Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Mastozoología neotropical
versión impresa ISSN 0327-9383versión On-line ISSN 1666-0536
Mastozool. neotrop. v.17 n.1 Mendoza ene./jun. 2010
ARTÍCULOS Y NOTAS
The karyotype of Noctilio Albiventris (Chiroptera, Octilionidae) from the northern pantanal of Brazil and its taxonomic implications
Rossana de Paula Vilamiu1, 2, Margaret Maria de O. Corrêa1, Leila Maria Pessôa 1 , João A. de Oliveira3, and Luiz Flamarion B. Oliveira3
1 Laboratório de Mastozoologia, Departamento de Zoologia, Instituto de Biologia, CCS, Universidade Federal do Rio de Janeiro, 21941-590 Rio de Janeiro - RJ, Brazil [Corresponding author: Margaret Maria de O. Corrêa <margaret@biologia.ufrj.br>].
2 Programa de Pós-graduação em Zoologia, Museu Nacional/UFRJ, Rio de Janeiro, RJ, Brazil.
3 Setor de Mamíferos, Departamento de Vertebrados, Museu Nacional/UFRJ, Rio de Janeiro, RJ, Brazil, CEP: 20940-040, Rio de Janeiro, Brazil.
ABSTRACT: The complete karyotype of Noctilio albiventris cabrerai is described on the basis of specimens from the northern Pantanal region of central Brazil. The G-banded karyotype comprises a diploid number (2n) of 34, an autosomal fundamental number (FNa) of 62, and respectively submetacentric and acrocentric X and Y chromosomes. Active nucleolar organizer regions (NORs) were located in pair 12. C-banding showed constitutive heterochromatin in the pericentromeric regions of all autosomes and in the X chromosome. Differences were found with respect to specimens from Honduras, referable to N. a. minor, which had a pair of autosomes with totally heterochromatic short arms, and from Colombia, referable to N. a. affinis, which had a metacentric X chromosome.
RESUMEN: El cariotipo de Noctilio albiventris (Chiroptera, Noctilionidae) del norte del Pantanal brasileño y sus implicancias taxonómicas. Se describe el cariotipo completo de Noctilio albiventris cabrerai en base a especímenes del norte del Pantanal, Brasil. El cariotipo en bandeo G presentó un número diploide (2n) de 34, un número fundamental autosómico (FNa) de 62, el cromosoma X submetacéntrico y el Y acrocéntrico. Las regiones organizadoras del nucléolo (RONs) activas se ubican en el par 12. El bandeo C reveló bloques de heterocromatina constitutiva en las regiones pericentroméricas de todos los autosomas y en el cromosoma X. Se resumen las diferencias con respecto a cariotipos asignables a N. a. minor (Honduras), con un par cromosómico de brazos cortos totalmente heterocromático, y a N. a. affinis (Colombia), con un cromosoma X metacéntrico.
Key words. G- and C-banding; Lesser bulldog bat; Nucleolar organizer regions; Pantanal.
Palabras clave. Bandeo G y C; Murciélago pescador menor; Pantanal; Regiones organizadoras del nucléolo.
The lesser bulldog bat, Noctilio albiventris, is considered a polytypic species (Simmons, 2005; Gardner, 2008). In the latest comprehensive revision, Davis (1976) recognized four subspecies, as follows: N. a. albiventris Desmarest, 1818, from Rio São Francisco, Bahia, Brazil, ranging from southern Venezuela and southern Guyana through the lower Amazon Basin to the coastal area of eastern Brazil; N. a. affinis d'Orbigny, 1837, with type-locality Beni, Bolivia, ranging from the upper reaches of the Amazon drainage system northward along the eastern base of the Andes to northwestern Venezuela, and thence eastward along the coast to Suriname; N. a. minor Osgood, 1910, from Zulia, Venezuela, ranging through Colombia (west of the Cordillera Oriental) and Central America to Honduras; and N. a. cabrerai Davis, 1976, from Olimpo, Paraguay, ranging through Brazil and Argentina within the Paraná basin. In his description of N. a. cabrerai, Davis (1976) included two specimens collected at "Fazenda Santa Isabel, Cuiabá" and "Fazenda São João Borges, 240 km, Cuiabá", in the Brazilian state of Mato Grosso, and provided a qualitative diagnosis, as well as cranial and external measurements for further identification of this taxon in Brazil.
Karyotype descriptions are available for specimens from Leticia, Colombia (Baker and Jordan, 1970; Baker et al., 1982; Hood and Pitocchelli, 1983), Nacaome, Honduras (Patton and Baker, 1978; Baker and Bickham, 1980), and Northwestern São Paulo State, Brazil (Varella-Garcia et al., 1989). These samples are referable on geographical grounds to N. a. affinis, N. a. minor and N. a. cabrerai, respectively, following Davis (1976).
