referees for the valuable corrections on the manuscript. Financial support was provided by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo a Pesquisa do Rio de Janeiro (FAPERJ), Fundação Universitária José Bonifácio (FUJB), PRONEX, PROBIO/MMA/GEF, Programa PROCIÊNCIA/UERJ.
LITERATURE CITED
1. ADLER GH. 1998. Impacts of resource abundance on populations of a tropical forest rodent. Ecology 79:242-254. [ Links ]
2. ALLISON LM, LA GIBSON, and JG ABERTON. 2006. Dietary strategy of the swamp antechinus (Antechinus minimus maritimus) (Marsupialia: Dasyuridae) in coastal and inland heathland habitats. Wildlife Research 33:67-76. [ Links ]
3. ALVES-COSTA CP, GAB DA FONSECA and C CHRISTOFARO. 2004. Variation in the diet of the brown-nosed coati (Nasua nasua) in southeastern Brazil. Journal of Mammalogy 85:478-482. [ Links ]
4. ASTÚA DE MORAES D, RT SANTORI, R FINOTTI, and R CERQUEIRA. 2003. Nutritional and fibre contents of laboratory-established diets of neotropical opossums (Didelphidae). Pp. 221-237, in: Predators with pouches: The Biology of Carnivorous Marsupials (M Jones, C Dickman and M Archer, eds.). CSIRO Publishing, Australia. [ Links ]
5. BAGNOULS F and H GAUSSEN. 1957. Les climats biologique et leur classification. Annales de Géographie 355:193-220. [ Links ]
6. BUTLER JRA and JT DUTOIT. 2002. Diet of free-ranging domestic dogs (Canis familiaris) in rural Zimbabwe: implications for wild scavengers on the periphery of wildlife reserves. Animal Conservation 5:29-37. [ Links ]
7. CÁCERES NC, VAO DITTRICH, and ELA MONTEIRO-FILHO. 1999. Fruit consumption, distance of seed dispersal and germination of solanaceous plants ingested by common opossum (Didelphis aurita) in Southern Brazil. Revue d'Ecologie (Terre et Vie) 54:1-10. [ Links ]
8. CÁCERES NC and ELA MONTEIRO-FILHO. 2001. Food habits, home range and activity of Didelphis aurita (Mammalia, Marsupialia) in a Forest fragment of Southern Brazil. Studies on Neotropical Fauna and Environment 36:85-2. [ Links ]
9. CÁCERES NC. 2002. Food Habits and seed dispersal by white-eared opossum, Didelphis albiventris, in Southern Brazil. Studies on Neotropical Fauna and Environment 37:97-104. [ Links ]
10. CÁCERES NC, IR GHIZON-JR, and ME GRAIPEL. 2002. Diet of two marsupials, Lutreolina crassicaudatus and Micoureus demerarae, in a coastal Atlantic Forest island of Brazil. Mammalia 66:331-340. [ Links ]
11. CÁCERES NC. 2004. Diet of three didelphid marsupials (Mammalia, Didelphimorphia) in southern Brazil. Mammalian Biology 69:1-4. [ Links ]
12. CARVALHO FMV, PS PINHEIRO, FAS FERNANDEZ, and JL NESSIMIAN. 1999. Diet of small mammals in Atlantic forest fragments in southeastern Brazil. Revista Brasileira de Zoologia de Juiz de Fora 1:91- 101. [ Links ]
13. CARVALHO FMV. 2003. Ecologia alimentar de três espécies simpátricas de marsupiais em fragmentos de Mata Atlântica no Estado do Rio de Janeiro, Dissertação de Mestrado - Departamento de Ecologia - Universidade Federal do Rio de Janeiro, Rio de Janeiro, 53 pp. [ Links ]
14. CARVALHO FMV, FAS FERNANDEZ, and JL NESSIMIAN. 2005. Food habits of simpatric opossums coexisting in small Atlantic Forest fragments in Brazil. Mammalian Biology 70:366-375. [ Links ]
15. CHARLES-DOMINQUE P, M ATRAMENTOWICZ, M CHARLES-DOMINQUE, H GERARD, CM HLADIK, and MF PRÉVOST. 1981. Les mammifères frugivores arboricoles nocturnes d'une forêt guyanaise: inter-relations plantes-animaux. Revué d'Ecologie (Terre et Vie) 35:341-435. [ Links ]
