Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Mastozoología neotropical
versión impresa ISSN 0327-9383versión On-line ISSN 1666-0536
Mastozool. neotrop. v.16 n.2 Mendoza jul./dic. 2009
ARTÍCULOS Y NOTAS
On the diagnostic characters, ecogeographic distribution, and phylogenetic relationships of Gracilinanus emiliae (Didelphimorphia: Didelphidae: Thylamyini)
Robert S. Voss1, David W. Fleck2, and Sharon A. Jansa3
1 Department of Mammalogy, American Museum of Natural History, New York, NY 10024, U.S.A. [Corresponding author: <voss@amnh.org>].
2 Department of Anthropology, American Museum of Natural History, New York, NY 10024, U.S.A.
3 Bell Museum of Natural History and Department of Ecology, Evolution, and Behavior, University of Minnesota, St. Paul, MN 55108, U.S.A.
ABSTRACT: Gracilinanus emiliae is a species of gracile mouse opossum that is currently known from fewer than a dozen specimens. The material we examined exhibits a distinctive suite of external and craniodental characters that are unlike those of any other congeneric species. We review all published records for G. emiliae, several of which are based on misidentifications or lost material, and we report a new specimen from northeastern Peru, the first to be recorded from that country, and the first to be unequivocally associated with primary lowland rain forest. The available distributional data suggest that G. emiliae (or a complex of cryptic taxa with the same morphological characters) is very widely distributed in Amazonia and perhaps also in Venezuelan coastal rain forests. Phylogenetic analyses of morphological and molecular data suggest that G. emiliae is the sister taxon to other analyzed species of Gracilinanus, the biogeographic history of which is likely to be complex.
RESUMEN: Sobre los caracteres diagnósticos, distribución ecogeográfica y relaciones filogenéticas de Gracilinanus emiliae (Didelphimorphia: Didelphidae: Thylamyini). Gracilinanus emiliae es una especie de marsupial conocida a partir de menos de una docena de especímenes. El material examinado presenta rasgos externos y craniodentales distintivos, que difieren de los correspondientes a las restantes especies congenéricas. Revisamos todos los registros publicados de G. emiliae, algunos de los cuales son basados en identificaciones erróneas o material actualmente perdido, y reportamos un nuevo espécimen del noroeste de Perú, el primero registrado para este país, y el primero asociado inequívocamente con bosque húmedo primario de tierra baja. Los datos geográficos disponibles sugieren que G. emiliae (o un complejo de especies crípticas) tiene una distribución muy amplia en Amazonía y quizás también en los bosques húmedos de la costa de Venezuela. Los análisis filogenéticos de caracteres morfológicos y moleculares sugieren que G. emiliae es el grupo hermano de las restantes especies de Gracilinanus. La historia biogeográfica de este género es probablamente compleja.
Key words. Amazonia; Mammal; Marsupial; Neotropical; Rainforest.
Palabras claves. Amazonía; Mamífero; Marsupial; Neotropical; Selva.
INTRODUCTION
Gracilianus emiliae was originally described by Thomas (1909) as a species of the didelphid marsupial genus Marmosa. Thomas's material consisted of a single specimen collected by Dr. Emilia Snethlage near Belém in the northeastern Brazilian state of Pará, and no additional material was reported by Tate (1933) when he classified emiliae as a member of the "Microtarsus Group" of Marmosa in his monographic revision of the genus. Cabrera (1958) subsequently assigned emiliae (along with many other species in Tate's Microtarsus Group) to the subgenus Thylamys, but Kirsch and Calaby (1977) transferred it without comment to the subgenus Marmosa. Gardner and Creighton (1989) placed emiliae in their new genus Gracilinanus, where it has remained to date (Hershkovitz, 1992; Gardner, 2005; Voss et al., 2005; Creighton and Gardner, 2008).
Gracilinanus emiliae is biogeographically unusual because it is one of only two species of Gracilinanus that have been reported from Amazonian lowland rainforests. Although a few specimens resembling G. agilis have been reported from lowland Amazonian rainforest in SE Peru (e.g., by Solari et al., 2006), most species of Gracilinanus occur in other biomes, including montane forest (in the Andes and the Venezuelan coastal cordilleras), Atlantic rain forest (in eastern Brazil, northeastern Argentina, and eastern Paraguay), and the Cerrado (see maps in Hershkovitz, 1992 [fig. 1]; Brown, 2004 [figs. 16-23]). Unfortunately, some Amazonian records of G. emiliae are based on misidentifications (Voss et al., 2001), and it has therefore been suggested that the genus is absent from the central part of the Amazon Basin (Costa et al., 2003; Patton and Costa, 2003). Because most records of G. emiliae that can be confirmed on the basis of examined specimens (mapped by Voss et al., 2001: fig. 10) are from localities adjacent to savanna landscapes, it is possible that this species only occurs along the rainforest edge and not in mature, closed-canopy habitats.
