Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Mastozoología neotropical
versão impressa ISSN 0327-9383versão On-line ISSN 1666-0536
Mastozool. neotrop. v.16 n.2 Mendoza jul./dez. 2009
ARTÍCULOS Y NOTAS
Influence of biotic and abiotic factors on the structure of burrows of the cavy Microcavia australis
Paula Taraborelli1, Natalia Borruel1, Ana J. Sandobal2 and Stella Giannoni3
1 Grupo de Investigaciones de la Biodiversidad, Instituto Argentino de Investigaciones de Zonas Áridas, CONICET. CC507, 5500 Mendoza, Argentina [Correspondence: P. Taraborelli <paulataraborelli@gmail.com>].
2 Parque Nacional El Leoncito, San Juan, Argentina.
3 Instituto y Museo de Ciencias Naturales, San Juan, Argentina.
ABSTRACT: Burrows provide a stable microclimate and give small mammals protection from extreme temperatures and from predators on the ground surface. The objective was to determine the influence of biotic and abiotic factors on the structure of burrows used by the cavy Microcavia australis. The study was conducted on two sites with different climate conditions, predation risk and size of plant patches. A total of 18 main burrows and 13satellite burrows were characterized at Ñacuñán, and 12 main and 3 satellite burrows at El Leoncito. The larger number of holes and higher development of main and satelliteburrows at Ñacuñán is likely related to higher risk of raptor predation. At both sites burrows would function as shelter from the environment since temperature in the galleries is lower than soil temperature at the hottest time of the day. Moreover, active holes are east-oriented at Ñacuñán, avoiding SE and S winds, and northwest-oriented at El Leoncito, receiving the warm dry wind from the NW. Also due to gallery inclination the sun goes deeper into the tunnels in the coldest season (winter) than in the warmest one (summer). Burrows would afford cavies a refuge from predators and a stable microclimate.
RESUMEN: Influencia de los factores bióticos y abióticos sobre la estructura de las cuiseras de Microcavia australis. Las cuiseras proveen de un microclima estable y dan protección a pequeños mamíferos de temperaturas extremas del ambiente y de depredadores. El objetivo fue determinar la influencia de los factores bióticos y abióticos sobre la estructura de las cuiseras utilizadas por Microcavia australis. El estudio se realizó en dos sitios con diferentes condiciones climáticas, riesgo de depredación y tamaño de parches de vegetación. Se caracterizaron un total de 18 cuiseras principales y 13 cuiserassatélites en Ñacuñán, y 12 cuiseras principales y 3 cuiseras satélites en El Leoncito. El mayor número de entradas y el gran desarrollo de cuiseras principales y satélites enÑacuñán estarían relacionados con un mayor riesgo de depredación por rapaces. En ambos sitios, las cuiseras podrían tener la función como refugio ante temperaturas extremas externas debido a que la temperatura en los túneles es menor a la temperatura del suelo en el período del día más cálido. Además, en promedio las entradas activas están orientadasal Este en Ñacuñán, evitando los vientos predominantes del SE y S, y hacia el Noroeste en El Leoncito, recibiendo los vientos cálidos y secos del Noroeste. Debido a la inclinación de los túneles el sol ingresa en ellos con mayor profundidad en la estación fría (invierno) que en la estación cálida (verano). Entonces las cuiseras serían para los cuises un refugio ante depredadores y otorgarían un microclima estable.
Key words: Biotic and abiotic factors; Burrow structure; Microcavia australis; Monte desert.
Palabras clave: Desierto del Monte; Estructura de cuiseras; Factores bióticos y abióticos; Microcavia australis.
INTRODUCTION
The majority of rodents live in systems of galleries. Burrows are used for nesting, food storage, and hibernating, and provide rodents with shelter from predators during their surface activity (King, 1984; Reichman and Smith, 1987; Kinlaw 1999). Most desert rodents escape the excessive heat imposed by high solar radiation, and high air and soil temperature by remaining below ground in their burrows during the heat of the day (Ghobrial and Nour, 1975). Underground refuges, with their relatively stable microclimate, afford small animals protection from extreme temperatures on the surface (Kucheruk, 1983). Other authors suggest for Dipodomys spectabilis and D. compactus a relationship between their burrow architecture and abiotic factors such as air and soil temperature, surface-wind velocity, relative humidity and precipitation (Kay and Whitford, 1978; Best, 1988; Baumgardner, 1991). Orientation of holes was found to be not randomly distributed and seems to be related to environmental factors such as sunlight and prevalent direction of cold winds (Kay and Whitford, 1978; Best, 1988; Baumgardner, 1991). On the other hand, the various functions of burrows are associated with their complex structure represented by a number of functionally different chambers connected by a tunnel system (Shenbrot et al., 2002). Satellite burrows could be distinguished from main burrows by their location close to the foraging area where animals can hide when threatened (Armitage, 1988; Branch et al., 1994). The higher number of entrances is important for animals to hide from predators (Ebensperger and Bozinovic, 2000a). Predation risk would be related to the structure of vegetation. Ebensperger and Hurtado (2005) describe a differential effect of shrubs and herbaceous plants on the vigilance behavior of Octodon degus linked to the costs and benefits of each type of plant cover. Shrubs provide higher vertical protection than herbaceous plants, which in turn provide lateral cover but visually obstruct detection of predators and conspecifics, hindering escape.