In this note, we describe the complete karyotype of N. a. cabrerai from the Northern Pantanal Ecoregion, Mato Grosso, Brazil, using conventional Giemsa staining , G- and C-banding, and silver staining techniques. This karyotype is then compared with the other karyotypes found for N. albiventris. Fifteen males and seven females of N. albiventris were used in this study, collected during 1999 and 2003 in the Private Natural Heritage Reserve (RPPN - Reserva Particular do Patrimônio Natural) of the Serviço Social do Comércio (SESC) in the Pantanal. This area is located between the Cuiabá and São Lourenço rivers in the municipality of Barão de Melgaço, nearly 120 km South of Cuiabá, Mato Grosso State, central Brazil (16°43' S; 56°11' W).
Cytogenetic analyses were carried out using mitotic metaphase chromosomes from bone marrow preparations of 12 specimens, eight males and four females, following Ford and Hamerton (1956), with modifications. Nearly 300 metaphase cells were analyzed with Giemsa conventional staining and chromosomes were classified following Levan et al. (1964). Metacentric, submetacentric and subtelocentric chromosomes were regarded as biarmed and acrocentrics uniarmed. Trypsin-Giemsa banded chromosomes (G-bands) were obtained according to Seabright (1972) and 39 metaphase cells were analyzed. Nucleolar organizer regions (NORs) were detected by silver nitrate staining (Ag-NOR) following the procedure of Howell and Black (1980), with 66 metaphases analyzed. Constitutive heterochromatin distribution patterns (C-bands) were revealed by the barium hydroxide method (Sumner, 1972), with approximately 100 metaphase cells analyzed.
Voucher specimens were deposited in the mammal collection of the Museu Nacional in Rio de Janeiro (MN), Brazil under the following accession numbers: 64084*; 64085*; 64140; 64145; 64146*; 64184*; 64185*; 64186; 64187; 64188*; 64189; 64192*; 64204; 64534*; 64552*; 64578*; 64580*; 64581; 64583*; 64793; 64794; 64795 (karyotyped specimens are marked with an asterisk).
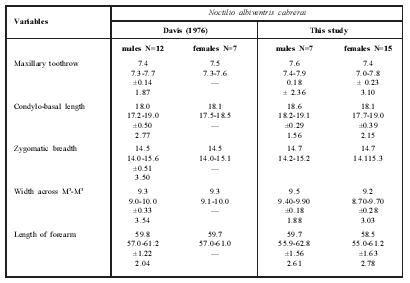
Based on the diagnosis and external and cranial measurements provided by Davis (1976) for the type series, we were able to identify our specimens as belonging to N. a. cabrerai. The specimens analyzed in the present study are very similar in external color pattern to Davis's (1976) description of the type series: grayish-brown above and buff to orange-buff below or completely brownishorange. The means of the cranial measurements for 15 males and seven females were very close to those given by Davis (1976) for the type series (Table 1).
Table 1 Mean, range, standard deviation and coefficient of variation for selected measurements of Noctilio albiventris cabrerai from Brazil, Paraguay and Argentina (Davis, 1976) and from Barão de Melgaço, Mato Grosso, central Brazil (this study).
Noctilio a. cabrerai has a diploid number (2n) = 34 and an autosomal fundamental number (FNa) = 62, comprising 13 metacentric and submetacentric pairs, two subtelocentric pairs, and one acrocentric pair of chromosomes. Chromosome pair 12 carries a secondary constriction on the long arm. The X chromosome is a medium-sized submetacentric and the Y is a small acrocentric (Fig. 1a). G-banding, in addition to conventional staining, helped in the identification of the homologous chromosome pairs (Fig. 1b).

Fig. 1. Karyotypes of Noctilio albiventris cabrerai (2n = 34, FNa = 62) from Barão de Melgaço, Mato Grosso, Brazil; a) Giemsa-stained female karyotype. The chromosome pair carrying the secondary constricitons and the NORs sites is shown in the inset; b) G-banded female karyotype; c) C-banded male karyotype. Scale = 10 µm.
Noctilio a. cabrerai has the same 2n and FNa as previously karyotyped specimens from areas as distant as Honduras (Patton and Baker, 1978), Southern Colombia-for which the FNa had been originally described as 58 (Baker and Jordan, 1970), but was later corrected to 62 by Hood and Pitocchelli (1983) following Patton and Baker (1978)-and Northwestern São Paulo State, Brazil (Varella-Garcia et al., 1989).
