Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Mastozoología neotropical
versión impresa ISSN 0327-9383
Mastozool. neotrop. vol.17 no.2 Mendoza dic. 2010
ARTÍCULOS
Seasonal Spatial Distribution Patterns Of A Capybara (Hydrochoerus Hydrochaeris) Population In The Flooded Savannas Of Colombia
Adriana Maldonado-Chaparro and Pedro Sánchez Palomino
Departamento de Biología, Universidad Nacional de Colombia, Ciudad Universitaria, Bogotá D.C., Colombia [Correspondencia: Adriana Maldonado-Chaparro <maldonado.aa@gmail.com>].
Recibido 17 noviembre 2008.
Aceptado 7 setiembre 2009.
Editor asociado: J Polop
ABSTRACT: Little is known about spatial induced processes regulating population dynamics in capybara (Hydrochoerus hydrochaeris), a social rodent from the lowlands of South America, an aspect that may explain the causative mechanism involved in a spatial density-dependent process like mortality and dispersal. We investigated and compared the spatial pattern of herds in a capybara population in the eastern savannas of Colombia. Herd locations were mapped during two contrasting periods of the year and changes in herd spatial distribution were measured using scale-dependent point pattern analyses, pair correlation function g(r) and the normalize K-function, (L(r)). Our results show that 1) herd size increases during the dry season; 2) herd spatial distribution followed a scale-dependent pattern; 3) regularity at small scales provides evidence of intra-specific competition between herds; and 4) clumped distribution was probably caused mainly by behavioral responses and habitat heterogeneity. This study highlights the importance of spatial statistics in the study of seasonal spatial distribution patterns of capybara herds, and their behavioral and ecological causes. It sheds light on ecological aspects such as space use and habitat influence.
RESUMEN: Patrón de ditribución espacio-temporal de una población de capibaras (Hydrochoerus hydrochaeris) en las sabanas inundables de Colombia. Poco se conoce sobre los procesos espaciales que regulan la dinámica de las poblaciones de capibara (Hydrochoerus hydrochaeris), un roedor social que se distribuye ampliamente en las tierras bajas de Sur América. Este aspecto es importante ya que permite explicar mecanismos involucrados con procesos espaciales y denso-dependientes como la mortalidad y la dispersión. En este trabajo estudiamos y comparamos el patrón espacial de las manadas en una población de capibaras localizada en los llanos orientales de Colombia en dos épocas climáticas contrastantes. A partir de localizaciones de las manadas referenciadas con GPS, cuantificamos los cambios en la distribución espacial de las manadas usando la metodología de análisis de patrón de puntos. Los resultados indican que: 1) la distribución espacial de las manadas sigue un patrón dependiente de la escala; 2) a pequeñas escalas la presencia de un patrón de distribución regular aporta evidencia a favor de procesos de competencia intra-específica entre manadas; 3) el patrón de distribución agregado puede ser una repuesta a comportamientos y a heterogeneidad en el hábitat.
Key words. Capybara; L-function; Pair Correlation Function; Point Pattern Analysis; Spatial distribution patterns.
Palabras clave. Análisis de Patrón de Puntos; Capibaras; Función de Correlación Pareada; Función-L; Patrón de distribución espacial.
INTRODUCTION
Distribution or spatial arrangement of individuals within a population is a fundamental characteristic of populations (Clarks and Evans, 1954). It creates the setting for future interactions among individuals (Gordon and Kulig, 1996) and often has implications for community structure and ecosystem function (Crist and Wiens, 1996). Individuals may exhibit different spatial arrangements, such as random, regular or aggregated distributions. Individuals are randomly distributed if the position of each individual is independent of others, are regularly distributed if individuals are evenly spaced and are aggregated if they occur in clumps (Pielou, 1960). Within a population, competition for space might induce regular spatial patterns more frequently than aggregated or random; however, competition for space would be expected to occur between very close neighbors within smaller scale clumps or aggregations (Campbell, 1992). Aggregated spatial distributions in animal species may be generated by favorable environmental conditions (Perry, 1995), such as suitable habitat for growth and reproduction or mutual attraction between individuals (Campbell, 1996).
The distribution and abundance of individuals can be represented by spatial points in a plot (Byers, 1992). Spatial patterns can be described, measured, and evaluated using various methods of point pattern analyses. Techniques in this area have been applied to explore spatial structure of communities and populations, but little is known about the understanding of the interaction among individuals or groups of individuals (Mane et al., 2005). Previous studies in spatial pattern analysis at the individual level have included different mammal species; thus, herd and individual spatial distributions of large herbivore species in a savanna community (Stein and Georgiadis, 2006) and female chimpanzee locations (Mane et al., 2005) concluded that spatial methods are useful to analyze space use as well as ecosystem influence and temporal information with the advantage of using GPS technology.
The main objective of this study is to determine and explain the spatial distribution of capybara herds in flooded savannas of Eastern Colombia in relation to habitat resources' availability. We hypothesize that capybara herd distribution is expected to follow a uniform distribution pattern in a habitat where food and water are not limiting resources, a situation observed during the rainy season in the eastern savannas of Colombia; however, if resource availability diminishes, as occurs in swamps during the dry season, spatial distribution will be modified to follow a clumped pattern.
