Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Mastozoología neotropical
versión impresa ISSN 0327-9383versión On-line ISSN 1666-0536
Mastozool. neotrop. v.16 n.2 Mendoza jul./dic. 2009
ARTÍCULOS Y NOTAS
Jumping ability in the arboreal locomotion of didelphid marsupials
Ana Cláudia Delciellos and Marcus Vinícius Vieira
Laboratório de Vertebrados, Departamento de Ecologia, Instituto de Biologia, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, CP 68020, CEP 21941-590 [Correspondence: Ana Delciellos <delciellos@biologia.ufrj.br>].
ABSTRACT: Didelphid marsupials are considered to cross discontinuities between arboreal supports using a cautious locomotion, using the prehensile tail as fifth limb. However, this ability was only described for Caluromys philander. We describe and compare the locomotory performance and postural behavior of seven species of didelphid marsupials crossing discontinuities between artificial supports representing arboreal gaps. Individuals were captured in areas of Atlantic Forest in Rio de Janeiro State, Brazil. Animals were stimulated to jump from a fixed horizontal support one meter above the ground to an inclined support of same diameter. We measured the maximum distance of jump (40, 60, 80 or 100 cm) and reach or distance actually reached by the jump. Arboreal species (Marmosops incanus, Gracilinanus microtarsus, Micoureus paraguayanus, and C. philander) jumped longer distances and had longer relative reach in jumps than semi-terrestrial species (Didelphis aurita, and Philander frenatus). Only the specialized terrestrial Metachirus nudicaudatus did not jump in the tests. The relation between absolute reach and body size was weak and non significant. This study did not corroborate the view that didelphid marsupials cross discontinuities between arboreal supports only through a cautious locomotion, without jumping. On the contrary, we identified patterns of jumping performance and behavior of didelphid marsupials related to their use of the vertical strata.
RESUMO: Habilidade de salto na locomoção arborícola de marsupiais didelfídeos. Os marsupiais didelfídeos são tidos como animais que cruzam descontinuidades entre suportes arbóreos através de uma locomoção cautelosa, utilizando a cauda preênsil como quinto membro. Entretanto, esta habilidade somente foi descrita para Caluromys philander. O objetivo desse estudo foi descrever e comparar o desempenho e comportamento de sete espécies de marsupiais didelfídeos na transposição de descontinuidades entre suportes artificiais representando descontinuidades arbóreas. Indivíduos capturados em áreas de Mata Atlântica no Estado do Rio de Janeiro, Brasil, foram submetidos a testes que consistiam em estimulá-los a saltarem de um suporte horizontal fixo a um metro do chão para um suporte inclinado de mesmo diâmetro. Foram medidas a distância máxima de salto (40, 60, 80 ou 100 cm) e o alcance ou distância efetiva atingida com o salto. As espécies arborícolas (Marmosops incanus, Gracilinanus microtarsus, Micoureus paraguayanus e C. philander) saltaram prontamente maiores distâncias e tiveram maiores alcances relativos no salto do que as semiterrestres (Didelphis aurita e Philander frenatus). Apenas a especializada terrestre Metachirus nudicaudatus não realizou saltos. A relação entre alcance e peso corporal foi fraca e não significativa. Esse estudo não corroborou a visão de que os marsupiais didelfídeos cruzam descontinuidades entre suportes arbóreos somente através de uma locomoção cautelosa, sem saltar. Ao contrário, identificamos diferentes padrões de desempenho e comportamento no salto relacionados ao uso dos estratos verticais da mata pelos marsupiais didelfídeos estudados.
Key words. Atlantic Forest; Body size; Didelphimorphia; Ecomorphology; Locomotion.
Palavras-chave. Didelphimorphia; Ecomorfologia; Locomoção; Mata Atlântica; Tamanho corporal.
INTRODUCTION
Locomotory abilities are of major importance for animals to escape from predators, to capture prey, and to find partners for reproduction, food and shelter (Bennett, 1989; Van Damme and Van Dooren, 1999). At one end, individual locomotory abilities contribute to determine the fitness of an organism, and at another, affect population dynamics and community structure (Essner, 2002; Irschick, 2003).