A second problem concerns the phylogenetic relationships of Gracilinanus emiliae, which is notably divergent from other congeneric species in several morphological traits (Voss et al., 2001, 2005). Although the monophyly of Gracilinanus was weakly supported in one recent analysis of sequence data from the nuclear IRBP gene (Voss et al., 2005), subsequent analyses incorporating sequence data from the nuclear DMP1 locus (Jansa and Voss, 2005; Jansa et al., 2006) suggested that emiliae may be more closely related to Cryptonanus than to other species of Gracilinanus. In addition to being a biogeographic outlier, emiliae thus appears to be evolutionarily divergent as well.
This paper reviews the diagnostic characters of Gracilinanus emiliae, evaluates ecogeographic information associated with examined specimens, and reports a new specimen from Peru that provides unambiguous evidence of habitat association. We also report parsimony analyses of sequence data from five nuclear loci that definitively resolve the relationships of G. emiliae to other sequenced species of Gracilinanus and Cryptonanus. Together, these results substantially clarify the biogeographic and evolutionary significance of this elusive and interesting marsupial.
MATERIALS AND METHODS
The morphological specimens we examined and others mentioned below are preserved in the following collections (listed in order of their standard institutional abbreviations): AMNH, American Museum of Natural History (New York); BMNH, Natural History Museum (London); FMNH, Field Museum (Chicago); MNRJ, Museu Nacional (Rio de Janeiro); MUSM, Museo de Historia Natural, Universidad Nacional Mayor de San Marcos (Lima); ROM, Royal Ontario Museum (Toronto); and USNM, National Museum of Natural History (Washington, D.C.).
We transcribed total length (nose to fleshy tail-tip, TL) and length of tail (basal flexure to fleshy tip, LT) from the labels of specimens obtained by American collectors (who use the field measurement protocol described by Hall, 1981), and we computed head-and-body length (HBL) by subtracting LT from TL. Alternatively, we transcribed headand-body length and tail length directly from the labels of specimens obtained by British collectors (who follow a different field measurement protocol). We also transcribed length of hind foot (heel to tip of longest claw, HF), length of ear (from notch, Ear), and weight from specimen labels or field notes, but we sometimes remeasured HF on fluid-preserved specimens to check the accuracy of values recorded by the collector, and we used our values whenever large discrepancies were found. All external measurements are reported to the nearest millimeter (mm), and all weights are reported to the nearest gram (g).
Craniodental measurements were taken with digital calipers and recorded to the nearest 0.01 mm, but values reported herein are rounded to the nearest 0.1 mm. The following dimensions were recorded as illustrated by Voss et al. (2004: fig. 2): Condylo-Basal Length (CBL), measured from the occipital condyles to the anteriormost point of the premaxillae; Nasal Breadth (NB), measured between the triple-point sutures of the nasal, frontal, and maxillary bones on each side; Least Interorbital Breadth (LIB), measured at the narrowest point across the frontals between the orbits, even when the postorbital constriction (between the temporal fossae) is narrower; Zygomatic Breadth (ZB), measured at the widest point across both zygomatic arches; Palatal Length (PL), measured from the anteriormost point of the premaxillae to the postpalatine torus, including the postpalatine spine (if present); Palatal Breadth (PB), measured across the labial margins of the M4 crowns, at or near the stylar A position; Maxillary Toothrow Length (MTR), measured from the anterior margin of C1 to the posterior margin of M4; Length of Molars (LM), measured from the anteriormost labial margin of M1 to the posteriormost point on M4; Length of M1-M3 (M1-M3), measured from the anteriormost labial margin of M1 to the posteriormost point on M3; Width of M4 (WM4), measured from the labial margin of the crown at or near the stylar A position to the lingual apex of the protocone.
Following Voss et al. (2001), a specimen was judged to be juvenile if dP3 is still in place; subadult if dP3 has been shed but P3 is still incompletely erupted; and adult if the permanent dentition is complete. Qualitative character variation is described herein using terminology that is explained or referenced by Voss and Jansa (2003, 2009). An exception (not defined by those authors) is the prefix "self-" as used in combination with descriptors of ventral pelage color, such as self-white or self-cream (Tate, 1933). This usage applies to hairs that have the same coloration from base to tip, as opposed to hairs that are basally gray and distally white, cream, or buffy.
