Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Mastozoología neotropical
versión impresa ISSN 0327-9383versión On-line ISSN 1666-0536
Mastozool. neotrop. v.16 n.1 Mendoza ene./jun. 2009
ARTÍCULOS Y NOTAS
Evaluating microhabitat selection by Calomys musculinus (Rodentia: Cricetidae) in western Aargentina using luminous powders
Valeria Corbalán* and Guillermo Debandi
Instituto Argentino de Investigaciones de las Zonas Áridas (IADIZA, CCT-CONICET Mendoza), Av. Ruiz Leal s/n, Parque Gral. San Martín, 5500 Mendoza, Argentina
*[Correspondencia: <corbalan@mendoza-conicet.gov.ar>
ABSTRACT: The aim of this study was to use fluorescent powder tracking techniques to study the use of space and the patterns of microhabitat selection in Calomys musculinus at the Ñacuñán Biosphere Reserve. The examination of over 3000 microhabitat records from the trails of eight individuals indicates that, in general, the species does not discriminate among major habitat types; however, we found significant differences in the specific categories of substrate used by the species within habitat. In addition, we also note that C. musculinus uses vertical habitats up to 50 cm.
RESUMEN: Evaluación de la selección de microhábitat por Calomys musculinus (Rodentia: Cricetidae) en el oeste de Argentina usando polvos luminosos. El objetivo de este trabajo fue evaluar el uso de microhabitat de Calomys musculinus en la Reserva de Biosfera Ñacuñán (Mendoza) utilizando la técnica de polvos fluorescentes. La especie es bastante generalista en el uso de los tipos de ambientes presentes, pero parece preferir algunos substratos específicos a nivel de microhabitat. Además, algunos animales utilizaron ramas y arbustos hasta medio metro de alto.
Key words. Monte desert; Ñacuñán Biosphere Reserve; Sigmodontinae.
Palabras clave. Desierto del Monte; Reserva de Biosfera Ñacuñán; Sigmodontinae.
Habitat selection is a process in which an animal chooses from alternative habitats available to it (Johnson, 1980; Litvaitis et al., 1994). It is a hierarchical process that can occur at a variety of spatial scales (Hutto 1985), ranging from the macrohabitat to the microhabitat level (Kotler and Brown, 1988).
Calomys musculinus is an omnivorous small rodent with tendency towards granivory (Campos et. al., 2001) found in central and northwestern Argentina (Redford and Eisenberg 1992). It is the main reservoir of the Junín virus, the etiologic agent of the Argentine Hemorrhagic Fever (AHF; Morse, 1993; Levins et al., 1994) and has been mostly studied in agricultural areas. Busch et al. (2000) found that this species showed macrohabitat selection, being significantly more abundant on the borders of cultivated areas where plant cover was higher than in agricultural fields. Nevertheless, associations of this species with measured habitat variables changed depending on the habitat and the scale considered. Ellis et al. (1997) demonstrated that increased cover and richness of grass species, as well as the vertical profile of vegetation, were significant predictors for the presence of this species in agricultural ecosystems.
However, in natural areas information on this species is scarce; Gonnet and Ojeda (1998) reported that on the foothills of the Andean Precordillera in Mendoza province, C. musculinus uses sites with high cover of shrubs, cacti, and particularly, grass. Contreras and Rossi (1980) also highlight the importance of dense vegetation with tender leaves in the areas occupied by this species. Recent studies in the Monte desert (Corbalán and Ojeda, 2004) have demonstrated that C. musculinus selects habitats with high vegetation cover, either of shrubs (e.g., Larrea cuneifolia) or trees (e.g., Prosopis flexuosa), and avoids open habitats (e.g., sand dunes). All of these studies were conducted using live traps, and attempts to find associations between microhabitat variables and capture sites of individuals of this species were unsuccessful (Corbalán, 2006.).
In this study we applied the technique of luminous powders to follow the tracks of animals and estimate microhabitat selection. Developed by Duplantier et al. (1984), this technique allowed us to follow the exact movement of the individuals, avoiding possible biases in information due to the effect of baited traps on the behavior of the animals (Lemen and Freeman, 1985).
