Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Mastozoología neotropical
versão impressa ISSN 0327-9383versão On-line ISSN 1666-0536
Mastozool. neotrop. v.16 n.2 Mendoza jul./dez. 2009
ARTÍCULOS Y NOTAS
Sexual maturation and reproductive activity of spring-born female corn mice, Calomys musculinus, in absence of adults
Lucía Sommaro, Daniela Gomez, Andrea Steinmann, and José Priotto
Departamento de Ciencias Naturales, Universidad Nacional de Río Cuarto, Agencia Postal N° 3, 5800 Río Cuarto, Córdoba, Argentina [Correspondence: L. Sommaro <lucisommaro@yahoo.com.ar>]
ABSTRACT: The aim of this study was to analyze the effects of parents on maturation and reproductive activity of spring-born females in fenced populations of Calomys musculinus, at the beginning of the breeding period. The field study was carried out in four 0.25-ha enclosures (two control and two experimental), each situated on natural pasture. This study had two periods: 1) Absence of fathers (AF) (from September 2003 to January 2004), and 2) Absence of mothers (AM) (from October 2004 to February 2005). In both periods, in control enclosures both parents remained with the offspring. During AF period, in experimental enclosures only the mothers remained with their offspring. In AM period, only the adult males remained with their offspring in experimental enclosures. Three trapping sessions of 8 successive nights every fortnight were carried out. Sexual maturation and reproductive activity of spring-born females were compared between treatments using repeated-measures ANOVA. The repeated measures factor was the age of young females. In both periods the number of spring-born females in each reproductive condition was independent of treatments. The females matured according to their physiological times (30-40 days of age). The removal of fathers and mothers did not affect either the timing of sexual maturation or the reproduction of C. musculinus spring-born females. Future research to test the effect of adult female density on juveniles should be done to test the same parameters.
RESUMEN: Maduración sexual y actividad reproductriva en ausencia de adultos en hembras de Calomys musculinus nacidas en primavera. El objetivo de este estudio fue analizar los efectos de los padres sobre la maduración y la actividad reproductiva de hembras nacidas en primavera, en poblaciones de clausura de Calomys musculinus al comienzo del periodo reproductivo. El estudio fue llevado a cabo en cuatro clausuras de 0.25-ha cada una (dos controles y dos experimentales), ubicadas en una pastura natural. El estudio estuvo dividido en 2 etapas: 1) Ausencia de padres (AP) (desde septiembre de 2003 a enero de 2004), y 2) Ausencia de madres (AM) (desde octubre de 2004 a febrero de 2005). En AP y AM, en las clausuras controles ambos padres permanecieron con sus crías. Durante AP, en las clausuras experimentales solo las madres permanecieron con sus crías. Por otro lado, durante AM solo los padres permanecieron con sus crías en las clausuras experimentales. Se realizaron tres sesiones de muestreo de 8 noches consecutivas de duración cada quince días. La maduración sexual y la actividad reproductiva de las hembras nacidas en primavera fueron comparadas entre tratamientos mediante un ANOVA de medidas repetidas. El factor de medidas repetidas fue la edad de las hembras. En ambas etapas el número de hembras de cada condición reproductiva fue independiente del tratamiento. La maduración de las hembras se correspondió con el tiempo fisiológico (3040 días de edad). La remoción de los padres y las madres no afectó ni el tiempo de maduración ni la reproducción de las hembras de C. musculinus, sin embargo el efecto de la densidad de hembras debe ser evaluado en futuros estudios.
Key words. Adult removal; Mating system; Reproductive activity.
Palabras clave. Actividad reproductiva; Remoción de adultos; Sistema de apareamiento.
INTRODUCTION
Calomys musculinus is the dominant rodent species of central and north-western Argentina, and the reservoir of Junin virus, the etiological agent of Argentine hemorrhagic fever (AHF) (Mills and Childs, 1998). It inhabits Pampean agrarian ecosystems and is found in a variety of habitats including natural pastures, crop-field edges, cultivated fields undisturbed after harvest, border areas protected by wire enclosures with little agricultural disturbance, roadsides, and railway banks (Busch et al., 2000). C. musculinus populations are characterized by seasonal density changes, with low density during winter (16 mice/ha) and peaks during summer or early autumn (260 mice/ha) (Mills et al., 1991, 1992).