The phylogenetic data we analyzed include 129 characters that were scored from morphological comparisons of skins, skulls, and fluid-preserved material representing 43 didelphid species in 17 genera. In order to root our analyses, we also scored character data from seven species of nondidelphid marsupials that served as outgroups in our analyses: one microbiotheriid (Dromiciops gliroides), two caenolestids (Caenolestes fuliginosus, Rhyncholestes raphanurus), two dasyurids (Murexia longicaudatus, Sminthopsis crassicaudata), and two peramelids (Echymipera kalubu, Perameles gunni). The nonmolecular characters that we scored for these 50 terminal taxa (defined by Voss and Jansa, 2009: appendix 3) include 39 from the integument (rhinarium, pelage, manus, pes, mammae, pouch, cloaca, tail, etc), 49 from the cranium and mandible (foramina, processes, sutures, etc), and 37 from the dentition (occlusal morphology, eruption sequences); additionally we scored four characters representing chromosomal fission/fusion events from published karyotypic studies.
We obtained nuclear gene sequences from a single tissue sample of Gracilinanus emiliae (MUSM 15292), and from tissue samples representing all of the other ingroup and outgroup taxa from which morphological character data were scored, using procedures for DNA extraction, amplification, and sequencing explained by Voss and Jansa (2009). The molecular data analyzed herein consist of 7320 aligned base pairs (bp) from five unlinked protein-coding genes: Breast Cancer Activating 1 Gene (BRCA1 exon 11; 2163 bp), Dentin Matrix Protein 1 (DMP1 exon 6; 1176 bp), Interphotoreceptor Retinoid Binding Protein (IRBP exon 1; 1158 bp), Recombination Activating 1 Gene (RAG1; 2790 bp), and von Willebrand Factor (vWF exon 28; 963 bp). However, we did not analyze RAG1 third codon positions (which are afflicted by severe base-compositional heterogeneity; Gruber et al., 2007), nor did we analyze DMP1 sequences from nondidelphid outgroups (which cannot be unambiguously aligned with opossum sequences; Jansa et al., 2006).
We analyzed these data (morphological, karyotypic, and molecular) simultaneously using maximum parsimony (MP) as implemented by PAUP* ver. 4.0b10 (Swofford, 1998). All molecular characters were treated as unordered and equally weighted, and the heuristic tree search employed 5000 replicates of random stepwise taxon addition followed by tree bisection-reconnection (TBR) branch swapping. We assessed nodal support using nonparametric bootstrapping (Felsenstein, 1985) as implemented by PAUP* (from 1000 pseudoreplicated datasets, each heuristically analyzed with 10 random-addition replicates and TBR branch swapping), and by computing Bremer support values (Bremer, 1994) as implemented by TreeRot (Sorenson, 1996).
RESULTS
Diagnostic characters and phenotypic variability
Gracilinanus emiliae can be unambiguously distinguished from other congeneric species by a unique combination of morphometric and qualitative characters (Table 1). Externally, G. emiliae is distinctively long-tailed, with a ratio of tail to head-and-body length that averages 1.80, much larger than any value we have computed from other congeners. Additionally, G. emiliae is the only currently recognized species of Gracilinanus with self-white underparts, all other species having gray-based buffy or brownish ventral fur. Mature adult specimens of G. emiliae have distinctly beaded supraorbital margins but no postorbital processes (Fig. 1). Whereas other congeners have large and distinct maxillary vacuities, these palatal openings are very small or absent in G. emiliae. The unworn upper canine (C1) of G. emiliae has a posterior accessory cusp that is absent in other species of Gracilinanus, and the molars of G. emiliae are distinctively small.
Table 1
Selected diagnostic characters of Gracilinanus species. Tabulated traits are those of adult specimens (juveniles and subadults may not exhibit all features).

Fig. 1. Dorsal, ventral, and lateral views of the skull and mandible of Gracilinanus emiliae. This drawing (modified from Voss et al., 2001: figs. 11, 12) is a composite based on several specimens, none of which is anatomically complete (AMNH 203363, 267006; ROM 35466).
External and craniodental measurements of adult and subadult specimens of Gracilinanus emiliae exhibit remarkably little intraspecific variation (Table 2). Close scrutiny of these specimens, including side-by-side comparisons of skulls from the Guianas, Colombia, Brazil, and Peru did not reveal any conspicuous morphological differences among them that could not be attributed to age or individual variation. In short, there seems to be no compelling phenotypic evidence that more than a single species is represented in the material at hand. However, given the broad distribution of this taxon, it is possible that two or more morphologically cryptic but genetically distinct forms are present. At the moment, we are unable to evaluate this possibility.