The X chromosome of specimens from Northern Pantanal (this study), Northwestern São Paulo (Varella-Garcia et al., 1989), and Honduras (Patton and Baker, 1978) is submetacentric. In the specimen from Leticia, Colombia, referable to N. a. affinis, the X chromosome is metacentric (Baker and Jordan, 1970, Hood and Pitocchelli, 1983). This difference in the X chromosome among populations may be a result of a pericentric inversion and deletion or addition of heterochromatic chromosome segments. The Y chromosome in our specimens is a small acrocentric, similar to the condition described for all other studied samples.
Until now, G- and C-bands were not available for Brazilian samples of N. albiventris. C-banding in N. a. cabrerai from Northern Pantanal reveals pericentromeric heterochromatic regions in all autosomes, with pairs 13, 15 and 16 presenting more conspicuous blocks of constitutive heterochromatin. In addition to pericentromeric bands, the X chromosome also shows C-bands on the long arms. The Y chromosome is totally heterochromatic (Fig. 1c).
In the specimen from Honduras, the only other for which C bands were available, conspicuous pericentromeric C-bands were visible in all chromosomes, except in one small pair that had totally heterochromatic short arms (Patton and Baker, 1978). These differences in C-band intensity suggest a distinction in chromosome microstructure between N. a. cabrerai from Northern Pantanal and N. a. minor.
Silver staining marks the secondary constriction in the pericentromeric region of the long arms of chromosome pair 12 in specimens from Northern Pantanal. NORs are coincident with the secondary constrictions observed in these chromosomes (Fig. 1a, inset). In a previously karyotyped specimen from Northwestern São Paulo, also assignable to N. a. cabrerai on geographical grounds, NORs were detected near the centromeres in the long arms of the smallest medium-sized submetacentric chromosomes (Varella-Garcia et al., 1989). These results suggest a similar pattern of Ag-NOR sites in specimens from areas far apart in the Parana river basin, which delimits the range of N. a. cabrerai as originally proposed by Davis (1976).
The present study reveals differences in the chromosome macro- and microstructure of N. a. cabrerai from Northern Pantanal compared to that of specimens from other areas in the species range, thus documenting differentiation at the cytogenetic level among N. albiventris populations.
Based on morphometric data and pelage color variation, Davis (1976) assumed that populations living in the drainage basin of the Rio Parana (N. a. cabrerai) were isolated from those inhabiting the Amazon Basin (N. a. affinis). Confirming this view, Noctilio a. cabrerai specimens from the Pantanal region of Brazil are found to have a submetacentric X chromosome, as compared to a metacentric X in the specimen from Leticia, Colombia.
A sample of 16 specimens from an area of the Amazon Basin extending from Leticia (Colombia) to the mouth of Rio Negro (Manaus, Brazil) had a larger mean condylobasal length than 10 other geographic samples covering the entire range of the species (Davis, 1976). This finding, and also the darker pelage color of this sample, led Davis (1976) to recognize N. a. affinis as a distinct taxon, with its type locality in Beni, Bolivia, and including two other pooled samples, one from Bolivia (22 specimens) and the other from Peru (47 specimens). Davis (1976) also tentatively included samples of large specimens from Caracas to Suriname, along a relatively narrow coastal belt in the Guiana region, in this subspecies, but pointed out that they might constitute a separate taxonomic unit.
Recent taxonomic compilations (Simmons, 2005; Gardner, 2008) have regarded N. a. affinis as a junior synonym of N. a. albiventris, while recognizing N. a. cabrerai and N. a. minor as valid. This taxonomic assignment is based on morphologically intermediate material (two specimens from the coastal localities of Paracou and Sinnamary, French Guiana) with respect to Davis's (1976) morphometric definitions of N. a. affinis and N. a. albiventris based on samples from the eastern and northernmost parts of their ranges, respectively (Simmons and Voss, 1998).
The karyotypic data summarized here, nevertheless, suggest that the sample from Leticia, unequivocally assigned by Davis (1976) to N. a. affinis, is distinct from the other available karyotyped samples, namely N. a. minor from Honduras and N. a. cabrerai from the Pantanal and the Rio Paraná Basin, São Paulo, Brazil.
The other species in the genus, N. leporinus, has been shown to have a constant 2n of 34 but a variable FNa. Specimens from São Paulo (eastern Brazil) had FNa = 60, while specimens from Pernambuco (northeastern Brazil) had FNa = 54 (Varella-Garcia et al., 1989). Lewis-Oritt et al. (2001) compared samples referred to N. leporinus and N. albiventris on the basis of molecular data (Cytochrome b and RAG-2 sequences). They suggested a deep divergence within N. albiventris between populations from Peru and populations from Venezuela, Suriname and Guiana, raising the possibility that two species are present within N. albiventris in this region. This finding, together with the karyological information summarized above, is also supportive of the distinctness of N. a. affinis and points to the need for more detailed analyses of variation among populations currently referred to N. albiventris, insofar as the limits of morphological, karyological and molecular divergence have yet to be fully understood.