Individuals of Microcavia australis live in systems of galleries that they actively build on highly compact to soft soils with high organic contents; however, this cavy shows no characteristic morphological adaptations such as claws or developed incisors typical of species that burrow in hard soils (Nowak and Paradiso, 1983; Tognelli et al., 2001). This cavy has diurnal habits and a social structure made up of several females, one or a few males, and the young and juveniles (Rood, 1967 and 1972; Redford and Eisenberg, 1992; Tognelli et al., 1995; Taraborelli and Moreno, accepted). In the Monte desert, burrows of M. australis reach their highest density in the mesquite community, under Prosopis chilensis and/or P. flexuosa trees with arched branches touching the soil (Rood, 1967; Tognelli et al., 1995; Campos, 1997), and according to Contreras and Roig (1979) they present numerous entrances, approximately 26 per burrow. Cavies do not need the burrow for their daily forage for food, but use it as shelter during relatively long periods of time. The response of these cavies to predators like foxes was to escape toward their satellite burrows, showing strong fidelity to them (Contreras and Roig, 1979). Burrow systems are also presumably used for sleeping at night, napping during the day, parturition and litter rearing, group nesting (social thermoregulation) and avoiding inclement weather (Ebensperger et al., 2006; Taraborelli and Moreno, accepted).
Torres et al. (2003) determined that environment factors such as incidence of the sun, cold winds, and water drainage on the surface would be determinant in the architecture of burrows built by the red vizcacha rat (Tympanoctomys barrerae). M. australis possibly makes an adjustment in burrow construction in response to environment factors. Therefore the objective was to determine the influence of biotic and abiotic factors on the structure of burrows used by the cavy M. australis. We compared the structure and use of gallery systems of cavies between two habitats (ElLeoncito and Ñacuñán) with different climate conditions, plant cover and predation risk (Table 1) to test whether there are habitat-dependent differences in the architecture and microclimate of the burrows, and to relate burrow structure to some climate factors (e.g. predominant cold or warm winds, insolation, etc.). Predictions proposed would be that at both sites burrow architecture will depend on climate factors, size of plant patches and predation risk. For example, at Ñacuñán cold winds will negatively affect the layout of burrows, resulting in fewer holes aligned with the direction of the wind. At El Leoncito, a cold site, warm winds will positively affect the layout of burrows, resulting in more holes aligned with the direction of the wind. And, at both sites, the sun incidence will have a negative influence in summer, but a positive one in winter, with less or more holes being opened in a northward direction. Burrows will afford a stable microclimate at both sites, as temperature inside the burrows will be lower than soil temperature at the hottest time of the day. On the site with higher predation risk and cover that visually obstruct detection of predators,Ñacuñán, there will be more holes in main burrows and more satellite burrows to provide shelter from predators.
Table 1
Biotic and abiotic factors at El Leoncito and Ñacuñán. Mean ± standard error.
MATERIALS AND METHODS
Study areas
The study was carried out in two populations in the Monte desert. One population of M. australis is located in the semiarid Monte desert, in the Man and Biosphere Reserve of Ñacuñán (34° 2' S, 67° 58' W, 12 300 ha, 540 m a.s.l.) in the center west of Mendoza Province (Ojeda et al., 1998). Soils are sandy clay, deep; the surface soil is soft and relatively steady (Tanquilevich, 1971; Abraham, 2001; Table 1). The climate is semiarid, warm and dry. Approximately 50% of precipitations occur in the summer months (Cabrera, 1976; Estrella et al., 2001; Table 1). The mesquite community is the habitat preferred by M. australis, because of its structural complexity and the food supply it provides (Campos, 1997; Taraborelli, 2006). This community is composed of three plant layers: the tree layer, dominated by Prosopis flexuosa, the shrub layer, and the herbaceous layer (Roig, 1971). The second population is located in the arid Monte (scrubland) of El Leoncito National Park (31º 47' S, 69º 17' W; 76 000 ha), in the southwest of San Juan Province (Márquez, 1999). Ciénaga del Medio (2484 m a.s.l.) is the habitat preferred by M. australis at El Leoncito. In this locality soils are sandy and present the typical desert varnish on the surface (Bracco and Contreras, 2000; Table 1). The climate is arid, cold and dry, with marked diurnal, nocturnal and seasonal temperature ranges (winter -4-20 ºC, summer 8-32 ºC; Bracco and Contreras, 2000; Márquez and Dalmasso, 2003). Precipitations are in the form of snow and hail and reach 75 mm in winter and in the form of rain and lower than 10 mm in summer (Le Houérou, 1999; Márquez et al., 2000; Márquez and Dalmasso, 2003; Table 1). Predominant winds are dry and warm from the NW (Bracco and Contreras, 2000; ). In Ciénaga del Medio there occurs a shrubland of Larrea nitida with low cover, and the herb layer is lower than 10 cm in height (Márquez et al., 2000; Márquez and Dalmasso, 2003; Taraborelli, 2006).