The reproductive period of this species begins in mid-September and finishes at the end of April (Mills et al., 1998). C. musculinus has a short gestation length (21 days) and each female can produce many pups in her lifetime (6 pups per litter). Females show a high frequency of postpartum estrus, which implies that a new pregnancy may overlap with the lactation of the previously produced litter (Mills et al., 1992; Buzzio and Castro-Vasquez, 2002). Juveniles of C. musculinus reach sexual maturity between 30 and 40 days of age, with a mean weight close to 16.5g. (de Villafañe, 1981). In the laboratory, C. musculinus females typically build covered nests, males do not contribute to the construction of the nest, and there is no nest co-habitation by a male-female pair (Yunes et al., 1991). Lactating females display much more aggression towards sexually mature females than towards stranger or familiar males, and the presence of another female near the nest is deleterious for litter survival (Laconi, 1998; Laconi and Castro-Vázquez, 1998; Laconi et al., 2000). During the breeding period, females are territorial and their home ranges are both crossed by transient and by resident males but never by breeding females (Steinmann et al., 2008). On the other hand, males have home ranges that are twice as large as those of females and they fully share them with both sexes and their spatial distribution is strongly influenced by searching for mates (Steinmann et al., 2005, 2008). This spacing behaviour pattern of C. musculinus agrees with a promiscuous mating system (Steinmann et al., 2008). In promiscuous species, in which females typically mate with more than one male during each estrous period, males are expected to evolve large testes relative to their body size (Heske and Ostfeld, 1990). C. musculinus male's testis present an unusual development of Leydig cells, and testosterone levels in plasma higher than monogamous mouse and vole species (Castro-Vázquez et al., 1987; Buzzio and Castro-Vázquez, 2002).
The age at which sexual maturation is reached, fecundity and the duration of breeding season are the most important reproductive variables in the determination of rodent population abundances (Dapson, 1979; Mihok et al., 1985; Rodd and Boonstra, 1988). In rodent populations the sexual maturation of juvenile cohorts can be related to the presence of adult animals (Wasser and Barash, 1983; Wolff et al., 2001; Wolff et al., 2002). Thus, the removal of specific segments of rodent populations affects reproduction in younger cohorts (Saitoh, 1981; Gilbert et al., 1986; Rodd and Boonstra, 1988; Pusenius and Viitala, 1993). In promiscuous species, territorial females are generally assumed to have a greater impact on inhibiting juveniles than males, due to the fact that females typically compete for exclusive offspring-rearing space (Wolff, 1993; Bond and Wolff, 1999). Thus, young females that cannot acquire an exclusive breeding site may delay sexual maturation until space becomes available (Wolff, 1997). Delayed sexual maturation or reproductive suppression of juveniles may result from either direct contact with adults generally through their intrasexual intolerant behaviour, or from chemical signals from urine of related or grouped females (Getz et al., 1983; Heise and Rozenfeld, 1999). However, Wolff et al. (2001) did not observe that the presence of mothers suppressed reproduction in their daughters for Microtus ochrogaster and M. pennsylvanicus. Besides, in Calomys venustus (a promiscuous-polygynous South American rodent) Priotto et al. (2006) observed that both juvenile females and males matured in relation to physiological times independently of the presence of adults.
The aim of this study was to test the hypothesis that the presence of parents causes a delay in maturation and reproductive activity of spring-born females in fenced populations of C. musculinus, at the beginning of the breeding period.
METHODS
Field procedures
This study was carried out on Espinal Reservation of the campus of the National University of Río Cuarto (33º 07´S, 64º 14´W). The study area was a natural pasture interspersed with bushy and weedy species, and it had high vegetal cover (about 100% throughout the year) being similar to natural habitats of C. musculinus.
We set up four 0.25 ha enclosures (two control and two experimental) made of galvanized iron sheets extending 0.3 m underground and 0.7 m above ground. In each enclosure, six reproductive shelters were enclosed with a concrete circle of 1 m diameter and 0.7 m height and were covered by iron mesh. On the inner margin of each enclosure, a 1 m-wide grass strip was devegetated with herbicide. For a detailed description of the study area and enclosure construction see Priotto and Polop (2003), and Priotto et al. (2004).