Table 2
Provenance, age, sex, measurements (mm) and weights (g) of subadult and adult specimens of Gracilinanus emiliae. Notes: a Type of Marmosa emiliae Thomas. b Type of Gracilinanus longicaudus Hershkovitz. c Remeasured from fluid specimen. d Estimated value. e Tate's (1933: table 1) value of 5.3 mm for the interorbital breadth of this specimen (repeated in Hershkovitz, 1992: table 5) is erroneous. f Value from Hershkovitz (1992: table 5); the right zygomatic arch of this specimen is now broken.

Ecogeographic distribution
Eight localities from which we have examined specimens that exhibit the diagnostic characteristics of Gracilinanus emiliae as described above are mapped in Fig. 2. Most other localities from which G. emiliae has previously been reported by authors are based on misidentified or lost specimens. Although Voss et al. (2001: 29-30) provided correct identifications for some of these and emphasized the dubious validity of others, their results were ignored by Brown (2004: fig. 20), whose distribution map for G. emiliae included five erroneous or dubious records. Among the invalid records mapped by Brown are two Brazilian localities originally reported by Patterson (1992) based on juvenile specimens of Marmosa lepida (see Voss et al., 2001: 2930). A third invalid record is the type locality of "Marmosa" agricolai, Moojen, 1943, a taxon that Gardner and Creighton (1989) considered a junior synonym of G. emiliae but which exhibits many inconsistent morphological characters (Voss et al., 2001: 29). Recently, Voss et al. (2005) referred agricolai to the genus Cryptonanus.
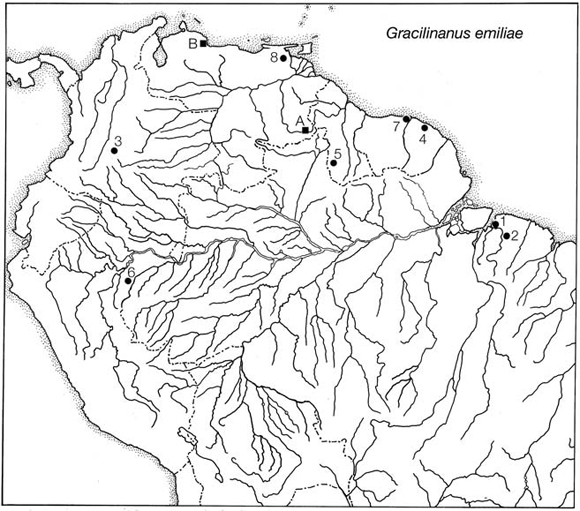
Fig. 2. Geographic distribution of collection localities based on specimens examined by the authors (.) and others reported in the literature (?). Specimens that we examined are from: 1. Brazil, Pará, "Para" (= Belém, type locality; 1o27'S, 48o29'W, 10 m); 2. Brazil, Pará, Capim (1o41'S, 47o47'W, 25 m); 3. Colombia, Meta, Los Micos (3o17'N, 73o53'W, 450 m); 4. French Guiana, Paracou (5o17'N, 52o55'W, 25 m); 5. Guyana, Upper Takutu-Upper Essequibo, 12 km E Dadanawa (ca. 2o50'N, 59o31'W, ca. 150 m); 6. Peru, Loreto, Nuevo San Juan (5o15'S, 73o10'W, 150 m); 7. Surinam, Marowijne, Langamankondre (5o43'N, 54o01'W, sea level); and 8. Venezuela, Monagas, Hato Mata de Bejuco (ca. 9o19'N, 62o56'W, 18 m). Localities reported by Linares (1997, 1998) based on specimens that we have not seen are: A. Venezuela, Bolívar, San Ignacio de Yuruaní (5o01'N, 61o09'W, 850 m); and B. Venezuela, Falcón, 19 km NW Urama (10o34'N, 68o21'W, 25 m).