Records of predators were searched and taken from footprints, feces and aegagropiles (Taraborelli et al., in revision). Also the records were from direct observations during the day (8:00-20:30 hrs) during 7-11 days at three times of the year (November-February, April-August and September-March) from 2003 through 2005. The ratios of predators to cavies were calculated for each time of the year, and then Taraborelli et al. (in revision) estimated the mean and standard error for all data. At Ñacuñán, diurnal raptors were Buteo polyosoma and Milvago chimango; and mammalian carnivores were Lycalopex gymnocercus, Galictis cuja, Conepatus chinga and Felis catus. Predators recorded throughout the year at El Leoncito were crepuscular and nocturnal mammalian carnivores; such as Lycalopex sp. (records of Lycalopex culpaeus are the highest) and P. concolor. Diurnal raptors recorded were Athene cunicularia, Geranoaetus melanoleucus, Buteo polysoma, Falco femoralis, Falco sparverius and Circus cinereus (Taraborelli et al., in revision).
Characterization of burrows
We considered the compound structure with several entrances to the galleries, close to one another and connected by active trails, as the main burrow. Main burrows were generally guarded by patches of vegetation and inhabited by a social group. Only the main burrows were used continually by the cavies during the night (Ebensperger et al., 2006). Satellite burrows have a few entrances to the galleries, are at great distance from the vegetation, and are not stably occupied by the cavies.
Condition of burrow holes (active or inactive) was determined, inactive holes were recognized by the presence of spider webs, or for being covered with leaves or earth, and active holes were identified by signs such as fresh excrements, urine and footprints. Areas and number of active and inactive holes were determined for main and satellite burrows. Orientation (N, E, W, S, SE, SW, NE and NW) was defined for all holes. Height of active holes was recorded, and for the galleries, depth of the first tunnel section up to the first tunnel forking, tilt (Fig. 1) and inner temperature at 50 cm from the entrance. We used the methodology applied by Hoogland (1995) and Torres et al. (2003), and observed and recorded the same burrow systems at different times of year during 20032005. Temperature records were established using a thermometer (0-50 ºC), during three days on each trapping occasion (morning, midday, afternoon, evening), and also measurements of surface soil temperature and environment temperature were made to enable comparisons to be drawn. Environmental temperature was recorded at 1.5 m from the ground. Size of plant patches, plant species present and cover (%) on burrows (Hays et al., 1981; Matteucci and Colma, 1982) were recorded for each plant layer (herb, shrub and tree layers) affording protection to burrows, for comparison with plant availability in the environment. For this purpose, we used a method of strip transects divided into rectangular segments (Matteucci and Colma, 1982) randomly setting up ten 50-m long transects, covering the whole site area. These samplings were performed at three times of the year: time of food abundance (November-February), time of food shortage (April-July), and reproductive season (September-October) for each study site.