ACKNOWLEDGEMENTS
The authors would like to thank L. Brandão as well as the management and staff of RPPN SESC Pantanal for granting permission and providing support for the study of small mammals in the reserve area. This work was supported by grants from SESC (Serviço Social do Comércio) and the Universidade Federal do Rio de Janeiro. J. A. Oliveira, L. M. Pessôa, and L. F. B. Oliveira are supported by research fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). We are grateful to Dr. Marcos Palatnik and Dra. Cecilia Teixeira de Aguilar (UFRJ) for revising a first draft of this manuscript, and to two anonymous reviewers for providing important suggestions and corrections. We thank Stella Franco for her help in processing specimens in the MN collection and Christopher Tribe for revising the English version. Collecting permits were provided by Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA).
LITERATURE CITED
1. BAKER RJ and JW BICKHAM. 1980. Karyotypic evolution in Bats: evidence of extensive and conservative chromosomal evolution in closely related taxa. Systematic Zoology 29:239-253. [ Links ]
2. BAKER RJ and RG JORDAN. 1970. Chromosomal studies of some Neotropical bats of the families Emballonuridae, Noctilionidae, Natalidae and Vespertilionidae. Caryologia 23:595-604. [ Links ]
3. BAKER RJ, MW HAIDUK, LW ROBBINS, A CADENA, and BF KOOP. 1982. Chromosomal studies of South American bats and their systematic implications. Pp.303-327, in: Mammalian biology in South America (MA Mares and HH Genoways, eds.). Special Publication Series Pymatuning Laboratory of Ecology 6. [ Links ]
4. DAVIS WB. 1976. Geographic variation in the lesser noctilio, Noctilio albiventris (Chiroptera). Journal of Mammalogy 57:687-707. [ Links ]
5. FORD CE and JL HAMERTON. 1956. A colchicine, hypotonic citrate, squash sequence for mammalian chromosomes. Stain Technology 31:247-251. [ Links ]
6. GARDNER AL. 2008. Family Noctilionidae Gray, 1821. Pp. 384-389, in: Mammals of South America, Volume 1 Marsupials, Xenarthrans, Shrews, and Bats. (AL Gardner, ed.). The University of Chicago Press, Chicago and London. [ Links ]
7. HOOD C and J PITOCCHELLI. 1983. Noctilio albiventris. Mammalian Species 197:1-5. [ Links ]
8. HOWELL WM and DA BLACK. 1980. Controlled silver staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Experientia 36:1014-1015. [ Links ]
9. LEVAN A, K FREDGA, and AA SANDBERG. 1964. Nomenclature for centromeric position on chromosomes. Hereditas 52:201-220. [ Links ]
10. LEWIS-ORITT N, RA VAN DEN BUSSCHE, and RJ BAKER. 2001. Molecular evidence for evolution of piscivory in Noctilio (Chiroptera: Noctilionidae). Journal of Mammalogy 82:748-759. [ Links ]
11. PATTON JC and RJ BAKER. 1978. Chromosomal homology and evolution of phyllostomatid bats. Systematic Zoology 27:449-462. [ Links ]
12. SEABRIGHT MA.1972. A rapid banding technique for human chromosomes. Lancet 2:971-972. [ Links ]
13. SIMMONS NB. 2005. Order Chiroptera. Pp.312-529, in: Mammal species of the World: a taxonomic and geographical reference. Third Edition, Vol 1 (DE Wilson and DM Reeder, eds.). Johns Hopkins University Press, Baltimore. [ Links ]
14. SIMMONS NB and RS VOSS. 1998. The mammals of Paracou, French Guiana: A Neotropical lowland rainforest fauna. Part1. Bats. Bulletin of the American Museum of Natural History 237:1-219. [ Links ]
15. SUMNER AT. 1972. A simple technique for demonstrating centromeric heterochromatin. Experimental Cell Research 75:304-306. [ Links ]
16. VARELLA-GARCIA ME, E MORIELLE-VERSUTE and VA TADDEI. 1989. A survey of cytogenetic data on Brazilian bats. Revista Brasileira de Genética 12:761-793. [ Links ]
Recibido 8 julio 2009.
Aceptado 4 diciembre 2009.
Editor asociado: M Díaz