The study was carried out between September 2003 and February 2005. It had two periods: 1) Absence of fathers (AF) (from September 2003 to January 2004), and 2) Absence of mothers (AM) (from October 2004 to February 2005). Between AF and AM periods (February-September 2004) all animals captured in the enclosures were removed. The rodent populations were from an area located 30 km away from the place of study. In September and October 2003 (AF), and October and November 2004 (AM), 24 and 16 mates respectively were preserved in the laboratory in individual reproductive cages. In both periods, adults and their offspring were weighed and ear-tagged for permanent identification. Both, sex and birth date of the offspring were also recorded. In both periods, after the offspring were weaned in the laboratory, adults and their offspring were carried to the enclosures and then each family group was located in a reproductive shelter. After three days the reproductive shelters were opened and the animals dispersed into the enclosures. During AF period, in control enclosures both parents remained with the offspring (12 mates and 58 juveniles). In experimental enclosures only the adult females remained with their offspring (12 adult females and 45 juveniles). In AM period, there were 8 positive mating less than in AF period. In control enclosures both parents remained with their offspring (8 mates and 47 juveniles) whereas, in experimental enclosures only the adult males remained with their offspring (8 adult males and 49 juveniles).
In each enclosure there was a CMR grid of 6 x 10 traps with an interstation interval of 6 m. One Sherman live-trap was placed at each station and baited with a mixture of peanut butter and cow fat. Three trapping sessions of 8 successive nights every fortnight were carried out from November to January in AF and from December to February in AM. In each devegetated edge, in order to detect those voles that were not able to settle in the habitat area of the plot, 28 Sherman live traps were placed at 6 m intervals. Animals that were trapped three consecutive times in devegetated edge areas within each trapping sessions were removed from the population since we assumed that they were not able to settle within the enclosures. Traps were checked each morning and trapped animals were weighed, and sex and reproductive state were recorded. New animals were individually marked with a numerical code in their ears and released in the site of capture.
Reproductive condition of spring-born females was judged from vagina perforation (perforate or imperforate vagina), pregnancy and size of nipples. When reproductive condition of female could not be judged by external characters, the condition of vaginal smears was analysed. Vaginal smears were taken with a small Pasteur pipette and then a smear was made on a glass slide and observed under a microscope. Vaginal smears were classified as estrous smears when the number of cornified cells was greater than epithelial cells and leucocytes (Buzzio and Castro-Vazquez, 2002). Spring-born females were classified as immature if they had imperforate vagina or no estrous vaginal smear, mature but non-active if they had an estrous vaginal smear or perforate vagina but non-evidence of pregnancy or suckling, or mature and active if they were pregnant or suckling.
Data analyses
The population size for each trapping session was based on the minimum number of animals known to be alive (MNKA), expressed as the number of animals per hectare. The population density was compared among treatments using repeated-measures ANOVA. Each trapping session was the repeated-measure factor. To analyze the number of cohort 1 females in relation to their reproductive condition (immature, mature non-active and mature active females) and treatment (AF and AM), repeated-measures ANOVA was also used (Von Ende, 2001). The repeated-measure factor was the age range at which reproduction condition was measured in each enclosure (e.g.: <20, 20-30, 3040, 40-50, 50-60, 60-70, and >70 days). Because the F statistics for within-subject factors (and their interactions) are inflated in repeated-measures ANOVA when the sphericity assumption is not met (Von Ende, 2001), Greenhouse-Geisser corrected probability was used when interactions were statistically significant.
RESULTS
AF period
From November 2003 to January 2004 a total of 1267 captures were recorded in 5760 night traps. A total of 384 individuals (187 males and 197 females) were ear-tagged. The population density did not vary between control and experimental enclosures (F = 15.212; d.f: 1,2; P = 0.599), but varied in relation to the trapping session (F = 26.642; d.f: 1.2; P = 0.004). In November, the mean population densities were 35 mice per ha in the control enclosures and 28 in experimental enclosures, and towards January they decreased to 25 and 22 in control and experimental enclosures respectively. During this study, no animal was trapped three consecutive times in live traps placed in the devegetated edge areas within each weekly census, therefore all studied animals were able to settle in the plots.