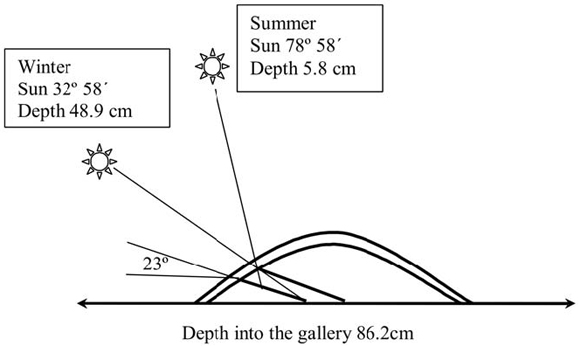
Fig. 1. Incidence of sunbeams on burrows in winter and summer at Ñacuñán
Statistical analysis
ANOVA with repeated measures in time was used to determine existing differences in both number of holes and burrow area among times of the year (time of food abundance or shortage, reproductive season) for the different kinds of burrows (main and satellite). The different kinds of burrows were included as a factor with two levels during the analyses. The same method was used to compare mean tilt and mean depth of burrow tunnels between sites (El Leoncito and Ñacuñán) and among times of the year. It was also used at both sites to check for differences in mean temperature records (environment, soil and inside the galleries) between times of the year and periods of the day (morning, midday, afternoon, evening). Post ANOVA tests (Tukey test, p<0.05) were performed among the variables considered. Results are shown as sampling mean ± standard error. Results are shown as sampling mean ± standard error. Circular Statistics (Batschelet, 1981) was used to verify whether distribution of active holes in burrows at Ñacuñán and El Leoncito was at random (Rayleigh test). The Watson-Williams test was employed to determine whether there were differences in the orientation of active burrow holes between sampled sites. It was established whether orientation of burrow holes differed significantly from that ofinactive holes at Ñacuñán and at El Leoncito using the Pairwise Chi-Square Test. Circular Statistical analysis was also used by Torres et al. (2003). The depth up to which the sunbeams reach inside the galleries at midday in winter and summer was determined by trigonometric calculations. Such theoretical calculations were made for both sites, which are located at different latitudes. Every calculation was obtained by relating the angle of the sun at midday to gallery depth, for two times of the year (winter and summer) with two different inclinations of the Earth's axis. The X2 test was used to compare the cover of plant species present in the burrows and in the study area for both sites. Pearson Residuals (r=fobs-fexp/ªfexp) were used to test for differences between frequencies.
RESULTS
A total of 18 main and 13 satellite burrows were characterized at Ñacuñán, in a 2.7-ha plot, and 12 main and 3 satellites burrows at El Leoncito, in a 2.1-ha plot. All the holes were shielded by patches of vegetation (shrubs, herbs, trees) in both sites. At Ñacuñán, satellite burrows had lower number of holes than main burrows (Table 2; F=85.56; p<0.0001; df=1; N=31). Also the area covered was smaller for satellite burrows (; F=85.56; p<0.0001; df=1; N=31). At El Leoncito it was possible to locate satellite burrows with a single hole, some near the plant cover, at a distance of 1-3.5 m, and others farther away, at 5-7 m. Main burrows were more numerous than satellite burrows (F=8.48; p=0.0048; df=1; N=15; Table 2). The average number of holes per main and satellite burrow at El Leoncito was significantly lowerthan it was at Ñacuñán (main burrow: F=98.31; p<0.0001; df=1; N=31; satellite burrow: F=6.9; p=0.039; df=1; N=16; Table 2). On both sites, the number of holes per burrow remained constant throughout the year.
Table 2
Burrow system characteristics at El Leoncito and Nacunán. Mean ± standard error.
The area of plant patches covered was smaller at El Leoncito than at Nacunán (F=53; p<0.0001; df=1; N=31, El Leoncito 54.61 ± 3.1 m2, Ñacuñán 102.05 ± 6.52 m2). Significant differences were found in the cover of plant species present in the burrow and in the study area (X2=95.35; p<0.0001; df=17; N=31). For example at El Leoncito, the only shrub present, Larrea nitida, occurs on the burrows in a much higher percentage than in the entire environment (99.9% versus 24.7%).At Ñacuñán, plant species showing higher cover on the burrow than in the area are herbs such as Lecanophora ecristata and Parietaria debilis, and those showing lower cover on the burrow than in the area are sub-shrubs like Acantholippia seriphioides and Verbena aspera, the remaining species occur in the same proportions in both burrows and area. The mean number of plant species forming therefuge at Ñacuñán was 5.3 ± 0.28, always including the presence of all three plant layers (tree, shrub and herb layers). At El Leoncito only one shrub species afforded cover to the burrows, namely L. nitida.
With respect to the design of galleries, theirtilt angle was significantly higher at Ñacuñán than at El Leoncito (Table 2; F=36.77; p<0.0001; df=1; N=46), and in no case did they show differences throughout the year (F=0.25; p=0.7826; df=2; N=46), or between orientations of burrow holes (F=0.77; p=0.6139; df=7; N=46). The depth of galleries was significantly greater at Ñacuñán compared to El Leoncito (Table 2; F=20.46; p=0.0001; df=1; N=46). No differences in depth were found between orientations of burrow holes for either site (F=1.72; p=0.1021; df=7; N=46). Height of holes was equal for both sites (Table 2). At Ñacuñán, midday sunbeams arrive in winter at an angle of 32º 58' coming into the galleries up to 48.86 cm; whereas in summer midday sunbeams do not enter the burrows directly (78º 58') because of their inclination (23º), penetrating only 5.8 cm into them (Fig. 1). At El Leoncito, midday sunbeams in winter fall at an angle of 35º 13', reaching a depth of 27.74 cm into the gallery that has a tilt angle of 18º; at summer midday hours the sunbeams would arrive at an angle of 81º 13' and would penetrate up to 4.38 cm into the gallery.