For arboreal animals, the ability to cross discontinuities between arboreal supports is a vital component of its locomotive repertoire (Gunther et al., 1991; James et al., 2007). In terrestrial animals this ability is frequently associated with the need to cross obstacles in the ground (Gunther et al., 1991), and to escape from predators (Henry et al., 2005). Arboreal animals use supports that are discontinuous, limited, changeable in diameter, mobiles, and oriented in all the possible angles (Cartmill, 1974). Discontinuities may be crossed by simply "bridging" two supports extending fore and hindlimbs, when a prehensile tail acts as a fifth limb (Youlatos, 1993). Another possibility is jumping, which allows the animal to cross larger distances than bridging. Jumping ability is frequently considered essential for daily activities in a three-dimensionally complex environment (James et al., 2007), allowing to optimize its energy expenses when fast covering long distances (Gunther et al., 1991; Higham et al., 2001), for example.
The majority of studies that evaluated vertebrate behavior and performance in jumping was done with terrestrial lemurs which move by vertical jumps on the ground (Aerts, 1998), gliders such as flying squirrels (Essner, 2002), anuran amphibians (Marsh and John-Alder, 1994; Wilson et al., 2000; McCay, 2003), and lizards (Higham et al., 2001; Melville and Swain, 2003; Kohlsdorf and Navas, 2007). The consensus in these studies was that jumping ability of an animal is a result of a set of physiological (e.g. anaerobic metabolism), morphological (e.g. body size, limbs length), behavioral (e.g. motivation), and environmental (e.g. structural complexity) factors (Bennett, 1989; Aerts, 1998; Irschick and Jayne, 1999; Harris and Steudel, 2002; James et al., 2007).
Didelphid marsupials are quadruped (Jenkins and McClearn, 1984), and predominantly arboreal or scansorial in their morphology (Cartmill, 1974; Szalay, 1994). Didelphids have been traditionally considered to move slowly and carefully along tree branches, without jumping, bridging discontinuities between branches with the help of their grasping ability and prehensile tail (Charles-Dominique et al., 1981). However, this generalization is based on descriptions of Caluromys philander locomotion, an arboreal specialist of the canopy (Charles-Dominique et al., 1981). The ability to cross discontinuities between arboreal supports for the majority of didelphid marsupials is still unknown. Besides, even if bridging supports is more frequently used, jumping may also be part of the locomotory repertoire of didelphid marsupials.
One way to evaluate jumping ability of didelphids is through performance tests in an ecomorphological approach (Delciellos and Vieira, 2002; Delciellos et al., 2006). Arboreal didelphids are generally faster than terrestrial species in arboreal walking (Delciellos and Vieira, 2006, 2007) and climbing slender supports (Lemelin, 1999; Delciellos and Vieira, 2009), but there are exceptions to this general pattern. For example, C. philander is known as a canopy specialist (Charles-Dominique, 1983; Schmitt and Lemelin, 2002), but its performance (velocity) in arboreal walking and climbing is more similar to the semi-terrestrial Philander frenatus (Delciellos and Vieira, 2006, 2007, 2009).
We describe and compare the locomotory performance and behavior of seven species of didelphid marsupials crossing discontinuities between arboreal supports. We expect that the jumping behavior will be more elaborated in arboreal than in terrestrial didelphids, and that arboreal species will be capable of longer jumps without falling. Postural behavior involved in the crossing of discontinuities by jumping is described for the first time. The same species of didelphids have been previously studied regarding arboreal walking (Delciellos and Vieira, 2006, 2007) and climbing performances (Delciellos and Vieira, 2009), and climbing behavior (Antunes, 2003). Arboreal walking behavior was studied only for C. philander (Schmitt and Lemelin, 2002).
MATERIAL AND METHODS
Study area
Marsupials were captured in remnants of Coastal Atlantic Forest on the border of the Serra dos Órgãos National Park (PARNASO, 22°94' - 22°32' S, 42°69' - 43°06' W), Rio de Janeiro State, Brazil, in the municipalities of Guapimirim, Teresópolis, and Cachoeiras de Macacu, from January 1999 to February 2004. Captures were part of the small mammal surveys of the Project of Conservation and Use of Brazilian Biological Diversity (PROBIO, MMA-GEF), and part of the Small Mammal Population Monitoring Program of the Laboratório de Vertebrados, Universidade Federal do Rio de Janeiro.
The Coastal Forest of the Serra do Mar is a subdivision of the Atlantic Forest Biome (Dinerstein et al., 1995). The canopy reaches 20- 25 m, with tree-trunk diameter varying from 40 to 60 cm, sometimes reaching 1 m. A sub canopy and understory are present, but generally it is not possible to distinguish them clearly. Lianas, palm trees, epiphytes, ferns, and bromeliads are common. Common tree species belong to the genera Sloanea, Ficus, Cedrela, Cariniana, Vochysia, and Cecropia. Common species of the sub canopy and understory include tree ferns of the genera Alsophila, Cyathea, and Hemitelia, and the palm tree Euterpe edulis (Rizzini, 1979).