The study was conducted at the Ñacuñán Biosphere Reserve (12 800 ha), 200 km southeast of Mendoza city, Argentina (34º02'S, 67º58'W), in October 2001 and May 2002. The area belongs to the Monte biome. The climate at the Reserve is semiarid and seasonal, and the vegetation is xerophytic (Morello, 1958; Roig, 1971). Mean annual precipitation is 345.29 mm (period 1972-2001) and rainfall events occur mainly during the spring and summer months (Estrella et al., 2001).
There are three distinctive habitats in the Reserve. The mesquite forest is the most heterogeneous habitat (Corbalán and Ojeda, 2004) and is characterized by three strata (trees, shrubs and grasses), and high cover of Prosopis flexuosa trees. The creosotebush community is next in heterogeneity and is dominated by shrubs of Larrea cuneifolia and high herbaceous cover. Sand dunes are the least heterogeneous habitat, characterized by low shrub cover and high percentage of bare soil (Roig, 1971; Corbalán and Ojeda, 2004). Calomys musculinus use all of these habitats, but their abundance is higher at the creosotebush community (Corbalán and Ojeda, 2004). In the study area, it coexists with other four small-mammals: Eligmodontia typus, Graomys griseoflavus, Akodon molinae (Rodentia: Cricetidae), and Thylamys pallidior (Didelphimorphia: Didelphidae).
For purposes of this study, a total of eight individuals of C. musculinus were captured using Sherman live traps set in transects in all three habitats during three consecutive nights in two periods (October 2001 and May 2002). Traps were baited with rolled oats in the evening, and checked three or four hours later (22:00-23:00). Captured individuals of C. musculinus were weighed, sexed, carefully hand-dusted with luminous powder (BioQuip, Gardena, CA), and released at the capture site. Only the animals that had been captured at least 50 m apart were dusted. No more than three individuals were dusted per night because of the long time spent in following the tracks the next day. Color used was red (#1162R) or blue (#1162B), according to the distance among dusted animals and in order to prevent confusing the tracks from different individuals. Captured individuals from other species were released and traps were closed.
In the morning of the next day, the trails left by the individuals were marked with flags until the powder was no longer visible. As powders were more visible in the sunlight than under UV light, we were able to work during the daytime.
Characteristics of microhabitat use by the animals were recorded every 15 cm along the path, recording bare soil, litter, trees, shrubs, subshrubs, or herbs intercepting a vertical stick 1.5 m long. In order to evaluate microhabitat availability, a 50-m random transect was established near each capture site, and microhabitat characteristics were recorded every 15 cm along that transect, as previously detailed for microhabitat use.
We recognized different categories of use and availability: 1) «uncovered» (bare soil or litter on the ground), 2) «herbs» (grasses or forbs), 3) «subshrubs», 4) «shrubs», and 5) «complex» (when a combination of categories 2 to 4 intercepts the stick). Categories 2 to 5 can contain bare soil or litter on the ground.
In order to estimate microhabitat selection, frequency of the categories of use was contrasted with frequency of categories available in the environment. Since lengths of trails were different for each animal assessed, the frequency of each category was expressed as the proportion of total records for each individual. Thus, availability also was calculated as proportions of each category in the total records from random transects. Data were analyzed following a GLM procedure with Binomial as distribution, and logits as link function (i.e., logistic regression), using GenStat Discovery Edition 2.0. When residual errors in the models showed overdispersion (i.e., when the residual deviance was higher than the degree of freedom of the residual), models were rescaled to correct for biases in the statistical test of hypotheses (Crawley, 1993), and F tests were used instead of χ2 as measure of fit of the models.
We arranged the data using two factor variables, «treatment» with two levels (individual trails and random transects), and «category» with five levels (the five categories described above). We used the «treatment-category » interaction as indicative of differential use of microhabitat categories. When this interaction was significant, i.e., when some category was selected or avoided, we performed t tests using the parameter estimates and its standard error to determine its probabilities. In the interaction, each category that was significantly different between individual trail and random transect was considered as «selected» or «avoided» if the sign of the parameter estimate was positive or negative, respectively, in the logistic model. We used the 5% probability to reject the null hypothesis when contrasting categories, without adjusting for multiple comparisons. We followed this convention due to the general lack of agreement about the methodology for such comparisons (Cabin and Mitchell, 2000; Moran, 2003; García, 2004). Nevertheless, all except one of the comparisons were well under the p=0.001 level, whereas the level of rejection using Bonferroni correction is near p=0.005.