There were differences in the orientation of active burrow holes between the sites sampled (Watson-Williams test, F= 157.21; p<0.0001;N=46). At Ñacuñán, the orientation distribution of active burrow holes is not homogeneous; holes are most frequently easterly-oriented (mean angle of orientation 88º 01'± 8º 15'; Rayleigh test of uniformity p<0.0001; N=31; Table 2). At El Leoncito, active burrow holes are not homogeneously distributed either, most of them are oriented toward the northwest (mean angle 33º 33'± 10º 37'; Rayleigh test of uniformity p<0.0001; N=15; Table 2).
At El Leoncito, temperature within the burrows differs from the soil temperature at midday and in the afternoon (F=3.56; p=0.0024; df=6; N=46; Fig. 2B), and only at midday atÑacuñán (F=3.13; p=0.0065; df=6; N=46; Fig. 2A).
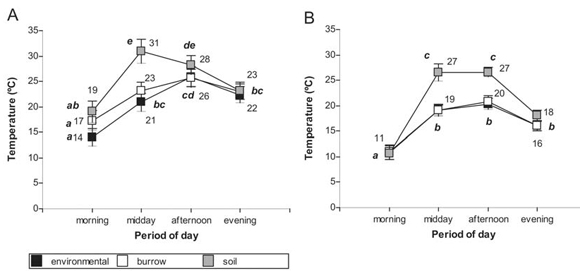
Fig. 2. Mean temperature of environment, soil and burrow galleries at Ñacuñán (A) and El Leoncito (B) along the day; a, b, c, d, e indicate significant differences among the different temperatures (Tukey test; p<= 0,05).
DISCUSSION
External factors would be influencing the structure and microclimate of burrows used by the cavy Microcavia australis. Among the biotic factors that might affect burrow structure would be the risk of predation. At Ñacuñán, the number of holes per satellite and main burrow was significantly higher than at El Leoncito. Satellite burrows were clearly distinguished from main burrows because they presented atÑacuñán 2-3 holes per burrow and were one third of the area covered by main burrows; and because they were not stably occupied by cavies. At El Leoncito satellite burrows had only one hole. At any rate they would have the same function on both sites, a refuge from predators. The highest predation risk occursat Ñacuñán, and comes from raptors, as these predators were recorded from morning to afternoon. Mammalian carnivores, instead, would be overlapping in the evening, and records of these predators were few (Taraborelli et al., in revison). Only Galactis cuja (Mustelidae, Carnivore) may be able to penetrate the burrows because these carnivores have an elongated body, as described by Taraborelli (2006)for Ñacuñán. The higher number and development of main and satellite burrows at Ñacuñán is likely related to the higher risk of predation by raptors that overlap during the period of activity of cavies, as well as to the risk of ferret attacks (Taraborelli, 2006). Predation risk would be related to vegetation structure.At Ñacuñán, the individuals displayed their behavioral patterns in the burrow area, beneath the cover provided by trees, shrubs and herbaceous plants, where shrubs and trees would afford vertical protection from raptors, but herbaceous plants would obstruct visual detection of terrestrial mammalian predators and of the shadow of raptors on the ground (Taraborelli et al., 2008). At El Leoncito, the shrub Larrea nitida is the only species present in the burrows, reaching a much higher percentage than in the whole environment (99.9%to versus 24.7%). At Ñacuñán all three plant layers (tree, shrub and herb layers) are always present, with an average number of 5-9 plant species forming the refuge. Taraborelli et al. (2008) described that cavies responded to a threat by fleeing toward the burrow at greater speed, they reacted at a lower distance from predators, and the latency time until the first antipredator response was longer than in individuals from El Leoncito. The vision of cavies would be more impaired by shrubs andherbaceous plants at Ñacuñán (Taraborelli et al., 2008). Contreras and Roig (1979) have reported that sometimes the antipredator response of M. australis was to take refuge in the nearest satellite burrows rather than in their own burrow systems. When confronted with the fake predators, at both sites the cavies fled towards the burrow and/or hid in the galleries (Taraborelli et al., 2008). Ebensperger and Bozinovic (2000a) suggested that a higher number of entrances would be important for animals to hide more quickly from potential predators. Satellite burrows of Lagostomus maximus and Marmota flaviventris could also be distinguished from main burrows by their location close to the foraging area, where animals can hide when threatened (Armitage, 1988; Branch et al., 1994). Main burrows of M. australis were inhabited by a social group and used continually during the night for resting, napping during the day, parturition and litter rearing, and group nesting (social thermoregulation; Ebensperger et al., 2006; Taraborelli and Moreno, accepted). Use of the refuge would increase survival of the individuals in the presence of a predator, and if the refuge were also the nesting site it would directly increase the fitness of rodents (Kramer and Bonenfant, 1997; Sundell and Ylönen, 2004).