At the beginning of the breeding period there were not significant differences in the number of cohort 1 females in relation to treatment (F = 3.116; d.f: 1, 6; P = 0.123), but there were significant differences in relation to reproductive condition (F = 16.337; d.f: 2, 6; P = 0.004) and age (F = 4.152; d.f: 6, 36; P = 0.003). However, the interaction between reproductive condition and age was statistically significant (F = 25.903; d.f: 12, 36; P = 0.000). The number of mature non-active females was higher than the number of immature and mature active females. In control and experimental enclosures most females were mature between 30 and 50 days of age, and the reproductive activity started between 50 and 70 days of age (Fig. 1). The other first order interactions (treatment × reproductive condition; treatment × age) and the second order interaction (treatment × reproductive condition × age) were not statistically significant (P values > 0.05).
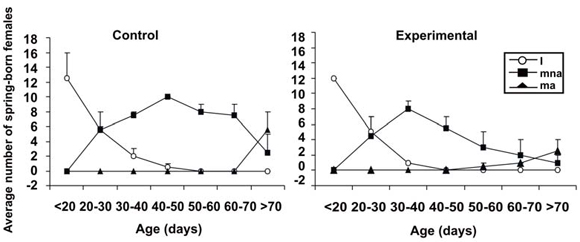
Fig. 1. AM period. Average numbers of spring-born females of Calomys musculinus in relation to age, treatment and reproductive condition. (i) Immature females, (mna) mature but non active females and (ma) active females. Control: presence of both fathers and mothers. Experimental: absence of fathers.
AM period
From the beginning of December 2004 to February 2005 a total of 1335 captures were recorded in 5760 night traps. A total of 422 C. musculinus individuals were ear-tagged (232 males and 190 females). Population density did not vary either between control and experimental enclosures (F = 13.4102; d.f: 1,2; P = 0.6148) or in relation to the trapping session (F = 14.2312; d.f: 1,2; P = 0.6201). In December, the mean population densities were 22 mice per ha in control enclosures and 23 in experimental enclosures, and towards February they increased to 28 and 29 in control and experimental enclosures, respectively. During this study, only ten animals were removed of the enclosure populations because they were trapped three consecutive times in live traps placed in the devegetated edge areas. We assumed that the rest of the animals were able to settle within the enclosures.
At the beginning of the breeding period there were not significant differences in the number of cohort 1 females in relation to treatment (F = 0.1608; d.f: 1, 6; P = 0.7023), but there were significant differences in the number of cohort 1 females in each reproductive condition (F = 6.859; d.f: 2, 6; P = 0.028), and first order interaction (age × reproductive condition) was observed (F = 26.520; d.f: 12, 36; P = 0,000). In this study period, the number of mature active females was higher than the number of immature and mature non-active females. In control and experimental enclosures the majority of females were mature between 20 and 40 days of age, and the reproductive activity started between 30 and 40 days of age (Fig. 2). The other first order interactions (treatment × reproductive condition; treatment × age) and the second order interaction (treatment × reproductive condition × age) were not statistically significant (P values > 0.05).
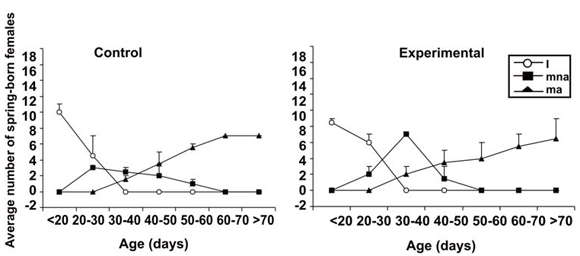
Fig. 2. reproductive condition. (i) Immature females, (mna) mature but non active females and (ma) active females. Control: presence of both fathers and mothers. Experimental: absence of mothers.
DISCUSSION
In this field study we achieved that the control and experimental populations comprised individuals with known relatedness and early life history. In addition, our approach allowed us to control the age distribution of the population and to standardize the post-weaning social environment. These manipulations might have caused some obscure side effects and thus reduced the compatibility of the result with respect to a completely natural situation. Nevertheless, due to the specific nature of the aim of this study, the requirement of strict control was given priority.
In the present study the absence of a relationship between population densities and treatment (father or mother removal) allowed us to analyse the reproduction of young females assuming independence of population densities.
In our study young females reached maturation between 20 and 50 days of age, independently of treatment. These physiological times were similar to those reported by de Villafañe (1981) and Buzzio and Casto-Vazquez (2002). In both control and experimental enclosures, the reproductive activity started between 30 and 50 days of age.