Another erroneous locality for Gracilinanus emiliae mapped by Brown (2004) was originally reported by Ávila-Pires (1964). Although Ávila-Pires did not provide a museum catalog number for the specimen in question and did not say where it came from or who collected it, the only possible match appears to be MNRJ 20918 (originally INPA 1045), which was collected at or near Manaus by M.C. Mello in 1957 (J.A. de Oliveira, pers. comm.). This specimen was recently reidentified by Astúa (2006) as Hyladelphys kalinowskii. A fifth locality for Gracilinanus emiliae mapped by Brown (2004) that is almost certainly erroneous is Santarem. This record is based on a specimen in the British Museum that Thomas (1888: 349) identified as "Didelphys pusilla" and that Tate (1933) identified as "Marmosa microtarsus". Neither Thomas nor Tate provided a catalog number, but handwritten annotations in Thomas's personal copy of his 1888 monograph indicate that it was BMNH 54.11.14.3, corresponding to material originally purchased from Stevens—a commercial dealer in natural history specimens—and apparently now lost (P. Jenkins, pers. comm.). It is not clear why Hershkovitz (1992) identified this specimen as emiliae sight unseen, given that Tate (1933) had not done so when he saw it in London with the holotype of emiliae at hand.
We are currently unable to evaluate two Venezuelan records of Gracilinanus emiliae reported by Linares (1997, 1998). Although his morphometric data are largely consistent with that identification (we assume that his measurement "m2-4" corresponds to m2-5 of Hershkovitz [1992], which is equivalent to LM of the present study), some diagnostic traits were omitted from his description, and no specimen numbers were provided. Only one of his records falls slightly outside the known geographic range of G. emiliae as documented by specimens that we have personally examined.
Our new material, which represents a range extension of about 950 km, consists of a single adult female specimen (MUSM 15292) collected on 7 September 1999 near Nuevo San Juan, a Matses Indian village on the Río Gálvez, a left-bank tributary of the Río Yavarí, in the Peruvian department of Loreto (Fig. 2: locality 6). This animal was shot at night by a Matses hunter who saw it perched on a branch of a small tree in the subcanopy of well-drained primary lowland rain forest. No savannas or other natural nonforest vegetation is present in the ca. 8000 km2 drainage basin of the Río Gálvez nor in any of the other adjacent lowland watersheds between the Río Ucayali and the Río Yavarí.
Phylogenetic relationships
The strict consensus of 12 equally most-parsimonious trees recovered by our combined analysis of morpholological, karyotypic, and molecular data is almost completely resolved, and most suprageneric nodes are strongly supported (Fig. 3). As in Bayesian and likelihood analyses of these data (Voss and Jansa, 2009), Gracilinanus was found to be deeply embedded within a monophyletic cluster of didelphine genera comprising the tribe Thylamyini. Although Gracilinanus and Cryptonanus were consistently recovered as sister taxa, support for this relationship is weak.
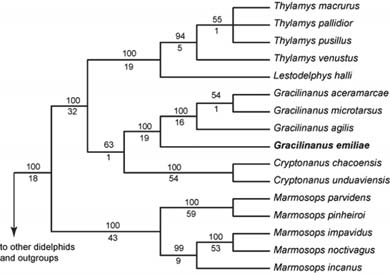
Fig. 3. Phylogenetic relationships among thylamyine opossums based on a heuristic parsimony analysis of morphological characters and DNA sequences. Bootstrap percentages are provided above each branch, decay (Bremer) support below. "Other didelphids" include 27 species belonging to the subfamilies Caluromyinae, Glironiinae, Hyladelphinae, and Didelphinae (tribes Marmosini, Metachirini, and Didelphini); "outgroups" include seven species of nondidelphid marsupials (caenolestids, Dromiciops, dasyurids, and peramelids).
Whereas previous analyses of nonmolecular (morphological + karyotypic) and molecular characters yielded conflicting and weakly supported hypotheses about the relationships of Gracilinanus emiliae to other thylamyines (Voss et al., 2005; Jansa and Voss, 2005; Jansa et al., 2006), the phylogenetic position of G. emiliae is now convincingly resolved as the sister taxon of all other Gracilinanus species in our analysis. Although G. dryas, G. marica, and at least one undescribed species remain to be analyzed phylogenetically, their morphological attributes suggest that they are more closely related to G. agilis, G. microtarsus, and G. aceramarcae than they are to G. emiliae.
DISCUSSION
The new Peruvian specimen provides incontrovertible evidence that Gracilinanus emiliae occurs in primary lowland rain forest. Several other examined specimens might also have been collected in this habitat (Table 3), but only two are accompanied by definite information about the vegetation in which they were taken. One of these (from Paracou, French Guiana; locality 4) was shot at night from a tree where it perched about 4 m above the ground in dense secondary growth next to a road through primary lowland rain forest, and the other (from 47 km E Maturín, Venezuela; locality 8) was trapped on a vine in lowland gallery forest. Lowland rain forest is the dominant vegetation formation in northeastern Pará (localities 1 and 2), and this habitat is present as riparian growth in the Rupununi savannas around Dadanawa (locality 5). Los Micos, Colombia (locality 3) is on the rainforested eastern flanks of the Serranía de la Macarena near the western margin of the Llanos. Langamankondre (locality 7) is on the west bank of the Marowijne River, in a part of Surinam where gallery forests commonly interrupt the coastal savannas. The most plausible interpretation of these data is that Gracilinanus emiliae is a lowland rainforest species that occurs in both primary and secondary formations and in gallery forests within some savanna-dominated landscapes.