Burrow architecture could be affected by some abiotic factors such as air and soil temperature, surface-wind velocity, and sunlight. Burrows would provide a stable microclimate for the cavies. On both sites burrows would function as a shelter from the environment, as temperature within the burrows is lower than soil temperature at the hottest time of the day(6 ºC at El Leoncito and 10 ºC at Ñacuñán). Soil temperature would directly affect the activity of cavies, more than does environment temperature. In other rodents, both fossorial and semifossorial, such as Pappogeomys castanops, Octodon degus, Meriones crassus and Tympanoctomys barrerae, burrows would also be used to avoid extreme temperatures and predators; moreover, air moisture in the burrows is higher than environmental moisture (Hickman, 1977; Ebensperger and Bozinovic, 2000b; Ebensperger, 2001; Shenbrot et al., 2002; Torres et al., 2003). Temperature stability inside the burrows could be due to the orientation of active holes, which are easterly oriented at Ñacuñán, thus avoiding winds from the S and SE (Estrella et al., 2001), that would diminish the inner temperature of burrows. The holes are northerly oriented at El Leoncito, receiving the dry and warm winds from the NW (Bracco and Contreras, 2000) that would maintain the inner temperature of burrows, softening the temperature range in the open. The sun would also have an effect on these orientations because in the morning hours its rays fall directly from the E and N, causing temperatures within burrows not to fall below the environment temperature. In this sense, El Leoncito is a place with harsher climate, strong temperature ranges for its being at 2484 m a.s.l., with a longer period of low temperatures that extends from autumn to spring inclusive (Taraborelli, 2006). Therefore, El Leoncito may be a more stressful place during wintercompared to Ñacuñán. In the burrows of Tympanoctomys barrerae, distribution of holes is also positively influenced by the incidence of the sun from the northern sector, with a higher number of holes located towards the N, NE and NW, and negatively affected by the cold southern winds, there being less holes with S and SE orientation (Torres et al., 2003). In the burrows of Spalacopus cyanus, holes are also oriented towards the NW and W, and none towards the S (Reig, 1970). Due to the tilt angle of burrow galleries on both sites, the sun does not reach higher depth into the galleries at midday in the coldest season (winter) than in the warmest season (summer). The sun enters some 6 to 9 times deeper into the galleries in winter, which would help maintain the inner temperature of burrows in the coldest season. Another burrowing species (T. barrerae) seems to present the same pattern of direct incidence of the sun into the tunnels in winter; these tunnels have a tilt angle of 19º-20º (Torres et al., 2003).
External factors, including abiotic factors such as wind, temperature, and sunlight during summer and winter as well as biotic factors, like predation risk and type of plant patches over the burrow, would influence the structure and microclimate of the burrows of M. australis. Burrows would also provide a refuge from predators. Another factor that could influence burrow structure is the productivity of the environment, but this would require further study.
ACKNOWLEDGMENTS
This study was partially financed by CONICET, PICT Nº 03281 and PIP 02884. The authors wish to express their thanks in the first place to A. Srur, P. Moreno, park ranger M. Martinez, M. C. González, M. F. Carballido and V. Bauni for their cooperation in the field. To N. Horak for translating the manuscript into English. To Dr. R. Ojeda and M. Martella for the literature provided and the corrections. To Dr. E. Martínez Carretero for identification of the vegetation.