A lot of studies have suggested that adult females regulate sexual maturation of juvenile females (Batzli et al., 1977; Redfield et al., 1978; Saitoh, 1981; Getz et al., 1983; Haigh, 1987; Bondrup-Nielsen, 1986; Carter et al., 1986; Gilbert et al., 1986; Rodd and Boonstra, 1988; Schadler, 1990). Because females typically compete for territories (Wolff, 1993; Wolff and Peterson, 1998), young females that cannot acquire an exclusive breeding site may delay sexual maturation until density decline or space becomes available (Wolff, 1997). Thus, adult female would affect sexual maturation of young females but not young males (Wolff et al., 2002). C. musculinus is a promiscuous species where females are territorial and do not share their home range with other females, whereas male home ranges overlap with both sexes (Steinmann et al., 2008). These characteristics suggest that mother removal (AM) more than father removal (AF) could affect the timing of sexual maturation of their daughters at the beginning of the breeding season. However, neither the absence of mothers nor fathers in C. musculinus populations affected the timing of sexual maturation and the proportion of young that become reproductive. This finding does agree with those registered in field by Priotto et al. (2006) in C. venustus and in laboratory by Wolff et al. (2001) in M. ochrogaster and M. pennsylvanicus. In our study, the absence of an inhibition effect of adult females on sexual maturation of juveniles could be due to the fact that: a) the initial density of adult females in each enclosure (0.25ha) was lower than a threshold density of twelve females per 0.25ha, considering the average home range size reported by Steinmann et al. (2005); b) adult females do not have any effect on sexual maturation of juveniles independently of their numbers. In this study we were able to test if adult female or male absence affected reproductive parameters of young females at low and constant number of adult rodents. Therefore, considering the space use of C. musculinus females, and the availability of exclusive breeding sites, future research to test the effect of adult female density on juveniles should be done to prove if the spacing behaviour of females limit the number of breeding females and therefore it acts as regulation factor of population density.
ACKNOWLEDGEMENTS
We thank Susana Vilor for the English version. This research was made possible by grants from the Consejo Nacional de Investigación Científica y Tecnológica (CONICET) and Secretaría de Ciencia y Técnica (SECyT), Universidad Nacional de Río Cuarto.
LITERATURE CITED
1. BATZLI G, L GETZ, and S HURLEY. 1977. Suppression of growth and reproduction of microtine rodents by social factors. Journal of Mammalogy 58:583-591. [ Links ]
2. BOND ML and JO WOLFF. 1999. Does access to females or competition among males limit male home-ranges in a promiscuous rodent? Journal of Mammalogy 80:1243-1250. [ Links ]
3. BRANT CL, TM SCHWAB, JG VANDENBERGH, RL SCHAEFER, and NG SOLOMON. 1998. Behavioural suppression of female pine voles after replacement of the breeding male. Animal Behaviour 55:615-627. [ Links ]
4. BONDRUP-NIELSEN S. 1986. Investigation of spacing behaviour of Clethrionomys gapperi by experimentation. Journal of Animal Ecology 55:269-279. [ Links ]
5. BUSCH M, MH MIÑO, JR DADON, and K HODARA. 2000. Habitat selection by Calomys musculinus (Muridae, Sigmodontinae) in crop areas of the Pampean region, Argentina. Austral Ecology 10:15-26. [ Links ]
6. BUZZIO O and A CASTRO-VAZQUEZ. 2002. Reproductive Biology of the corn mouse, Calomys musculinus, a Neotropical Sigmodontine. Mastozoología Neotropical 9:135-158. [ Links ]
7. CARTER C, L GETZ, and M COHEN-PAESONS. 1986. Relationships between social organization and behavioral endocrynology in monogamus mamad. Advances in the Study of Behavior 16:109-45. [ Links ]