Specimens of study
The seven didelphid marsupials studied are nocturnal and solitary (Nowak, 1999), varying from 40 g to 1300 g (Table 1). They are classified as omnivores with subtle differences in food habits, varying from frugivore-omnivore to insectivore-omnivore, but all consume fruits and arthropods to some degree (Astúa de Moraes et al., 2003). Differences in use of vertical strata of the forest by didelphid marsupials are more clearcut (Dickman and Vieira, 2006).
Table 1
Morphological measurements ( x ± sd) of the didelphid marsupials studied.
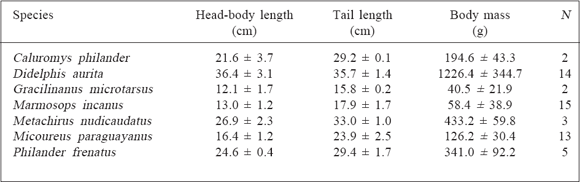
Caluromys philander (Linnaeus, 1758) is a canopy specialist (Cartmill, 1974; Charles-Dominique, 1983; Schmitt and Lemelin, 2002), but occasionally uses the ground, possibly searching for food; Didelphis aurita Wied-Neuwied, 1826 is semi-terrestrial (Cunha and Vieira, 2002); Gracilinanus microtarsus (Wagner, 1842) is an arboreal of the canopy, occasionally captured in the understory and ground (Charles-Dominique, 1983); Marmosops. incanus (Lund, 1840) is arboreal of understory, frequently captured on the ground, but never in the canopy (Cunha and Vieira, 2002; Loretto and Vieira, 2008); Metachirus nudicaudatus (Dermarest, 1817) is essentially terrestrial, the truly cursorial didelphid (Szalay, 1994; Cunha and Vieira, 2002); Micoureus paraguayanus (Moojen, 1943) is an arboreal of the canopy and understory, occasionally captured in the ground (Grelle, 2003; Vieira and Monteiro-Filho, 2003), and Philander frenatus (Olfers, 1818) is semi-terrestrial, occasionally captured in the understory (Cunha and Vieira, 2002).
Only individuals with four functional molars were considered adults and tested (Gentile et al., 1995; Macedo et al., 2006). Individuals physically debilitated, and females with pouch young were not tested.
Performance tests
Performance tests consisted of encouraging the animal to cross discontinuities between two supports of same diameter (2.54 cm). One horizontal support was kept fixed one meter above the ground, and the other initially inclined at an angle of 60 degrees (Fig. 1). The distance between the two supports was increased, initially set at 40 cm. The animal was taken out of its cage, placed onto the fixed support, and encouraged to jump the discontinuity by an observer approaching from the posterior direction, touching the tail of the animal or shaking a key ring. If the animal jumped the first distance (40 cm) successfully, it was encouraged to jump distances of 60 cm, 80 cm and 100 cm. These distances were obtained moving away the horizontal support and increasing the inclination of the other support, until a maximum of 75 degrees.
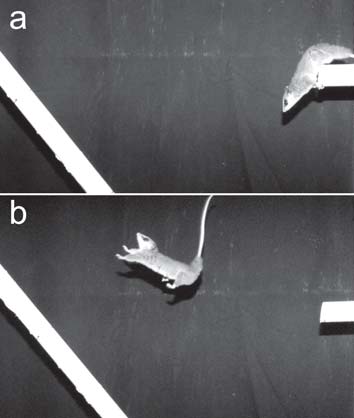
Fig. 1. Individual of Micoureus paraguayanus in the test of jump of 80 cm. (a) position of the head and posterior limbs for the jump, and (b)* aerial phase of the jump (Photos: Diogo Loretto). * Reproduced of Delciellos et al. (2006) with permission of the authors.
The jumps were recorded with a camcorder (NTSC standard, 30 complete frames • s-1, shutter speed = 0.01 s) fixed approximately two meters away perpendicularly to the plane of movement of the individual. Animals were tested during the afternoon in normal daylight conditions because no difference was observed between diurnal and nocturnal tests (Vieira, 1995). The floor was covered with five centimeters thick foam to prevent any injury to the animal in case of falling.