We analyzed data at two different levels. First, to see whether microhabitat selection is a general pattern, we considered all individuals as replicates and compared them with all random transects (which were considered replicates of available microhabitats). Second, we evaluated microhabitat selection on a finer scale, where the categories used by each individual were contrasted with the nearest random transect.
Data from eight individuals of C. musculinus yielded 3341 microhabitat records. Table 1 shows weight, sex, habitat of capture, and traveled distance for each individual.
Table 1. Data on individuals dusted with luminous powder in the Biosphere Reserve of Ñacuñán.
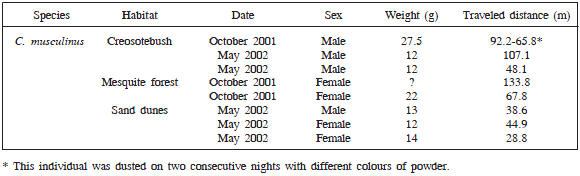
Luminous powder left by C. musculinus was found mainly on the ground. However, on several occasions the powder was found on grasses and subshrubs up to 0.5 m height. In the creosotebush community one individual used vertical space with 14% of data points recorded aboveground, whereas all the rest of individuals had between 0 and 1% of data points aboveground.
The global analysis (including all individuals and all random transects) did not detect microhabitat selection by C. musculinus as indicated by the non-significant Treatment- Category interaction (F4, 50 = 0.18, p = 0.947). The habitat variable was not included in the analysis because it did not improve the model, being non-significant per se or when taking into account the other categorical variables (treatment and category). Most of the comparisons of each individual with its nearest random transect were significant, indicating microhabitat selection at this level (Table 2). All three individuals from the creosotebush community selected complex microhabitats, but they differed regarding avoided categories (Table 2). In contrast, individuals from the mesquite forest selected open microhabitats (Table 2), whereas individuals from sand dunes selected shrub cover and avoided open microhabitats (bare soil and herbs; Table 2).
Table 2. Microhabitat selection by each individual of C. musculinus. Categories: 1: uncovered; 2: herbs; 3 subshrubs; 4: shrubs; 5: complex (see text). Columns 3 to 5 indicate results of the logistic model. All comparisons for selected or avoided categories were significant at p < 0.001 except the comparison marked with (*) that was significant with p = 0.038.

Previous studies on habitat use, as well as its typical cursorial locomotion, seem to indicate that C. musculinus is a species restricted to the ground space. However, the powder left by this species on grasses, and on some subshrubs (Lycium spp. and Acantholippia seriphioides), revealed that herbs and subshrubs are not always used as protection, and that individuals would climb probably to search for food. Trails left by C. musculinus on plants were in a horizontal direction, i.e., using the same stem, or stems of the same height, and never surpassing 0.5 m height.
In agreement with the study by Busch et al. (2000) in agricultural areas, the environmental variables selected by C. musculinus in the Monte desert changed depending on the habitat. The selectivity of this species was found only at the individual level (i.e. when tracks of each individual were compared with the nearest random transect), but some variables were shared by all individuals of each plant community. In the creosotebush community all individuals selected the more complex cover and avoided uncovered microhabitats. Conversely, in the mesquite forest, individuals selected uncovered microhabitats, whereas in sand dunes they selected shrub cover. Predation risk is one of the most important costs modulating rodent activities and patterns of habitat selection (Kotler, 1984, 1985; Brown, 1988; Longland and Price, 1991; Hughes and Ward, 1993; Vásquez, 1994; Brown et al., 1994; Kotler et al., 1994). Open areas, such as sand dunes, constitute an unsafe place for most small mammals, since predation risk is greater there than in bush habitats (Djawdan and Garland, 1988; Hughes and Ward, 1993). Probably, shrubs are safer microhabitats in absence of complex microhabitats, which explains a higher selectivity for this variable in sand dunes. An evidence of this is one record of a 14 meterlong continuous trail under shrubs left by one individual in this habitat. In more complex and heterogeneous habitats such as mesquite forests, predation risk is low and individuals can use uncovered microhabitats.