16. CHEN XL, CR DICKMAN, and MB THOMPSON. 2004. Selective consumption by predators of different body regions of prey: is rate of energy intake important? Journal of Zoology 264:189-196. [ Links ]
17. CORDERO GA and AN NICOLAS. 1987. Feeding habits of the opossum (Didelphis marsupialis) in Northern Venezuela. Pp. 125-131, in: Studies in Neotropical mammalogy - Essays in honor of Philip Hershkovitz (BD Patterson and RM Timm, eds.). Fieldiana, Zoology 34. [ Links ]
18. CORK SJ. 1996. Optimal digestive strategies for arboreal herbivorous mammals in contrasting forest types: Why Koalas and Colobines are different. Austral Ecology 21:10-20. [ Links ]
19. COSTA MLMN, ACS ANDRADE, and TS PEREIRA. 1997. Arboreal species phenology of montane forest in the Ecological Reserve of Macaé de Cima. Pp. 169-186, in: Serra de Macaé de Cima: Diversidade florística e Conservação em Mata Atlântica. Instituto de Pesquisas Jardim Botânico do Rio de Janeiro, Rio de Janeiro. 345 pp. [ Links ]
20. CUNHA AA and MV VIEIRA. 2002. Support diameter, incline, and vertical movements of four didelphid marsupials in the Atlantic Forest of Brazil. Journal of Zoology 258:419-426. [ Links ]
21. D´ANDREA PS, R GENTILE, R CERQUEIRA, CEV GRELLE, C HORTA, and L REY. 1999. Ecology of small mammals in a Brazilian rural area. Revista Brasileira de Zoologia 16:611-620. [ Links ]
22. DAY MG. 1966. Identification of hair and feather remains in the gut and feaces of stoats and weasels. Journal of Zoology 148:201-217. [ Links ]
23. FONSECA CSD and R CERQUEIRA. 1991. Water and salt balance in a South American marsupial, the Gray Four-eyed Opossum (Philander opossum). Mammalia 55:421-432. [ Links ]
24. FONSECA GAB and MC KIERULFF. 1989. Biology and natural history of Brazilian Atlantic Forest small mammals. Bulletin of Florida State Museum, Biological Sciences 34:99-152. [ Links ]
25. FREITAS SR, D ASTÚA DE MORAES, RT SANTORI, and R CERQUEIRA. 1997. Habitat preference and food use by Metachirus nudicaudatus and Didelphis aurita (Didelphimorphia, Didelphidae) in a restinga forest at Rio de Janeiro. Revista Brasileira de Biologia 57:93-98. [ Links ]
26. GENTILE R, PS D'ANDREA, and R CERQUEIRA. 1995. Age structure of two marsupials in a Brazilian restinga. Journal of Tropical Ecology 11:671- 677. [ Links ]
27. GENTILE R, PS D'ANDREA, R CERQUEIRA, and LS MAROJA. 2000. Population dynamics and reproduction of marsupials and rodents in a Brazilian rural area: a five-year study. Studies on Neotropical Fauna and Environment 35:1-9. [ Links ]
28. KIRSCH JA, FJ LAPOINTE, and MS SPRINGER. 1997. DNA-hybridization studies of marsupials and their implications for metatherian classification. Australian Journal of Zoology 45:211-280. [ Links ]
29. LEITE YL, JR STALLINGS, and L PIRES-COSTA. 1994. Partição de recursos entre duas espécies simpátricas de marsupiais na Reserva Biológica de Poço das Antas, Rio de Janeiro. Revista Brasileira de Biologia 54:525-536. [ Links ]
30. LEVINGS SC and DM WINDSOR. 1985. Litter arthropod populations in a tropical deciduous forest relationships between years and arthropod groups. Journal of Animal Ecology 54:61-69. [ Links ]
31. MAHER DO, IPF OWENS, and CN JOHNSON. 2001. The ecological basis of life history variation in marsupials. Ecology 62:3531-3540. [ Links ]
32. MARTINS EG and V BONATO. 2004. On the diet of Gracilinamus microtarsus (Marsupialia, Didelphidae) in an Atlantic Forest in southearstern Brazil. Mammalian Biology 69:58-60. [ Links ]