8. CHRISTIAN JJ. 1971. Fighting, maturity and population density in Microtus pennsylvanicus. Journal of Mammalogy 52:556-567. [ Links ]
9. DAPSON R. 1979. Phenologic influences on cohort-specific reproductive strategies in mice (Peromyscus polinotus). Ecology 60:1125-1131. [ Links ]
10. DE VILLAFAÑE G. 1981. Reproducción y crecimiento de Calomys musculinus murillus (Thomas, 1916). Historia Natural 1:237-256. [ Links ]
11. GAINES MS and RK ROSE. 1976. Population dynamics of Microtus ochrogaster in eastern Kansas. Ecology 57:1145-1161. [ Links ]
12. GETZ LL, D DLUZEN and JL MCDERMOTT. 1983. Suppression of reproductive maturation in male-stimulated virgin female Microtus by a female urinary chemosignal. Behaviour Process 8:54-64. [ Links ]
13. GILBERT C, C KREBS, D TALARICO, and D CICHOWKI. 1986. Do Clethrionomys rutilus females supress maturation of juvenile females? Journal of Animmal Ecology 55:543-552. [ Links ]
14. HAIGH G. 1987. Reproductive inhibition of female Peromyscus leucopus: female competition and behavioural regulation. American Zoologist 27:867-878. [ Links ]
15. HAMILTON WD. 1964. The genetical evolution of social behaviour I and II. Journal of Theoretical Biology 7:17-52. [ Links ]
16. HEISE SR and FM ROZENFELD. 1999. Reproduction and urine marking in laboratory groups of female common voles. Journal of Chemical Ecology 25:1671-1686. [ Links ]
17. KESNER MH and AL LINZEY. 1997. Modeling population variation in Peromyscus leucopus: and exploratory analysis. Journal of Mammalogy 78:643-654. [ Links ]
18. KREBS C. 1966. Demographic changes in fluctuating populations of Microtus californicus. Ecology Monographs 36:239-273. [ Links ]
19. LACONI MR. 1998. Mating system in Calomys musculinus and Calomys laucha (Muridae, Sigmodontinae). Mastozoología Neotropical 5:72-74. [ Links ]
20. LACONI MR and A CASTRO-VÁZQUEZ. 1998. Precopulatory fighting and other aggressive interactions during mating encounters in the corn mouse, Calomys musculinus (Muridae, Sigmodontinae). Mastozoología Neotropical 5:5-12. [ Links ]
21. LACONI MR, GA JAIIN. and A CASTRO-VÁZQUEZ. 2000. Influence of different social partners on the survival and growth of pups in two species of Calomys (Muridae Sigmodontinae). Ethology Ecology and Evolution 12:187-196. [ Links ]
22. MIHOK S, B TURNER, and S IVERSON. 1985. The caracterization of vole population dynamics. Ecology Monographs 55:399-420. [ Links ]
23. MILLS J, B ELLIS, K MCKEE, J MAIZTEGUI, and J CHILDS. 1991. Habitat associations and relative densities of rodent populations in cultivated areas of central Argentina. Journal of Mammalogy 72:470-479. [ Links ]
24. MILLS J, B ELLIS, J CHILDS, J MAIZTEGHI, and A CASTRO-VAZQUEZ. 1992. Seasonal Changes in mass and reproductive condition of the corn mouse (Calomys musculinus) on the Argentine Pampa. Journal of Mammalogy 73:876-884. [ Links ]
25. MILLS J and J CHILDS. 1998. Ecological studies of rodent reservoirs: the relevance for human health. Emerging Infectious Diseases 4:529-537. [ Links ]
26. PRIOTTO JW and JJ POLOP. 2003. Effect of overwintering adults on juvenile survival of Calomys venustus (Muridae: Sigmodontinae). Austral Ecology 28:281-286. [ Links ]
27. PRIOTTO JW, AR STEINMAN, MC PROVENSAL, and JJ POLOP. 2004. Juvenile dispersal in Calomys venustus (Muridae: Sigmodontinae). Acta Oecologica 25:205-210. [ Links ]
28. PRIOTTO JW, A STEINMANN, and JJ POLOP. 2006. Factors affecting home range size and overlap in Calomys venustus (Muridae: Sigmodontinae) in Argentine agroecosystems. Mammalian Biology 67:97-104. [ Links ]
29. PRIOTTO J, C PROVENSAL, and JJ POLOP. 2006. Effect of adults on juvenile reproduction of Calomys venustus (Muridae: Sigmodontinae). Austral Ecology 31:859-868. [ Links ]
