Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Mastozoología neotropical
Print version ISSN 0327-9383On-line version ISSN 1666-0536
Mastozool. neotrop. vol.17 no.1 Mendoza Jan./June 2010
ARTÍCULOS Y NOTAS
Two different vocalization patterns in Ctenomys (Rodentia, Ctenomyidae) territorial signals
Gabriel Francescoli1 and Verónica Quirici2
1 Sección Etología, Facultad de Ciencias. Iguá 4225, Montevideo 11400, Uruguay [Corresponding author: Gabriel Francescoli <gabo@fcien.edu.uy>].
2 Centro de Estudios Avanzados en Ecología y Biodiversidad and Departamento de Ecología, Pontificia Universidad Católica de Chile, Alameda 340, Santiago, Chile.
ABSTRACT: Two different vocalization patterns for long-range (S-Type) vocal signals were detected in species of the genus Ctenomys. These patterns were described separately for C. pearsoni, C. mendocinus, and C. talarum. This paper gathers information about the vocalizations of other species, such as C. sociabilis, C. rionegrensis and C. torquatus. We confirm the existence of these patterns, identify which of those two patterns each species uses, and suggest some possible explanations about how these patterns could have originated.
RESUMEN: Dos patrones diferentes de vocalización en señales territoriales de Ctenomys (Rodentia, Ctenomyidae). Dos patrones de vocalización diferentes fueron detectados en las señales vocales de larga distancia (señales Tipo S) en especies del género Ctenomys. Estos patrones fueron descritos separadamente para C. pearsoni, C. mendocinus y C. talarum. Este trabajo recoje información sobre las vocalizaciones de otras especies como C. sociabilis, C. rionegrensis y C. torquatus, confirmando la existencia de los dos patrones, identificando cuál de los dos patrones usa cada especie, así como también sugiriendo algunas posibles explicaciones sobre cómo estos patrones pudieron haberse originado.
Key words. Ctenomys; Differences; Rhythm patterns; Vocal signals.
Palabras clave. Ctenomys; Diferencias; Patrones rítmicos; Vocalizaciones.
Tuco-tucos (genus Ctenomys) are subterranean rodents endemic of South America with more than 50 species, mainly solitary, but with at least one confirmed social species, Ctenomys sociabilis (Lacey et al., 2000). This specific richness originated in an explosive speciation event occurred in relatively recent times (Lessa and Cook, 1998).
Communication signals are part of the behavioral repertoire of many species. Ctenomys are no exception and almost all known species produce vocalizations. Those species that were studied use their vocalizations as a way to communicate, especially at long distances. These territorial/warning signals (long-range signals or S-type vocalizations; Francescoli, 1999) are based on repeated notes, grouped into more general emission patterns. As a result of adaptation to subterranean life, and/or as a result of a common motivational background (Francescoli, 2000), main vocalization frequencies are similar among many species thus suggesting that at least some of the relevant information might be encoded in the rhythmic pattern. This information might be related to identification of sex and/or reproductive state through differential characteristics in S-type signals' emission rate and rhythmic structure (Francescoli, 2000; unpublished observations).
In a preliminary study aimed at describing the vocalizations of Ctenomys mendocinus, Francescoli and Camin (2000) have suggested that at least two different patterns exist for the S-type vocalizations in the genus Ctenomys. This difference, like other behavioural characteristics such as copulatory pattern (Altuna et al., 1991; Camin, 1999; Fanjul and Zenuto, 2008), have not yet been used as characters to explore phylogenetic relationships in Ctenomys.
The aim of this paper is to confirm the existence of those two patterns in other species than C. mendocinus and C. pearsoni, using available information about species that have been studied for their vocalizations or those for which anecdotal data about their vocalization pattern exists, and to discuss the possible origin of the patterns and their use as characters for phylogenetic studies.