Table 3
Ecological data associated with examined specimens of Gracilinanus emiliae. Numbered localities refer to those mapped in Fig. 2.

Gracilinanus emiliae is obviously hard to collect. No more than a single specimen is known from any locality, even those where intensive multi-year faunal inventory efforts have been carried out (e.g., Paracou, French Guiana; Voss et al., 2001). Apparently arboreal, it is unlikely to be captured unless a special effort is made to thoroughly sample the arboreal fauna, but weighing only 10 to 14 g (Table 2) it is probably too small to be taken in most commercially manufactured traps (e.g., those used by Malcolm, 1991). Additionally, like other small didelphids, it may be unattracted to most commonly used baits. Therefore, the absence of any specimens from central Amazonia may simply be an artifact of inadequate or inappropriate collecting efforts.
Given the geographic dispersion of collecting localities (Fig. 2) and the difficulty of collecting specimens even where the species is known to occur, it seems probable that Gracilinanus emiliae occurs throughout the Amazonian lowlands and (if Linares' [1997, 1998] specimens were correctly identified) it may also be widespread in Venezuelan coastal rainforests (sensu Voss and Emmons, 1996). If true, this would explain an otherwise puzzling gap in the distribution of the genus, various species of which inhabit almost every other forested region of tropical and subtropical South America (Table 4). No matter what phylogenetic hypothesis is supported when G. dryas, G. marica, and at least one undescribed species are included in future analyses, the biogeographic history of the genus has obviously covered most of the continental landscape at low latitudes. How the Amazonian distribution of G. emiliae fits into this puzzle remains to be determined.
Table 4
Ecogeographic distribution of Gracilinanus species. Notes: a See Voss et al. (2004: table 12). b See Costa et al. (2003). The taxonomic status of peruanus Thomas, 1931, remains to be convincingly established, but if it is a synonym of agilis Burmeister, 1854, then the range of the latter would extend into the premontane and lowland rainforests of SE Peru. We believe that the Venezuelan material identified as G. agilis by Linares (1997, 1998) is misidentified. c See Handley (1976) and Linares (1998). d This report. e See Handley (1976), Linares (1998), and Creighton and Gardner (2008). The unpublished Trinidadian record is based on AMNH 206763 from Bush Bush Forest (10º24'N, 61º03'W, near sea level; Downs et al., 1968). f See Costa et al. (2003). g Represented by three examined Colombian specimens, one from Unguía in Departamento Chocó (FMNH 69849), another from Turbaco in Departamento Bolívar (USNM 399950), and a third from San José in Departamento Valle de Cauca (AMNH 31693).

The recovered position of Gracilinanus emiliae as phylogenetically intermediate between Cryptonanus on the one hand and the clade formed by G. aceramarcae + G. agilis + G. microtarsus on the other is not altogether unexpected given the morphological distinctness of emiliae from other congeneric species. Indeed, G. emiliae could be interpreted as morphologically transitional in some characters. For example, whereas maxillary fenestrae (maxillary perforations that lie between the maxillopalatine fenestra and M1 on either side of the palate; Voss and Jansa, 2003: fig. 5) are absent in Cryptonanus and well developed in most species of Gracilinanus, they are always small and occasionally absent (unilaterally or bilaterally) in G. emiliae. Another example is the morphology of C1, which is often provided with both anterior and posterior accessory cusps in Cryptonanus (see Voss et al., 2005: fig. 3A); most species of Gracilinanus lack accessory canine cusps (Voss et al., 2005: fig. 3B), but a posterior accessory cusp is present on C1 in G. emiliae.
Although the relationships we recovered within Gracilinanus could be recognized informally (as the basis for "species groups") or formally (by naming subgenera), such actions seem premature given our still-incomplete taxon sampling and the likelihood that more species remain to be discovered or resurrected from synonymy. Plausibly, such discoveries may come from closer morphological study of available specimens, from sequence data representing hitherto unsampled nominal taxa (e.g., peruana Tate, 1931), and from new collecting efforts in landscapes where the genus is still unknown (e.g., most of the northern and central Andes). Future analyses based on additional characters and more comprehensive taxon sampling will doubtless provide a more secure basis for intrageneric classification.