Image analyses
Maximum Distance Jumped and reach by each individual were measured with software SigmaScan Pro 5.0 (SPSS Inc.) on the frames recorded and digitized with ATI All-in-Wonder 128 video card and software (ATI Technologies Inc.). Maximum distance jumped was the maximum distance between the two supports that the individual jumped, measured as a projection of the horizontal support reaching the inclined support (40, 60, 80, or 100 cm). Reach was the effective linear distance between the point on the fixed support where the left posterior limb was right before the jump, to the point on the inclined support where the left posterior limb landed after the jump. The distances differ because the jumping trajectory always made a descending arch, such that the point where the individual landed was always lower than the point in line with the horizontal support. The frame with the longest reach for each individual was chosen for the description of postural behavior.
Relative measures of jumping reach were calculated dividing reach measurements of each individual by its body size to emphasize differences between species independently of body size (Van Damme and Van Dooren, 1999). The effect of body mass was also evaluated in a multiple regression with absolute reach as a function of body size and distance between supports.
RESULTS
Jumping performance
Jumping ability to cross discontinuities between supports was higher in arboreal species (Table 2). Among them, only Marmosops incanus and Micoureus paraguayanus jumped the maximum distance of 100 cm between the two supports. However, the small species M. incanus and Gracilinanus microtarsus had the highest average relative reach (Table 2). The canopy specialist Caluromys philander, the largest arboreal species of the group (Table 2), had the smallest average relative reach (Table 2).
Table 2.
Maximum distance jumped and reach (![]() ± sd) of the didelphid marsupials studied. Relative reach is reach divided by body mass of each individual.
± sd) of the didelphid marsupials studied. Relative reach is reach divided by body mass of each individual.
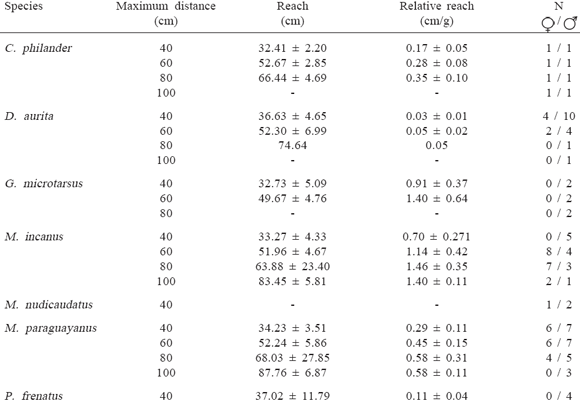
Among the terrestrial species, Metachirus nudicaudatus did not jump, and Philander frenatus and Didelphis aurita jumped a maximum distance of 60 cm, though an individual of D. aurita crossed a distance of 80 cm. Philander frenatus had the highest average relative reach among terrestrial species (Table 2).
Maximum distance jumped explained most of the variation in absolute reach (R2 = 0.82, F(2, 48) = 11.63, p < 0.001, beta (distance) = 0.94). The relationship between reach and body size was non-linear, with a critical point of reduction of reach around 200 g (Fig. 2). Above 200 g, reach distances were all shorter but showed no further reduction with increasing body mass. The adjustment to a potential curve was significant (y = 88.93x -0.10, R2 = 0.16, p = 0.004).
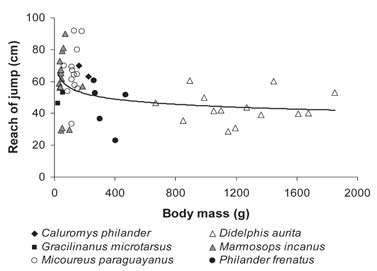
Fig. 2. Nonlinear relation between absolute reach of jump and body mass. The adjustment to a potential curve was significant (y = 88.93x -0.10, R2 = 0.16, p = 0.004).
A general behavioral pattern in crossing discontinuities between arboreal supports was observed for all the didelphids studied. Before jumping, the majority of individuals stopped at the extremity of the horizontal support apparently to evaluate the direction of the jump. The arboreal M. incanus, G. microtarsus, and M. paraguayanus made this evaluation quickly (less than 1s) and jumped, whereas the semi-terrestrial D. aurita and P. frenatus made a longer evaluation (more than 1s but less than 20s), moving the head up and down an from left to right.