LITERATURE CITED
1. ABRAHAM EM. 2001. Geomorfología y Suelos. Pp. 17-19, in: El Desierto del Monte: La Reserva de Biosfera de Ñacuñán (S Claver and S Roig-Juñent, eds.). IADIZA-UNESCO-MaB. [ Links ]
2. ARMITAGE KB. 1988. Resources and social organization of ground-dwelling squirrels. Pp. 131-155, in: The Ecology of social behavior (CN Slobodchikoff, ed.). Academic Press, Inc. Horcourt Brace Jovanovich, Publishers. San Diego, California. [ Links ]
3. BATSCHELET E. 1981. Circular Statistics in Biology. Academic Press, London. [ Links ]
4. BAUMGARDNER GD. 1991. Dipodomys compactus. Mammalian Species 369:1-4. [ Links ]
5. BEST TL. 1988. Dipodomys spectabilis. Mammalian Species 311:1-10 [ Links ]
6. BRACCO A and VH CONTRERAS. 2000. Caracterización Geológica. Pp. 3-11, in: Relevamiento de los recursos Naturales de la Reserva Estricta El Leoncito. Instituto y Museo de Cs. Naturales-UNSJ, Secciones de Geología y Biología. [ Links ]
7. BRANCH LC, D VILLARREAL, A SOSA, M PESSINO, M MACHICOTE, P LERNER, P BORRAZ, M URIOSTE, and JL HIERRO. 1994. Estructura de las colonias de vizcacha y problemas asociados con la estimación de densidad poblacional en base a la actividad de las vizcacheras. Argentina. Mastozoología Neotropical 1:135-142. [ Links ]
8. CABRERA AL. 1976. Regiones Fitogeográficas Argentinas. Buenos Aires, ACME. [ Links ]
9. CAMPOS CM. 1997. Utilización de recursos alimentarios por mamíferos medianos y pequeños del desierto del Monte. Doctoral Thesis in Biological Sciences, Universidad Nacional de Córdoba, Argentina. [ Links ]
10. CONTRERAS JR and VG ROIG. 1979. Observaciones sobre la organización social, ecología y estructura de los habitáculos de Microcavia australis en Ñacuñán, Provincia de Mendoza. Ecosur 10:191-199. [ Links ]
11. EBENSPERGER LA. 2001. A review of the evolutionary causes of rodent group-living. Acta Theriologica 46:155-144. [ Links ]
12. EBENSPERGER LA and F BOZINOVIC. 2000a. Communal burrowing in the hystricognath rodent, Octodon degus: a benefit of sociality? Behaviour, Ecology and Sociobiology 47:265-369. [ Links ]
13. EBENSPERGER LA and F BOZINOVIC. 2000b. Energetics and burrowing behaviour in the semifossorial degu Octodon degus (Rodentia: Octodontidae). Journal of Zoology 252:179-186. [ Links ]
14. EBENSPERGER LA, P TARABORELLI, SM GIANNONI, MJ HURTADO, C LEÓN, and F BOZINOVIC. 2006. Nest and space use in a highland population of the lesser cavy, Microcavia australis: implications for its social organization. Journal of Mammalogy 87:834-840. [ Links ]
15. ESTRELLA H, J BOSHOVEN, and M TOGNELLI. 2001. Características del clima regional y de lareserva de Ñacuñán. Pp. 25-34, in: El Desierto del Monte: La Reserva de Biosfera de Ñacuñán (S Claver and S Roig-Juñent, eds.). IADIZA- UNESCO-MaB. [ Links ]
16. GHOBRIAL LL and TA NOUR. 1975. The physiological adaptations of desert rodents. Pp. 413-444, in: Rodents in desert environments (I Prakash and PK Ghosh, eds.). Monographiae Biologicae, The Hague, Netherlands. [ Links ]
17. HAYS RL, C SUMMERS, and W SEITZ. 1981. Estimating Wildlife habitat variables. Fish and Wildlife Service, U. S. Department of the interior, Washington. [ Links ]
18. HICKMAN GC. 1977. Burrow system structure of Pappogeomys castanops (Geomyidae) in Lubbock Country, Texas. The American Midland Naturalist 97:50-58. [ Links ]
19. HOOGLAND JL. 1995. The Black-tailed Prairie dog: social life of a burrowing mammal. The University of Chicago Press, Chicago and London. [ Links ]
20. KAY FR and WG WHITFORD. 1978. The burrow environment of the bannertailed kangaroo rat, Dipodomys spectabilis, in south-central New Mexico. American Midland Naturalist 99:270-279. [ Links ]
21. KING JA. 1984. Historical ventilations on a prairie dog town. Pp. 447-456, in: The biology of ground-dwelling squirrels: annual cycles, behavioral ecology, and sociability (JO Murie and GR Michener, eds.). Lincoln, University of Nebraska Press. [ Links ]
22. KINLAW A. 1999. A review of burrowing by semi-fossorial vertebrates in arid environments. Journal of Arid Environments 41:127-145. [ Links ]
23. KRAMER DL and M BONENFANT. 1997. Direction of predator approach and the decision to flee to a refuge. Animal Behaviour 54:289-295. [ Links ]
24. KUCHERUK VV. 1983. Mammal burrows - their structure, topology and use. Pp. 5-54, in: Fauna and Ecology of Rodents. [ Links ]