30. PUSENIUS J and J ]VITALA. 1993. Demography and regulation of breeding density in the field vole, Microtus agrestis. Annales Zoologici Fennic 30:133-142. [ Links ]
31. REDFIELD JA, MJ TAITT, and CJ KREBS. 1978. Experimental alteration of sex ratios in populations of Microtus townsendii, a field vole. Canadian Journal of Zoology 56:7-27. [ Links ]
32. RODD HF and R BOONSTRA. 1988. Effects of adult meadow voles, Microtus pennsylvanicus, on young conspecifics in field populations. Journal of Animal Ecology 57:755-770. [ Links ]
33. SAITOH T. 1981. Control of female maturation in high density populations of the red-backed vole, Clethrionomys rufocanus belforidae. Journal of Animal Ecology 50:79-87. [ Links ]
34. SCHADLER MH. 1990. Social organization and population control in the pine vole, Microtus pinetorum. Pp. 121-30, in: Social Systems and Population Cycles in Voles (RH Tamarin, RS Ostfeld, SR Pugh, and G Bujalska, eds.). Birkhauser Verlag, Basel. [ Links ]
35. STEINMANN AR. 2006. Comportamiento de espaciamiento de Calomys musculinus (Rodentia: Muridae). Ph.D. Thesis. Facultad de Ciencias Exactas Físico-Químicas y Naturales. Universidad Nacional de Río Cuarto, Río Cuarto, Argentina. [ Links ]
36. STEINMANN AR, JW PRIOTTO, E CASTILLO, and JJ POLOP. 2005. Size and overlap of home range in Calomys musculinus (Muridae: Sigmodontinae). Acta Theriologica 50:197-206 [ Links ]
37. STEINMANN AR, JW PRIOTTO, LV SOMMARO, and JJ POLOP. 2006a. Spacing behaviour of juveniles corn mice, Calomys musculinus, at the beginning of the breeding period, in absence of adult males. Acta Oecologica 29:305-310. [ Links ]
38. STEINMANN AR, JW PRIOTTO, LV SOMMARO, and JJ POLOP. 2006b. The influence of adult female absence on the spacing behaviour of juvenile corn mice, Calomys musculinus: a removal experiment. Annales Zoologici Fennici 43:366-372. [ Links ]
39. STEINMANN AR, JW PRIOTTO, and JJ POLOP. 2008. Territorial behaviour in corn mice, Calomys musculinus (Muridae: Sigmodontinae), with regard to mating system. Journal of Ethology (in press). [ Links ]
40. VON ENDE CN. 2001. Repeated-measures analysis: growth and other time-dependent measures. Pp 134-157, in: Design and analysis of ecological experiments (SM Scheiner and J Gurevitch, eds.). Oxford University Press: Oxford. [ Links ]
41. WASSER S and D BARASH. 1983. Reproductive supression among female mammals. Implications for biomedicine and sexual selection theory. Review of Biology 58:513-538. [ Links ]
42. WOLFF JO. 1992. Parents suppress reproduction and stimulate dispersal in opposite-sex juvenile white-footed mice. Nature 359:409-410. [ Links ]
43. WOLFF J. 1993. Why are female small mammals territorial? Oikos 68:364-370. [ Links ]
44. WOLFF JO. 1997. Population regulation in mammals: an evolutionary perspective. Journal of Animal Ecology 66:1-13. [ Links ]
45. WOLFF JO and EM SCHAUBER. 1996. Space use and juvenile recruitment in gray-tailed voles in response to intruder pressure and food abundance. Acta Theriologica 41:35-43. [ Links ]
46. WOLFF JO and JA PETERSON. 1998. An offspring-defense hypothesis for territoriality in female mammals. Ethology Ecology and Evolution 10:227-239. [ Links ]
47. WOLFF JO, AS DUNLAP, and E RITCHART. 2001. Adult female prairie voles and meadow voles do not suppress reproduction in their daughters. Behavioural Processes 55:157-162. [ Links ]
48. WOLFF JO, DW EDGE, and G WANG. 2002. Effect of adult sex ratios on recruitment of juvenile gray-tailed voles, Microtus canicaudus. Journal of Mammalogy 83:947-956. [ Links ]
49. YUNES RMF, RA CUTRERA, and A CASTROVÁSQUEZ. 1991 Nesting and digging behavior in three species of Calomys (Rodentia; Cricetidae). Physiology & Behavior 49:489-492. [ Links ]
Recibido 29 abril 2008.
Aceptado 22 abril 2009.
Editor asociado: M Busch