MATERIALS AND METHODS
Capybara (Hydrochoerus hydrochaeris) is the world's largest rodent. It is distributed exclusively in the tropical region of South America, from Panama through the eastern savannas of Colombia, Venezuela, Brazil, Ecuador, Peru, Paraguay and Uruguay to northern Argentina (Mones and Ojasti, 1986; Ojasti, 1990). Capybaras are social rodents that typically live in groups varying from 10 to 30 individuals, although some groups have up to 100 individuals (Ojasti, 1990). Groups consist of one dominant male and two dominant females with several adults of both sexes and their offspring (Herrera and Macdonald, 1993). Groups are territorial and their home range varies from 5 to 16 ha (Herrera and Macdonald, 1989; Camargo et al., unpublished data).
Capybaras are semiaquatic grazing herbivores that live near riversides, lake shores or in flooded savannas, usually near a forest patch (Ojasti, 1991; Gonzalez-Jimenez, 1995). Capybaras need dry areas for resting and feeding, and water bodies for drinking, to copulate and for predator avoidance (Ojasti, 1973). Population size is influenced by environmental conditions determined by season, food quality, and predation and hunting intensity; low precipitation levels during the dry season result in water scarcity, a fact that may induce herd migration, reduction in escaping coverage availability and decrease in food availability which affects feeding behavior (Ojasti, 1973).
This study was carried out between October 2007 and January 2008, in a 482 ha area in the ranch "Hato Andalucia" (71º31'16.7"W, 06º0'52.0"N), approximately 120 km north-east of the village of Hato Corozal, Casanare, in the eastern Colombian savanna. Native landscape coverage of the region mainly consists of (1) swamps or 'esteros', most of which dry out during dry season; (2) flooded savannas or 'bajíos', areas that flood during the rainy season and are covered with highly palatable grasses, such as Leersia hexandra, Painum sp., and (3) savannas or 'bancos', which are permanently dry areas covered with palatable grasses, including Axonopus sp., tall grasses such as Andropogon sp. or bushes. Mean annual temperature and precipitation is 26.2 ºC and 1822 mm/yr (30-years period, IDEAM), respectively. The region has a unimodal regime with a rainy season from April to November and a dry season from November to April. This seasonality induces changes in vegetation phenology throughout the year, with extensive flooding in the most humid months, and water shortage and food depletion during the dry period (Ricaurte et al., 2007; Scholte, 2007).
Data Collection: We employed the line transect methodology for population surveying (Buckland et al., 2001) to assess the spatial herd distribution. We used four geographically referenced 2-km line transects, 500 m apart from each other. Observations were recorded from transects twice each season from 6 am to 6 pm. Surveys were carried out by two observers riding a horse at a constant speed of approximately 1 km/hour. The use of horses improves the observer's detection ability. In each survey observers estimated the perpendicular distance from the transect line to the geometric center of the herd and registered the number of individuals. A herd was previously defined as any observation of one or more animals at a given location.
Data analysis: We employed herd spatial location to construct point pattern plots for each of the seasons assessed and point pattern analysis (PPA) methodology to test whether the observed point pattern plot can be considered to have a regular, random, or aggregated pattern. For this analysis we explicitly used the locations and distance between herds. PPA is a non-parametric method used to indicate if the distribution of point samples deviates significantly from random (type of pattern) and identifies the scale at which non-random patterns occur (spatial structure). First, we constructed the point pattern plots by reducing capybara locations as a finite set of "points" (locations) in a region that corresponds to the study area.
Seasonal differences in herd sizes and first-nearest neighbor distances were calculated using the Mann-Whitney (Wilcoxon-W) test for medians with a 95% of confidence interval in Statgraphics Centurion XV software (StatPoint, Inc.). Statistical and point pattern analyses were done in Spatstat package in R software version 7.0 (Baddeley and Turner, 2005). In these analyses we assumed that the spatial distribution is invariant to translation within d-dimensional space (stationarity), and is invariant to rotation about the origin (isotropy). Point patterns were characterized by using the intensity (k), defmed as the expected number of points per unit of área (Stoyan and Pentinnen, 2000). The second order property of the point pattern was evaluated by using the normalized L-ñmction of the K-function and the pair correlation ñmction (Ripley, 1976). The K-function, K(r), relates the average number of extra events within distance r of a randomly chosen event to the average number of events per unit área (γ) (Waller and Gotway, 2004). To stabilize the variance, we normalized the K-function by using the square root transformation of K (r), L(r). We elected to use L-function because of its power to detect aggregated patterns (Barot et al., 1999).
The estimated L-function was compared to the theoretical function under the nuil hypothesis of complete spatial randomness. We estimated the significance of the test using a Monte Cario graphic procedure where we constructed rejection limits for the test as envelopes of the simulations at 5% level of significance based on 99 simulations by random arrangement of herd locations (Baddeley and Turner, 2005). When the nuil hypothesis is rejected, the sign of the difference between observed and theoretical distributions indicates a tendency towards aggregation (positive valúes) or to regularity (negative valúes). The test for spatial non-randomness was applied as a two-tailed test because we assumed that any departure from randomness will be either in the direction of aggregation or in the direction of regularity (Campbell, 1996).