25. LE HOUÉROU HN. 1999. Estudios e investigaciones Ecológicas de las Zonas Áridas y semiáridas de Argentina. IADIZA-CRICYT, Mendoza. [ Links ]
26. MÁRQUEZ J. 1999. Las áreas protegidas de la Provincia de San Juan. Multequina 8: 1-10. [ Links ]
27. MÁRQUEZ J and AD DALMASSO. 2003. Las comunidades vegetales de los ambientes húmedos del Parque Nacional El Leoncito, San Juan, Argentina. Multequina 12:55-67. [ Links ]
28. MÁRQUEZ J, G PASTRÁN, and G ORTIZ. 2000. Relevamiento de vegetación. Pp. 12-26, in: Relevamiento de los recursos Naturales de la Reserva Estricta El Leoncito. Instituto y Museo de Cs. Naturales-UNSJ, Secciones de Geología y Biología. [ Links ]
29. MATTEUCCI SD and A COLMA. 1982. Metodología para el estudio de la vegetación. Secretaría General de la Organización de los Estados Americanos (OEA). Programa Regional de Desarrollo Científico y Tecnológico. Monografía No 22. Serie Biología. [ Links ]
30. NOWAK R and JL PARADISO. 1983. Walker's Mammals of the world. Baltimore, Johns Hopkins Press. Second volume. [ Links ]
31. OJEDA RA, CM CAMPOS, JM GONNET, CE BORGHI, and V ROIG. 1998. The MaB Reserve of Ñacuñán, Argentina: its role in understanding the Monte Desert biome. Journal of Arid Environments 39:299-313. [ Links ]
32. PARERA A. 2002. Los mamíferos de la Argentina y la región austral de Sudamérica. Editorial El Ateneo, Buenos Aires, Argentina. [ Links ]
33. REDFORD KH and JF EISENBERG. 1992. Mammals of the Neotropics: the southern cone. Chicago, University of Chicago Press. [ Links ]
34. REICHMAN OJ and SC SMITH. 1987. Burrows and burrowing behavior by mammals. Current Mammalogy 2:197-244. [ Links ]
35. REIG OA. 1970. Ecological notes on the fossorial Octodont rodent Spalacopus cyanus (Molina). Journal of Mammalogy 51:592-601. [ Links ]
36. ROIG FA. 1971. Flora y vegetación de la reserva forestal de Ñacuñán. Mendoza, IADIZA. [ Links ]
37. ROOD J. 1967. Observaciones sobre la ecología y el comportamiento de los Caviinae de la Argentina (Mammalia, Rodentia). Zoología Platense 1:1-6. [ Links ]
38. ROOD JP. 1972. Ecological and behavioural comparisons of three genera of Argentine cavies. Animal Behaviour Monograph 5:1-83. [ Links ]
39. SHENBROT G, B KRASNOV, I KHOKHLOVA, T DEMIDOVA, and L FIELDE. 2002. Habitat-dependent differences in architecture and microclimate of the burrows of Sundevall´s jird (Meriones crassus) (Rodentia: Gerbillinae) in the Neveg Desert, Israel. Journal of Arid Environments 51:265-279. [ Links ]
40. SUNDELL J and H YLÖNEN. 2004. Behaviour and choice of refuge by voles under predation risk. Behaviour Ecology and Sociobiology 56:263-269. [ Links ]
41. TANQUILEVICH RF. 1971. Los suelos de la Reserva Ecológica de Ñacuñán. Deserta 2:131-206. [ Links ]
42. TARABORELLI P. 2006. Factores que afectan en la sociabilidad de Microcavia australis (Rodentia, Caviidae). Doctoral Thesis in Biological Sciences, Facultad de Ciencias Exactas, Físicas y Naturales, Universidad Nacional de Córdoba, Argentina. [ Links ]
43. TARABORELLI P, P MORENO, AM SRUR, AJ SANDOBAL, MG MARTINEZ, and S GIANNONI. 2008. Different antipredator responses by Microcavia australis (Rodentia, Hystricognate, Caviidae) under predation risk. Behaviour 145:829-842 [ Links ]
44. TARABORELLI P and P MORENO. Accepted. Composition of social groups and mating system of Microcavia australis (Rodentia, Caviidae). Mammalian Biology. [ Links ]
45. TARABORELLI P, P MORENO, AM SRUR, AJ SANDOBAL, MG MARTINEZ, MF CARBALLIDO, and SM GIANNONI. In revision. Influence of predation risk and plant structure on vigilance and intermittent locomotion in Microcavia australis (Rodentia, Caviidae) Journal of Ethology. [ Links ]
46. TOGNELLI MF, CM CAMPOS, RA OJEDA, and VG ROIG. 1995. Is Microcavia australis (Rodentia: Caviidae) associated with a particular plant structure in the Monte desert of Argentina? Mammalia 59:327-333. [ Links ]
47. TOGNELLI MF, CM CAMPOS, and RA OJEDA. 2001. Microcavia australis. Mammalian Species. 648:1-4. [ Links ]
48. TORRES MR, CE BORGHI, SM GIANNONI, and A PATÍN. 2003. Portal orientation and architecture of burrows in Tympanoctomys barrerae (Rodentia, Octodontidae). Journal of Mammalogy 84:541-546 [ Links ]
Recibido 25 septiembre 2008.
Aceptado 02 diciembre 2008.
Editor asociado: F Bozinovick