In this position that preceded the jump, individuals pointed the head to the direction of the inclined support, with the anterior portion of the body inclined downwards relative to the posterior portion (Fig. 1a). The posterior limbs were at the extremity of the support, being the responsible for the impulse forward for the jump (Fig. 1a). The tail was not used to grasp the support in the phase previous to the jump even in arboreal species. In the aerial phase, the tail was raised and moved sideways, and the anterior limbs were in a forward extended position (Fig. 1b). In all individuals, forelimbs landed before the hind limbs. In the landing of jumps of 60 and 80 cm, individuals of M. paraguayanus frequently rotated the body, and grasped the support with the tail to prevent falling. Many individuals of D. aurita were not able to hold firmly on the support after landing, and fell from the support. Individuals of M. nudicaudatus generally jumped directly to the floor, never to the inclined support. When they tried to jump to the inclined support, they did not grasp the support in landing, falling off to the floor. The tail moved erratically in the aerial phase in contrast to the sidelong tail movements of M. paraguayanus.
DISCUSSION
The ability to cross discontinuities between arboreal supports by jumps was clearly part of the didelphid locomotive repertoire. Jumping performance was related to the degree of use of the vertical strata of each species, arboreal species jumping the longest distances. Arboreal species also adopted a more appropriate postural behavior for jumping.
Different body sizes result in different morphological, physiological and behavioral solutions for the same problems posed by locomotion in a complex three-dimensional environment (Fleagle and Mittermeier, 1980; Cant, 1992; James et al., 2007). Body size explained a small (R2=0.16) but significant part of the jumping ability and behavior (Fig. 2). Indeed, stride length and frequency in arboreal locomotion by the same didelphid species was explained mostly by body size (Delciellos and Vieira, 2006, 2007). However, in jumping, body size set only an upper limit to performance (at ca. 200g), above which other factors were more important.
The major difference in jumping behavior between arboreal and terrestrial species was the prompt decision to jump of the arboreal species, whereas terrestrial species generally took more time exploring or evaluating the distance, and tended to go to the ground. Seemingly, these differences in behavior could be related to differences in visual and olfactory abilities of arboreal and terrestrial didelphid species. Studies on visual and olfactory capacities of didelphids are rare, restricted to species of Didelphis (Hokoç et al., 2006). However, the arboreal Caluromys philander and C. derbianus are considered the didelphids with more acute visual capacity because of its prominent and frontal eyes (Cartmill, 1974; Rasmussen, 1990).
The locomotion in the canopy of one of the more arboreal species of the group, C. philander, has been described as slow and careful, without jumps to cross discontinuities (Charles-Dominique et al., 1981). This type of behavior is also observed in primates of larger body size that distribute their body mass on several supports (Fleagle and Mittermeier, 1980; Fleagle, 1989). In this type of arboreal locomotion the individual firmly grasps the supports of the tree where it is with the posterior limbs and prehensile tail, which functions as a fifth limb (Fleagle, 1989). Next, it stretches the body and forelimbs to grasp with the hand a support of the tree it wants to pass. When successful, the posterior limbs are released, grabbing a support closer to the forelimbs, and finally the tail is released. This study demonstrates that C. philander is also capable of jumping to effectively cross discontinuities between supports. The use of jumping to cross discontinuities is probably more frequent when only large diameter supports are available, for instance near forest gaps or where the canopy is more discontinuous.
In contrast, the only truly cursorial didelphid Metachirus nudicaudatus (Szalay, 1989; Argot 2001, 2002, 2003) did not jump at all in any test. Individuals of M. nudicaudatus perform jumps on the ground of the forest, swiftly transposing obstacles (Miles et al., 1981). One of its specializations to its terrestrial habit is the increased length and muscle mass of hind limbs compared to other didelphids (Grand, 1983; Argot, 2002). Although long hind limbs provide longer strides, velocity, and jumping ability on the forest floor (Coombs, 1978 apud Macrini and Irschick, 1998), the lengthening of hind limbs also move the gravity center up. In locomotion in an arboreal environment a higher center of gravity increases oscillations of body, reducing stability (Cartmill, 1985).
Also, long hind limbs relative to forelimbs could restrict its climbing ability (Vanhooydonck et al., 2000). Thus, the specializations of M. nudicaudatus for high velocity and jumps on the ground may restrict its overall ability to use the arboreal environment.
Didelphis aurita and Philander frenatus use the forest floor most of time, but are not specialized as M. nudicaudatus (Cunha and Vieira, 2002). Accordingly, their jumping distances and relative reaches were shorter than arboreal species (Table 1). However, P. frenatus uses the arboreal strata less frequently than D. aurita (Cunha and Vieira, 2002), but had a higher jumping ability relative to its body mass (higher relative reach of P. frenatus in Table 2). The same result was found already for arboreal walking (Delciellos and Vieira, 2006, 2007), and climbing behavior (Antunes, 2003). These results indicate that the ability to use the arboreal environment of P. frenatus is higher than observed in the field.