There are only eight species or chromosomal forms of the genus Ctenomys for which we have some information about the characteristics of their S-type vocalizations. Some of them have been recorded and others were just heard during field work, but for all these some information have been published or, at least, gathered by the authors. The species are: i) C. mendocinus (Francescoli and Camin, 2000) from central-southern Argentina; ii) C. sociabilis (G. Francescoli, unpublished data) from NW Patagonia, Argentina; iii) C. pearsoni and two related forms: iv) "Solís" karyomorph and v) "Canelones" karyomorph, all from southern Uruguay (Francescoli, 1998, 1999, 2002; Novello and Altuna, 2002; but see Tomasco and Lessa, 2007); vi) C. rionegrensis, limited to a small region in western Uruguay and the Province of Entre Ríos, Argentina (G. Francescoli, unpublished data; Quirici et al., 2002 and V. Quirici, unpublished data); vii) C. talarum, from central Argentina (Schleich and Busch, 2002); viii) C. torquatus, from southern Brazil and northern Uruguay (G. Francescoli and V. Quirici, unpublished data).
The assignment of each species or form to a pattern was done by directly listening to the animals vocalizing in the field or by analyzing the obtained recordings or the published spectrograms (Table 1).
Table 1 Characteristics of the eight Ctenomys species or chromosomal forms with known vocalizations. Information sources are listed. (?)= doubt about information reliability.
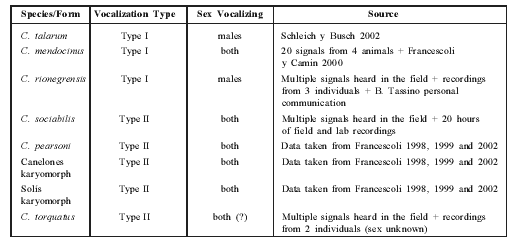
An examination of the general rhythmic structure of S-type vocalizations of these species revealed that all could be assigned to one of two different vocalization patterns: (a) vocalizations consisting of two parts (Part 1 and Part 2), each consisting of repeated notes (Type I or "continuous" pattern), and (b) vocalizations consisting of a repeated succession of note groups (Type II or "discrete" pattern) (Fig. 1).
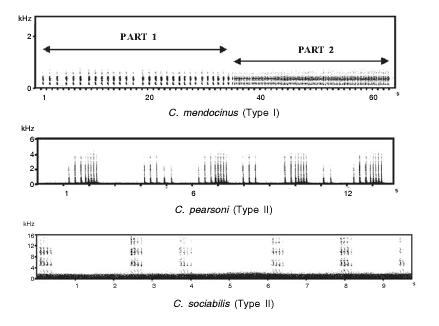
Fig. 1. Representative sonagrams of three species of Ctenomys included in the study. Differences of rhythmic organization between the two different patterns can be observed and are not obscured by differences in the frequency domain, even in a social species like Ctenomys sociabilis.
C. mendocinus, C. talarum and C. rionegrensis show Type I pattern, while C. sociabilis, C. torquatus, C. pearsoni, and the "Solís" and "Canelones" karyomorphs show Type II pattern (Table 1).
In Table 2 we present the data about vocalization type, together with other characters used to assess phylogenetic relationships in the genus Ctenomys.
Table 2 Characterization of eight Ctenomys species or forms with studied vocalization. The corresponding characters previously used to establish phylogenetic relationships among Ctenomys species are shown. (2n, Penial Morphology and sperm data taken from: Altuna and Lessa [1985], Balbontin et al [1996], D'Elia et al [1999], Ortells [1995], Vitullo et al [1988]).

There is some variability in the detailed signal structure among different species. Type I vocalizers have different number of notes in the units composing the Part I of their vocalizations, i. e. one note in C. talarum (Schleich and Busch, 2002 ) but groups of 2 or 3 notes in C. mendocinus (Francescoli and Camin, 2000 and unpublished data), while Part II has always the same structure: a "continuous" chain of individual notes. Among Type II vocalizers, there is also some variation in the number of notes composing the groups forming the emission; in Ctenomys pearsoni, groups can be composed by 3 to 7 notes arranged in a pattern of unique "blocks" or as a block with a variable number of "introductory" notes (Francescoli, 2002), whereas in Ctenomys sociabilis groups are usually built with four notes (G. Francescoli, unpublished data).