The close association of C. musculinus with grasses observed by different authors in the Monte desert (Contreras and Rosi, 1980; Gonnet and Ojeda, 1998) was not found in this study. The herb cover was selected only by one individual of C. musculinus in sand dunes, but this category was avoided or not selected by all other individuals. At the study site this species is more abundant in the creosotebush community, where shrubs and grasses are dominant (Corbalán and Ojeda, 2004). The evidence suggests that C. musculinus perceives different scales of habitat heterogeneity as found by Busch et al. (2000) in crop areas of the Pampean region of Argentina.
Corbalán (2006) did not find microhabitat selectivity for this species using trapping techniques in the same area. We think that differences in the results are due to the sampling methods, and that the use of luminous powders gives better insight to evaluate microhabitat selection. Although this method may affect the behavior of dusted animals by some kind of stress, we think that this effect might be similar to handling, toe clipping, and long sessions of trapping done in other studies. The advantage of this technique is that allowed us to follow the exact path left by the animals, and thus to quantify their movement patterns. Although the low number of individuals used in this paper did not allow us to get a deeper insight into microhabitat selection, we consider that this technique has a great potential for evaluating differences in microhabitat selection among sexes, ages and seasons of the year by many rodent species.
We thank Nelly Horak for assisting in the translation of this manuscript into English, and Ricardo Ojeda for providing us with the necessary materials. This study was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas of Argentina, CONICET (PIP 4684).
LITERATURE CITED
1. BROWN JS. 1988. Patch use as an indicator of habitat preference, predation risk, and competition. Behavioral Ecology & Sociobiology 22:37-47. [ Links ]
2. BROWN JS, BP KOTLER, and TJ VALONE. 1994. Foraging under predation: a comparison of energetic and predation cost in rodent communities of the Negev and Sonoran deserts. Australian Journal of Zoology 42:435-448. [ Links ]
3. BUSCH M, MH MIÑO, JR DADON, and K HODARA. 2000. Habitat selection by Calomys musculinus (Muridae, Sigmodontinae) in crop areas of the pampean region, Argentina. Ecología Austral 10:15-26. [ Links ]
4. CABIN RJ and RJ MITCHELL. 2000. To Bonferroni or not to Bonferroni: When and how are the questions. Bulletin of the Ecological Society of America 81:246-248. [ Links ]
5. CAMPOS CM, RA OJEDA, S MONGE, and M DACAR. 2001. Utilization of food resources by small and medium-sized mammals in the Monte desert biome, Argentina. Austral Ecology 26:142-149. [ Links ]
6. CONTRERAS JR and MI ROSI. 1980. Comportamiento territorial y fidelidad al hábitat en una población de roedores del centro de la provincia de Mendoza. Ecología Argentina 5:17-29. [ Links ]
7. CORBALÁN V and R OJEDA. 2004. Spatial and temporal organisation of small mammal communities in the Monte desert, Argentina. Mammalia 68:5-14. [ Links ]
8. CORBALÁN V. 2006. Microhabitat selection by murid rodents in the Monte desert of Argentina. Journal of Arid Environments 65:102-110. [ Links ]
9. CRAWLEY MJ. 1993. GLIM for Ecologists. Blackwell Scientific Publications, Oxford. [ Links ]
10. DJAWDAN M and T GARLAND. 1988. Maximal running speeds of bipedal and quadrupedal rodents. Journal of Mammalogy 69:765-772. [ Links ]
11. DUPLANTIER JM, J CASSAING, P ORSINI, and H CROSET. 1984. Utilisation de poudres fluorescentes pour l'analyse des deplacements des petits rongeurs dans la nature. Mammalia 48:293-298. [ Links ]
12. ELLIS BA, JN MILLS, JE CHILDS, MC MUZZINI, KT MCKEE, DA ENRIA, and GE GLASS. 1997. Structure and floristic of habitats associated with five rodent species in an agroecosystem in Central Argentina. Journal of Zoology (London) 243:437-460. [ Links ]
13. ESTRELLA H, J BOSHOVEN, and M TOGNELLI. 2001. Características del clima regional y de la Reserva de Ñacuñán. Pp. 24-34, in: El desierto del Monte: La Reserva de Biosfera de Ñacuñán. Mendoza (S Claver and S Roig-Junent, eds. [ Links ]).