ACKNOWLEDGMENTS
We thank the curators and collection support personnel at the BMNH, FMNH, MNRJ, ROM, and USNM for their hospitality when we visited their collections and for their indulgence in extending several specimen loans for unreasonable lengths of time. We are especially grateful to Paula Jenkins (at the BMNH) and João Alves de Oliveira (MNRJ) who kindly searched their collection at our request for old specimens previously misidentified as Gracilinanus emiliae. Our 1999 fieldwork in Peru was hosted by our Matses friends at Nuevo San Juan, and it was partially supported by a grant from the National Geographic Society; permits to collect and export specimens were provided by the Instituto Nacional de Recursos Naturales (INRENA). We thank Pat Wynne for her expert rendering of the cranial views and the map. Sergio Solari, Guillermo D'Elía, and an anonymous reviewer provided helpful suggestions on an earlier draft of our manuscript.
LITERATURE CITED
1. ASTÚA D. 2006. Range extension and first Brazilian record of the rare Hyladelphys kalinowskii (Hershkovitz, 1992) (Didelphimorphia, Didelphidae). Mammalia 2006:174-176. [ Links ]
2. ÁVILA-PIRES FD de. 1964. Mamíferos colecionados na região do Rio Negro (Amazonas, Brasil). Boletim do Museu Paraense Emílio Goeldi (nova ser.), Zoologia 42:1-23. [ Links ]
3. BREMER K. 1994. Branch support and tree stability. Cladistics 6:369-372. [ Links ]
4. BROWN BE. 2004. Atlas of New World marsupials. Fieldiana Zoology (new series) 102:i-viii, 1-308. [ Links ]
5. CABRERA A. 1958 ("1957"). Catálogo de los mamíferos de América del Sur [part 1]. Revista del Museo Argentino de Ciencias Naturales "Bernardino Rivadavia" (Ciencias Zoológicas) 4:1-307. [ Links ]
6. COSTA LP, YLR LEITE, and JL PATTON. 2003. Phylogeography and systematic notes on two species of gracile mouse opossums, genus Gracilinanus (Marsupialia, Didelphidae). Proceedings of the Biological Society of Washington 116:275-292. [ Links ]
7. CREIGHTON GK and AL GARDNER. 2008 ("2007"). Genus Gracilinanus Gardner and Creighton, 1989. Pp. 43-50, in: Mammals of South America, vol. 1 (AL Gardner, ed.), Chicago, University of Chicago Press. [ Links ]
8. DOWNS WG, THG AITKEN, CB WORTH, L SPENCE, and AH JONKERS. 1968. Arbovirus studies in Bush Bush Forest, Trinidad, W.I., September 1959-December 1964. American Journal of Tropical Medicine and Hygiene 17:224-236. [ Links ]
9. FELSENSTEIN J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783-791. [ Links ]
10. GARDNER AL. 2005. Order Didelphimorphia. Pp. 318, in: Mammal species of the world, 3rd ed, vol. 1 (DE Wilson and DM Reeder, eds.). Baltimore, Johns Hopkins University Press. [ Links ]
11. GARDNER AL and GK CREIGHTON. 1989. A new generic name for Tate's microtarsus group of South American mouse opossums (Marsupialia: Didelphidae). Proceedings of the Biological Society of Washington 102:3-7. [ Links ]
12. GRUBER KF, RS VOSS, and SA JANSA. 2007. Base-compositional heterogeneity in the RAG1 locus among didelphid marsupials: implications for phylogenetic inference and the evolution of GC content. Systematic Biology 56:83-96. [ Links ]
13. HALL ER. 1981. The mammals of North America (2 vols.). New York, John Wiley & Sons. [ Links ]
14. HANDLEY CO Jr. 1976. Mammals of the Smithsonian Venezuelan Project. Brigham Young University Science Bulletin (Biol. Ser.) 20(5):[i-iv], 1-89, map. [ Links ]
15. HERSHKOVITZ P. 1992. The South American gracile mouse opossums, genus Gracilinanus Gardner and Creighton, 1989 Marmosidae, Marsupialia): a taxonomic review with notes on general morphology and relationships. Fieldiana Zoology (new series) 39:1-56. [ Links ]
16. JANSA SA and RS VOSS. 2005. Phylogenetic relationships of the marsupial genus Hyladelphys based on nuclear gene sequences and morphology. Journal of Mammalogy 86:853-865. [ Links ]