It should be stressed that Type I vocalizers not always emit Part 2 (P2) of the signal, but both vocalization types could be still distinguished from each other using Part 1 (P1) of the Type I vocalizations. Type II vocalizers, also may present an ending part of their vocalization similar to the P2 pattern of Type I vocalizers, as a consequence of slowing down their emitting rhythm; this action seems to disaggregate the note successions (Francescoli, 1998).
The possible origin of this variability could be related to random variation and fixation of those variations (Francescoli, 2002). Bradbury and Vehrencamp (1998) stated that frequency, but not rhythm, is directly affected by attributes of the ecological or environmental differences between species. Following these authors, we can't attribute this rhythmic variation to environmental differences among the species.
Our results (see Table 2) suggest the existence of at least two groups of Ctenomys species presenting correlations between some states of characters used before in tuco-tucos phylogenetic studies and vocalization pattern.
Available information suggests that Type II pattern could have evolved from Type I pattern through increasing the duration of some silences to "build" discrete note groups, and/or by the loss of P2 in the Type I vocalization and an increase in P1 complexity. An interesting piece of evidence supporting this line of reasoning is the fact that Francescoli (1998) has found that two recently weaned individuals of C. pearsoni, a Type II vocalizer, emitted spontaneous S-type vocalizations with a continuous pattern. As the pattern found in adult C. pearsoni is discrete, an ontogenetical change in this direction can be assumed and, if confirmed, supports the hypothesis that Type II vocalizations have evolved from Type I vocalizations as suggested above. Nevertheless, if we place the data about vocalization type into a recent phylogeny based on molecular characters (Slamovits et al., 2001; Castillo et al., 2005) it can be seen that the species having Type II vocalization pattern are more basally related among them than those having the Type I pattern, thus challenging the elaborations made above. Clearly, more species should be included in the analyses to reach firmer conclusions in this respect.
Finally, the fact that some individuals of the studied Type I species sometimes emit only one part of the vocalization (usually P1) suggests that this could be because of a difference in individual level of awareness. This could also be the reason explaining the "slow ending" shown by some individuals of the Type II species, as referred above.
Additional research efforts on Ctenomys behaviour are needed in order to place behavioral characters into a phylogenetic context. We hope that this paper stimulates behavioral research in other tuco-tuco and subterranean rodent species.
ACKNOWLEDGEMENTS
We want to thank our friends at the Sección Etología for help during field and lab work, and CSIC (Uruguay) for partial funding. Sergio Camin recorded the vocalizations of C. mendocinus.
LITERATURE CITED
1. ALTUNA CA and EP LESSA. 1985. Penial morphology in Uruguayan species of Ctenomys (Rodentia, Octodontidae). Journal of Mammalogy 66:483-488. [ Links ]
2. ALTUNA CA, G FRANCESCOLI, and G IZQUIERDO. 1991. Copulatory pattern of Ctenomys pearsoni (Rodentia, Octodontidae) from Balneario Solís, Uruguay. Mammalia 55:316-318. [ Links ]
3. BALBONTIN J, S REIG and S MORENO. 1996. Evolutionary relationships of Ctenomys (Rodentia: Octodontidae) from Argentina, based on penis morphology. Acta Theriologica 41:237-253. [ Links ]
4. BRADBURY JW and SL VEHRENCAMP. 1998. Principles of animal communication. Sinauer, Sunderland. [ Links ]
5. CAMIN S. 1999. Mating behaviour of Ctenomys mendocinus (Rodentia, Ctenomyidae). Zeitschrift für Säugetierkunde 64:230-238. [ Links ]