The tradicional view that all didelphids cross discontinuities between arboreal supports using only cautious locomotion was not supported. Arboreal or semi-arboreal didelphid marsupials are capable of jumps between supports, using specific postural and locomotory behaviors. Jumping performance was also related to species habits, arboreal species outperforming terrestrial species. Didelphid marsupials move in the arboreal strata using cautions locomotion, but also jumping between supports when necessary.
ACKNOWLEDGMENTS
We thank to students and staff of the Laboratório de Vertebrados - Universidade Federal do Rio de Janeiro (UFRJ) for the invaluable assistance in the fieldwork and in the performance tests, particularly to Vanina Zini Antunes. Drs. Guillerme Muricy, Diego Astúa de Moraes, and David Flores made invaluable contributions on early versions of the manuscript. Angela Marcondes and Nélio Pereira de Barros provided invaluable logistical support. This study was supported by Fundação Universitária José Bonifácio (FUJB), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), e Projeto de Conservação e Utilização Sustentável da Diversidade Biológica Brasileira (MMA/ PRONABIO/PROBIO).
LITERATURE CITED
1. AERTS P. 1998. Vertical jumping in Galago senegalensis: the quest for an obligate mechanical power amplifier. Philosophical Transactions of the Royal Society of London B 353:1607-1620. [ Links ]
2. ANTUNES VZ. 2003. Comportamento postural e locomotor ao escalar de sete espécies de marsupiais (Didelphimorphia) da Mata Atlântica. Dissertação de Mestrado, Museu Nacional, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brasil. [ Links ]
3. ARGOT C. 2001. Functional-adaptive anatomy of the forelimb in the Didelphidae, and the paleobiology of the Paleocene marsupials Mayulestes ferox and Pucadelphys andinus. Journal of Morphology 247:51-79. [ Links ]
4. ARGOT C. 2002. Functional-adaptive analysis of the hindlimb anatomy of extant marsupials and the paleobiology of the Paleocene marsupials Mayulestes ferox and Pucadelphys andinus. Journal of Morphology 253:76-108. [ Links ]
5. ARGOT C. 2003. Functional-adaptive anatomy of the axial skeleton of some extant marsupials and the paleobiology of the paleocene marsupials Mayulestes ferox and Pucadelphys andinus. Journal of Morphology 255:279-300. [ Links ]
6. ASTÚA DE MORAES D, RT SANTORI, R FINOTTI, and R CERQUEIRA. 2003. Nutritional and fibres contents of laboratory-established diets of Neotropical opossums (Didelphimorphia, Didelphidae). Pp. 229-237, in: Predators with pouches: the biology of carnivorous marsupials (M Jones, C Dickman, and M Archer, eds.). CSIRO Publishing. [ Links ]
7. BENNETT AF. 1989. Integrated studies of locomotor performance. Pp. 191-202, in: Organismal functions: integration and evolution in vertebrates (DB Wake and G Roth, eds.). Wiley & Sons Ltd. [ Links ]
8. CANT GH. 1992. Positional behavior and body size of arboreal primates: a theoretical framework for field studies as an illustration of its application. American Journal of Physical Anthropology 88:273-283. [ Links ]
9. CARTMILL M. 1974. Pads and claws in arboreal locomotion. Pp. 43-83, in: Primate locomotion (FA Jenkins Junior, ed.). Academic Press. [ Links ]
10. CARTMILL M. 1985. Climbing. Pp. 73-88, in: Functional vertebrate morphology (M Hildebrand, DM Bramble, KF Liem, and DB Wake, eds.). Harvard University Press. [ Links ]
11. CHARLES-DOMINIQUE P. 1983. Ecology and social adaptations in didelphid marsupials: comparison with eutherians of similar ecology. American Society of Mammalogists Special Publication 7:395-422. [ Links ]
12. CHARLES-DOMINIQUE P, M ATRAMENTOWICZ, M CHARLES-DOMINIQUE, H GÈRARD, A HLADIK, CM HLADIR, and MF PRÉVOST. 1981. Les mamifères frugivores arboricoles nocturnes d'une forêt guyanaise: inter-relations plantes-animaux. Revue d'Ecologie 35:341-435. [ Links ]