17. JANSA SA, JF FORSMAN, and RS VOSS. 2006. Different patterns of selection on the nuclear genes IRBP and DMP-1 affect the efficiency but not the outcome of phylogeny estimation for didelphid marsupials. Molecular Phylogenetics and Evolution 38:363-380. [ Links ]
18. KIRSCH JAW and JH CALABY. 1977. The species of living marsupials: an annotated list. Pp. 9-26, in: The biology of marsupials (B Stonehouse and G Gilmore, eds.). Baltimore, University Park Press. [ Links ]
19. LINARES OJ. 1997. New locality records of mouse opossums from Venezuela (Marsupialia: Didelphidae). Mammalia 61:255-259. [ Links ]
20. LINARES OJ. 1998. Mamíferos de Venezuela. Caracas, Sociedad Conservacionista Audubon de Venezuela. [ Links ]
21. MALCOLM JR. 1991. Comparative abundances of Neotropical small mammals by trap height. Journal of Mammalogy 72:188-192. [ Links ]
22. PATTERSON BD. 1992. Mammals in the Royal Natural History Museum, Stockholm, collected in Brazil and Bolivia by A.M. Olalla during 1934-1938. Fieldiana Zoology (new series) 66:i-iv, 1-42. [ Links ]
23. PATTON JL and LP COSTA. 2003. Molecular phylogeography and species limits in rainforest didelphid marsupials of South America. Pp. 63-81, in: Predators with pouches: the biology of carnivorous marsupials (ME Jones, CR Dickman, and M Archer, eds.). Melbourne, CSIRO Press. [ Links ]
24. ROBBINS MB, MJ BRAUN, and DW FINCH. 2004. Avifauna of the Guyana southern Rupununi, with comparisons to other savannas of northern South America. Ornitología Neotropical 15:173-200. [ Links ]
25. SOLARI S, V PACHECO, L LUNA, PM VELASCO, and BD PATTERSON. 2006. Mammals of the Manu Biosphere Reserve. Fieldiana Zoology (new series) 110:13-22. [ Links ]
26. SORENSON M. 1996. TreeRot. Ann Arbor, University of Michigan. [ Links ]
27. SWOFFORD DL. 1998. PAUP*, phylogenetic analysis using parsimony, beta version 4.0b10. Sunderland, MA, Sinauer Associates. [ Links ]
28. TATE GHH. 1933. A systematic revision of the marsupial genus Marmosa with a discussion of the adaptive radiation of the murine opossums (Marmosa). Bulletin of the American Museum of Natural History 66:1-250 + 26 pls., folding tables. [ Links ]
29. THOMAS O. 1888. Catalogue of the Marsupialia and Monotremata in the collection of the British Museum (Natural History). London, Trustees of the British Museum (Natural History). [ Links ]
30. THOMAS O. 1909. New species of Oecomys and Marmosa from Amazonia. Annals and Magazine of Natural History (ser. 8) 3:378-380. [ Links ]
31. VOSS RS and LH EMMONS. 1996. Mammalian diversity in Neotropical lowland rainforests: a preliminary assessment. Bulletin of the American Museum of Natural History 230:1-115. [ Links ]
32. VOSS RS and SA JANSA. 2003. Phylogenetic studies of didelphid marsupials II. Nonmolecular data and new IRBP sequences: separate and combined analyses of didelphine relationships with denser taxon sampling. Bulletin of the American Museum of Natural History 276:1-82. [ Links ]
33. VOSS RS and SA JANSA. 2009. Phylogenetic relationships and classification of didelphid marsupials, an extant radiation of New World metatherian mammals. Bulletin of the American Museum of Natural History [in press]. [ Links ]
34. VOSS RS, DP LUNDE, and NB SIMMONS. 2001. The mammals of Paracou, French Guiana: a Neotropical rainforest fauna. Part 1. Nonvolant species. Bulletin of the American Museum of Natural History 263:1-236. [ Links ]
35. VOSS RS, DP LUNDE, and SA JANSA. 2005. On the contents of Gracilinanus Gardner and Creighton, 1989, with the description of a previously unrecognized clade of small didelphid marsupials. American Museum Novitates 3482:1-34. [ Links ]
36. VOSS RS, E YENSEN, and T TARIFA. 2004. An introduction to Marmosops (Marsupialia: Didelphidae), with the description of a new species from Bolivia and notes on the taxonomy and distribution of other Bolivian congeners. American Museum Novitates 3466:1-40. [ Links ]
Recibido 06 noviembre 2008.
Aceptado 02 febrero 2009.
Editor asociado: G D'Elía