33. MOURA MC. 2004. O papel de Didelphis aurita (Didelphimorphia, Didelphidae) na estruturação de duas comunidades de pequenos mamíferos da Mata Atlãntica. MSc dissertation, Departamento de Ecologia, Universidade Federal do Rio de Janeiro, Rio de Janeiro. [ Links ]
34. NIMER E. 1989. Climatologia do Brasil. IBGE, Rio de Janeiro. [ Links ]
35. PERISSÉ M, R CERQUEIRA, and CR SORENSEN. 1988. A alimentação na separação de nicho entre Philander opossum e Didelphis aurita (POLYPROTODONTIA, DIDELPHIDAE). Anais do Seminário Regional de Ecologia, São Carlos-SP, 6:283-294. [ Links ]
36. PERISSÉ M, CRSD FONSECA, and R CERQUEIRA. 1989. Diet determination for small laboratory-housed wild mammals. Canadian Journal of Zoology 67:775- 778. [ Links ]
37. REDFORD KH. 1984. Mammalian predation on termites: test with the burrowing mouse (Oxymycterus roberti) and its prey. Oecologia 65:145-157. [ Links ]
38. RODRIGUES PC. 1993. Bioestatística. Editora Universitária -Universidade Federal Fluminense. Rio de Janeiro. [ Links ]
39. SANTORI RT, D ASTÚA DE MORAIS and R CERQUEIRA. 1995a. Diet composition of Metachirus nudicaudatus and Didelphis aurita (Marsupialia, Didelphoidea) in Southeasten Brazil. Mammalia 59:511-516. [ Links ]
40. SANTORI RT, R CERQUEIRA, and CC KLESKE. 1995b. Digestive anatomy and efficiency of Philander opossum and Didelphis aurita (Didelphimorphia, Didelphidae) in relation to the feeding habits. Revista Brasileira Biologia 55:323-329. [ Links ]
41. SANTORI RT, D ASTÚA DE MORAIS, CEV GRELLE, and R CERQUEIRA. 1997. Natural diet at a Restinga Forest and Laboratory Food Preferences of the Opossum Philander frenatus in Brazil. Studies on Neotropical Fauna and Environment 32:12-16. [ Links ]
42. SETZER J. 1954. Isolinhas de umidade do clima no Estado do Rio de Janeiro e no Distrito Federal. Revista Brasileira de Geografia, 16(3):315-327. [ Links ]
43. SILVA SI. 2005. Trophic position of small mammals in Chile: a review. Revista Chilena de Historia Natural 78:589-599. [ Links ]
44. SMYTHE N. 1986. Competition and resource partitioning in the guild of neotropical terrestrial frugivourous mammals. Annual Review of Ecology and Systematics 17:169-188. [ Links ]
45. SOUTHWOOD TRE and PA HENDERSEN. 2000. Ecological Methods. Blackwell Science, Oxford. [ Links ]
46. STEPHENS DW and JR KREBS. 1986. Foraging Theory. Princeton University Press, Princeton. [ Links ]
47. STREILEN KE. 1982. Behavior, ecology, and distribution of South American marsupials. Pp. 251-256, in: Mammalian biology in South America (MM Mares and HH Genoways, eds.). Special Publications Pymatuning Laboratory of Ecology 6. [ Links ]
48. TYNDALE-BISCOE CH and RB MCKENZIE. 1976. Reproduction in Didelphis marsupialis and Didelphis albiventris in Colombia. Journal of Mammalogy 57:249-265. [ Links ]
49. VANSCHAIK RC, JW TERBORGH, and SJ WRIGHT. 1993. The phenology of tropical forests: adaptative significance and consequences for primary consumers. Annual Review of Ecology and Systematics 24:353-377. [ Links ]
50. VIEIRA EM and P IZAR. 1999. Interactions between aroids and arboreal mammals in the Brazilian Atlantic rainforest. Plant Ecology 145:75-82. [ Links ]
51. VIRGOS E, M LORENTE, and Y CORTES. 1999. Geographical variation in genet (Genetta genetta L.) diet: a literature review. Mammalian Review 29:117- 126. [ Links ]
52. ZAR JH. 1996. Biostatistical analysis. Prentice Hall, Englewood Cliffs, New Jersey. [ Links ]
53. WOLDA H. 1988. Insect seasonality. Annual Review of Ecology and Systematics 19:1-18. [ Links ]
Recibido 21 junio 2006.
Aceptado 27 diciembre 2007.
Editor asociado: D Flores