13. COOMBS WP. 1978. Theoretical aspects of cursorial adaptations in dinosaurs. The Quarterly Review of Biology 53:393-418. [ Links ]
14. CUNHA AA and MV VIEIRA. 2002. Support diameter, incline, and vertical movements of four didelphid marsupials in the Atlantic forest of Brazil. Journal of Zoology (London) 258:419-426. [ Links ]
15. DELCIELLOS AC, D LORETTO, and MV VIEIRA. 2006. Novos métodos no estudo da estratificação vertical de marsupiais neotropicais. Oecologia Brasiliensis 10:135-153. [ Links ]
16. DELCIELLOS AC and MV VIEIRA. 2002. Modelos ecomorfológicos para vertebrados arborícolas: o caso do marsupial Philander frenata. Holos Environment 2:195-207. [ Links ]
17. DELCIELLOS AC and MV VIEIRA. 2006. Arboreal walking performance in seven didelphid marsupials as an aspect of their fundamental niche. Austral Ecology 31:449-457. [ Links ]
18. DELCIELLOS AC and MV VIEIRA. 2007. Stride lengths and frequencies of arboreal walking in seven species of didelphid marsupials. Acta Theriologica 52:101-111. [ Links ]
19. DELCIELLOS AC and MV VIEIRA. 2009. Allometric, phylogenetic and adaptive components of climbing performance in seven species of didelphid marsupials. Journal of Mammalogy 90(1):104-113. [ Links ]
20. DICKMAN C and EM VIEIRA. 2006. Ecology and life histories. Pp. 199-228, in: Marsupials (P Armati, C Dickman, and I Hume, eds.). Cambridge University Press. [ Links ]
21. DINERSTEIN E, DM OSLON, DJ GRAHAM, AL WEBSTER, SA PRIMM, MP BOOKBINDER, and G LEDEC. 1995. A conservation assessment of the terrestrial ecoregions of Latin America and the Caribbean. WWF & World Bank. [ Links ]
22. ESSNER R. 2002. Three-dimensional launch kinematics in leaping, parachuting and gliding squirrels. The Journal of Experimental Biology 205:2469-2477. [ Links ]
23. FLEAGLE JG. 1989. Size and adaptation in Primates. Pp. 1-19, in: Size and scaling in primate biology (WL Jungers, ed.). Plenum Press. [ Links ]
24. FLEAGLE JG and RA MITTERMEIER. 1980. Locomotor behavior, body size, and comparative ecology of seven Surinam monkeys. American Journal of Physical Anthropology 52:301-314. [ Links ]
25. GENTILE R, PS D'ANDREA, and R CERQUEIRA. 1995. Age structure of two marsupial species in a Brazilian restinga. Journal of Tropical Ecology 11:679-682. [ Links ]
26. GRAND TI. 1983. Body weight: its relationship to tissue composition, segmental distribution of mass, and motor function. III. The Didelphidae of French Guyana. Australian Journal of Zoology 31:299-312. [ Links ]
27. GRELLE CEV. 2003. Forest structure and vertical stratification of small mammals in a secondary Atlantic Forest, southeastern Brazil. Studies on Neotropical Fauna and Environment 38:81-85. [ Links ]
28. GÜNTHER MM, H ISHIDA, H KUMAKURA, and Y NAKANO. 1991. The jump as a fast mode of locomotion in arboreal and terrestrial biotopes. Journal of Morphological Anthropology 78:341-72. [ Links ]
29. HARRIS M and K STEUDEL. 2002. The relationship between maximum jumping performance and hind limb morphology/physiology in domestic cats (Felis silvestris catus). The Journal of Experimental Biology 205:3877-3889. [ Links ]
30. HENRY HT, DJ ELLERBY, and RL MARSH. 2005. Performance of guinea fowl Numida meleagris during jumping requires storage. The Journal of Experimental Biology 208:3293-3302. [ Links ]
31. HIGHAM TE, MS DAVENPORT, and BC JAYNE. 2001. Maneuvering in an arboreal habitat: the effects of turning angle on the locomotion of three sympatric ecomorphs of Anolis lizards. The Journal of Experimental Biology 204:4141-4155. [ Links ]
32. HOKOÇ JN, SMA LIMA, AMM MORAES, and P AHNELT. 2006. A visão em marsupiais: características e evolução. Pp. 69-79, in: Os marsupiais do Brasil: biologia, ecologia e evolução (NC Cáceres and ELA Monteiro-Filho, eds.). Editora UFMS. [ Links ]
33. IRSCHICK D. 2003. Measuring performance in nature: implications for studies of fitness within populations. Integrative and Comparative Biology 43:396-407. [ Links ]