6. CASTILLO AH, MN CORTINAS, and EP LESSA. 2005. Rapid diversification of south american tuco-tucos (Ctenomys; Rodentia, Ctenomyidae): contrasting mitochondrial and nuclear intron sequences. Journal of Mammalogy 86:170-179. [ Links ]
7. D'ELÍA G, EP LESSA, and JA COOK. 1999. Molecular phylogeny of tuco-tucos, genus Ctenomys (Rodentia: Octodontidae): Evaluation of the mendocinus species group and the evolution of asymmetric sperm. Journal of Mammalian Evolution 6:19-38. [ Links ]
8. FANJUL MS and RR ZENUTO. 2008. Copulatory pattern of the subterranean rodent Ctenomys talarum. Mammalia 72:102-108. [ Links ]
9. FRANCESCOLI G. 1998. La comunicación acústica en poblaciones de Ctenomys (Rodentia, Octodontidae) de Uruguay, con algunas consideraciones sobre el uso del canal "vibratorio" por los roedores subterráneos. Unpublished Ph. D. Thesis. Universidad de la República - Pedeciba. Montevideo, Uruguay. [ Links ]
10. FRANCESCOLI G. 1999. A preliminary report on the acoustic communication in uruguayan Ctenomys (Rodentia, Octodontidae): basic sound types. Bioacoustics 10:203-218. [ Links ]
11. FRANCESCOLI G. 2000. Sensory capabilities and communication in subterranean rodents. Pp. 111-144, en: Life Underground. The biology of subterranean rodents (EA Lacey, JL Patton, and GN Cameron, eds.). The University of Chicago Press, Chicago. [ Links ]
12. FRANCESCOLI G. 2002. Geographic variation in vocal signals of Ctenomys pearsoni. Acta Theriologica 47:35-45. [ Links ]
13. FRANCESCOLI G and S CAMIN. 2000. Análisis primario de las vocalizaciones territoriales de Ctenomys mendocinus (Rodentia: Octodontidae). XV Jornadas Argentinas de Mastozoología. Resúmenes: 59. [ Links ]
14. LACEY EA, JL PATTON, and GN CAMERON. 2000. Introduction. Pp. 1-14, en: Life Underground. The biology of subterranean rodents (EA Lacey, JL Patton, and GN Cameron, eds.). The University of Chicago Press, Chicago. [ Links ]
15. LESSA EP and JA COOK. 1998. The molecular phylogenetics of tuco-tucos (genus Ctenomys, Rodentia: Octodontidae) suggests an early burst of speciation. Molecular Phylogenetics and Evolution 9:88-99. [ Links ]
16. NOVELLO A and C ALTUNA. 2002. Cytogenetics and distribution of two new karyomorphs of the Ctenomys pearsoni complex (Rodentia, Octodontidae) from southern Uruguay. Mammalian biology 67:188-192. [ Links ]
17. ORTELLS MO.1995. Phylogenetic analysis of G-banded karyotypes among the South American subterranean rodents of the genus Ctenomys (Caviomorpha: Octodontidae), with special reference to chromosomal evolution and speciation. Biological Journal of the Linnean Society 54:43-70. [ Links ]
18. QUIRICI V, C PASSOS, and B TASSINO. 2002. Vocalizaciones de Ctenomys rionegrensis (Rodentia: Octodontidae) durante el cortejo y la cópula. XVII Jornadas Argentinas de Mastozoología. Resúmenes:33. [ Links ]
19. SCHLEICH CE and C BUSCH. 2002. Acoustic signals of a solitary subterranean rodent Ctenomys talarum (Rodentia: Ctenomyidae): physical characteristics and behavioural correlates. Journal of Ethology 20:123-131. [ Links ]
20. SLAMOVITS CH, JA COOK, EP LESSA, and MS ROSSI. 2001. Recurrent amplifications and deletions of satellite DNA accompanied chromosomal diversification in South American tuco-tucos (Genus Ctenomys, Rodentia: Octodontidae): A phylogenetic approach. Molecular Biology and Evolution 18:1708-1719. [ Links ]
21. TOMASCO IH and EP LESSA. 2007. Phylogeography of the Tuco-tuco Ctenomys pearsoni: mtDNA variation and its implication for chromosomal differentiation. Pp. 859-882, in: The quintessential naturalist. Honoring life and legacy of Oliver P. Pearson (DA Kelt, EP Lessa, J Salazar-Bravo, and JL Patton, eds.). University of California Publications, Berkeley. [ Links ]
22. VITULLO AD, ERS ROLDAN, and MS MERANI. 1988. On the morphology of spermatozoa of tuco-tucos, Ctenomys (Rodentia: Octodontidae): New data and its implications for the evolution of the genus. Journal of Zoology, London 215:675-683. [ Links ]
Recibido 3 diciembre 2008.
Aceptado 21 abril 2009.
Editor asociado: E Lessa