14. IADIZA-MAB-UNESCO. GARCÍA LV. 2004. Escaping the Bonferroni iron claw in ecological studies. Oikos 105:657-663. [ Links ]
15. GONNET JM and RA OJEDA. 1998. Habitat use by small mammals in the arid Andean foothills of the Monte Desert of Mendoza, Argentina. Journal of Arid Environments 38:349-357. [ Links ]
16. HUGHES J and D WARD. 1993. Predation risk and distance to cover affect foraging behaviour in Namib desert gerbils. Animal Behaviour 46:1243-1245. [ Links ]
17. HUTTO RL. 1985. Habitat selection by nonbreeding migratory land birds. Pp. 455-476, in: Habitat selection in birds (ML Cody, ed.). Academic Press. [ Links ]
18. JOHNSON DH. 1980. The comparison of usage and availability measurements for evaluating resource preference. Ecology 61:65-71. [ Links ]
19. KOTLER BP. 1984. Risk of predation and the structure of desert rodent communities. Ecology 65:689-701. [ Links ]
20. KOTLER BP. 1985. Owl predation on desert rodents which differ in morphology and behavior. Journal of Mammalogy 66:824-828. [ Links ]
21. KOTLER BP and J BROWN. 1988. Environmental heterogeneity and the coexistence of desert rodents. Annual Review of Ecology and Systematics 19:281-307. [ Links ]
22. KOTLER BP, JS BROWN, and WA MITCHELL. 1994. The role of predation in shaping the behavior, morphology and community organisation of desert rodents. Australian Journal of Zoology 42:449-466. [ Links ]
23. LEMEN CA and PW FREEMAN. 1985. Tracking mammals with fluorescent pigments: a new technique. Journal of Mammalogy 66:134-136. [ Links ]
24. LEVINS R, T AWERBUCH, U BRINKMANN, I ECKARDT, P EPSTEIN, N MAKHOUL, C ALBUQUERQUE DE POSSAS, C PUCCIA, A SPIELMAN, and ME WILSON. 1994. The emergence of new diseases. American Scientist 82:52- 60. [ Links ]
25. LITVAITIS JA, K TITUS, and EM ANDERSON. 1994. Measuring vertebrate use of terrestrial habitats and foods. Pp. 254-262, in: Research and management techniques for wildlife and habitats (TA Bookhout, ed.). The Wildlife Society. [ Links ]
26. LONGLAND WS and MV PRICE. 1991. Direct observations of owls and heteromyid rodents: can predation risk explain microhabitat use? Ecology 72:2261- 2273. [ Links ]
27. MORAN MD. 2003. Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos 100:403-405. [ Links ]
28. MORELLO J. 1958. La Provincia fitogeográfica del Monte. Opera Lilloana 2:11-155. [ Links ]
29. MORSE SS. 1993. Emerging viruses. Oxford University Press, New York. [ Links ]
30. REDFORD K and JF EISENBERG. 1992. Mammals of the Neotropics. The Southern Cone. Chile, Argentina, Uruguay, Paraguay. Vol. 2. The University of Chicago Press, Chicago and London. [ Links ]
31. ROIG FA. 1971. Flora y vegetación de la Reserva Forestal de Ñacuñán. Deserta 1:25-232. [ Links ]
32. VÁSQUEZ RA. 1994. Assessment of predation risk via illumination level: facultative central place foraging in the cricetid rodent Phyllotis darwini. Behavioral Ecology and Sociobiology 34:375-381 [ Links ]
Recibido 4 agosto 2006.
Aceptado 28 abril 2008.
Editor asociado: J Salazar Bravo