34. IRSCHICK DJ and BC JAYNE. 1999. A field study of the effects of incline on the escape locomotion of a bipedal lizard, Callisaurus draconoides. Physiological and Biochemical Zoology 72:44-56. [ Links ]
35. JAMES R, CA NAVAS, and A HERREL. 2007. How important are skeletal muscle mechanics in setting limits on jumping performance? The Journal of Experimental Biology 210:923-933. [ Links ]
36. JENKINS JUNIOR FA and D MCCLEARN. 1984. Mechanisms of hind-foot reversal in climbing mammals. Journal of Morphology 182:197-219. [ Links ]
37. KOHLSDORF T and C NAVAS. 2007. Evolution of jumping capacity in Tropidurinae lizards: does habitat complexity influence obstacle-crossing ability? Biological Journal of the Linnean Society 91:393-402. [ Links ]
38. LEMELIN P. 1999. Morphological correlates of substrate use in didelphid marsupials: implications for primate origins. Journal of Zoology 247:165-175. [ Links ]
39. LORETTO D and MV VIEIRA. 2008. Use of space by the marsupial Marmosops incanus (Didelphimorphia, Didelphidae) in the Atlantic Forest, Brazil. Mammalian Biology 73:255-261. [ Links ]
40. MACEDO J, D LORETTO, MV VIEIRA, and R CERQUEIRA. 2006. Classes de desenvolvimento em marsupiais: um método para animais vivos. Mastozoología Neotropical 13:133-136. [ Links ]
41. MACRINI TE and DJ IRSCHICK. 1998. An intraspecific analysis of trade-offs in sprinting performance in a West Indian lizard species (Anolis lineatopus). Biological Journal of the Linnean Society 63:579-591. [ Links ]
42. MARSH R and H JOHN-ALDER. 1994. Jumping performance of Hylid frogs measured with high-speed cine film. The Journal of Experimental Biology 188:131-141. [ Links ]
43. MCCAY M. 2003. Winds under the Rain Forest canopy: the aerodynamic environment of gliding tree frogs. Biotropica 35:94-102. [ Links ]
44. MELVILLE J and R SWAIN. 2003. Evolutionary correlations between escape behaviour and performance ability in eight species of snow skinks (Niveoscincus: Lygosominae) from Tasmania. Journal of Zoology 261:79-89. [ Links ]
45. MILES MA, AA SOUSA, and MM PÓVOA. 1981. Mammal tracking and nest location in Brazilian forest with an improved spool-and-line device. Journal of Zoology 195:331-347. [ Links ]
46. NOWAK RM. 1999. Walker's mammals of the world. Johns Hopkins University Press. [ Links ]
47. RASMUSSEN DT. 1990. Primate Origins: Lessons from a Neotropical Marsupial. American Journal of Primatology 22:263-277. [ Links ]
48. RIZZINI CT. 1979. Tratado de Fitogeografia do Brasil: aspectos sociológicos e florísticos. EDUSP. [ Links ]
49. SCHMITT D and P LEMELIN. 2002. Origins of primate locomotion: gait mechanics of the woolly opossum. American Journal of Physical Anthropology 118:231-238. [ Links ]
50. SZALAY FS. 1994. Evolutionary history of the marsupials and an analysis of osteological characters. Cambridge University Press. [ Links ]
51. VAN DAMME R and JM VAN DOOREN. 1999. Absolute versus per unit body length speed of prey as an estimator of vulnerability to predation. Animal Behaviour 57:347-352. [ Links ]
52. VANHOOYDONCK B, R VAN DAMME, and P AERTS. 2000. Ecomorphological correlates of habitat partitioning in Corsican lacertid lizards. Functional Ecology 14:358-368. [ Links ]
53. VIEIRA EM and ELA MONTEIRO-FILHO. 2003. Vertical stratification of small mammals in the Atlantic rain forest of south-eastern Brazil. Journal of Tropical Ecology 19:501-507. [ Links ]
54. WILSON RS, CE FRANKLIN, and RS JAMES. 2000. Allometric scalling relationships of jumping performance in the striped marsh frog Limnodynastes peronii. The Journal of Experimental Biology 203:1937-1946. [ Links ]
55. YOULATOS D. 1993. Passages within a discontinuous canopy: bridging in the red howler monkey (Alouatta seniculus). Folia Primatologica 61:144-147. [ Links ]
Recibido 23 julio 2008.
Aceptado 17 noviembre 2008.
Editor asociado: D Flores