The pair correlation function g(r) is considered more powerful in detecting spatial patterns across scales because it takes into consideration pairs of neighbors separated by a distance r, and is related to the cumulative distribution function and probability density function of distances between pairs of points (Wiegand and Moloney, 2004). To estímate g(r), first, the K-function was estimated and then a numerical derivative was taken (Schabennerger and Gotway, 2005). Valúes of g (r) = 1 indicate a random distribution of the points, whereas valúes of g(r) > 1 indicate interpoint distances around r are more frequent (cluster distribution), and valúes of g (r) < 1 that they are less frequent than they would be under complete spatial randomness, i.e., a tendency toward regularity (Getiz et al., 2006).
In this study, the 'scale' of spatial patterns was defmed following Barot et al. (1999). Thus, valúes lower than the half of the máximum possible distance between pair of points was defmed as "small scale" and higher valúes were referred as "large scale". We used the valué of the function as an indicator of the degree of aggregation. For clumped patterns, when spatial randomness was rejected, the máximum valué of the function was used as a measure of "clumpness". This measure indicates the average distance between points within a clump, and was used to compare herd spatial aggregation among seasons.
RESULTS
Within the study área, we recorded a total of 2347 individuáis, in 97 and 99 herds in the rainy and dry season, respectively. The average number of herds per unit of área was similar in both seasons (Rainy: γ= 2.22xlOA-5; Dry: γ = 2.26 xl0A-5). During the rainy season we recorded 746 individual observations, 17 of which were solitary individuáis and the remaining were grouped in herds of up to 51 individuáis with a mean herd size of 7.69 ± 8.32. In contrast, dry season observations showed a higher number of capybaras (1601 individuals), as well as a significant increment in mean herd size (16.17±18.3 individuals; W = 1680.5; P < 0.0001).
The spatial point pattern (Fig. 1) shows the tendency of the distribution of the herds for each of the seasons evaluated and gives evidence for a seasonal variation of spatial distribution. Even though records are sparsely distributed throughout the whole area, we noticed some areas with especially shorter interherd distances during the dry season. This is consistent with the mean distance between the geometric center of a herd and its first neighbor, which was 73.59 m (C.V. = 0.98) for the rainy season and 93.99 m (C.V. = 0.80) in the dry season. Even though there was a significant increment of the mean distance to the first neighbor from rainy to dry season (W = 970.0; P = 0.0014), the maximum and minimum distances to the first neighbor were similar
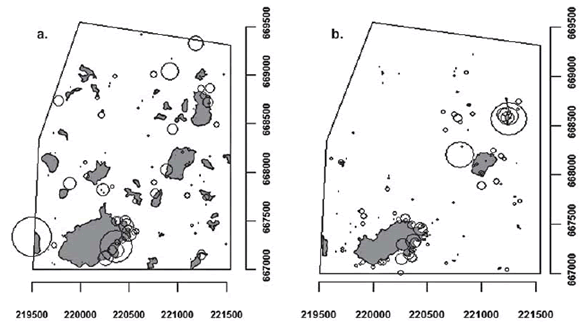
Fig. 1. Study área showing swamp distribution (gray) and position of the herds of Hydrochoerus hydrochaeris (circles). Circle size represents herd size. The solid line polygon endoses the área in which the herds were assessed; a. Herd spatial distribution during the rainy season; b. Herd spatial distribution during the dry season.
In general, dispersion patterns of capybara herds were scale-dependent. In both seasons herds were randomly distributed at small scales and exhibited a clumped pattern at large scales (Fig. 2). During the rainy season, herds were aggregated at scales from 120 to 370 m with the greatest degree of clumping at 100 m (Fig. 2a), whereas in the dry season herds were aggregated for all scales above 50 m (Fig. 2b). The rainy season pattern was only marginally different (p < 0.05) from the complete spatial randomness, showing a tendency towards clumpness in herd spatial distribution of capybaras.
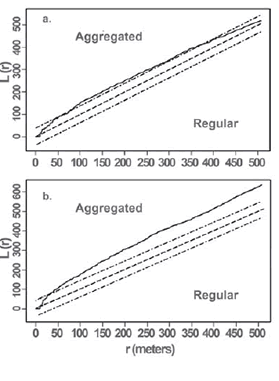
Fig. 2. L(r) values of spatial distributions of capybara during 2007-2008; a. Rainy season; b. Dry season. Continuous line, observed L(r) values; dotted lines, 95 % confidence envelopes for the pattern expected from a random distribution; dashed lines, the theoretical L(r) function for a random spatial pattern.
The pair correlation function shows an alternating sequence of values with a maximum peak at approximately 20 m and a regular pattern for distances below 10 m for both seasons (Figs. 3a, 3b). The rainy season function shows a tendency to a random distribution of the herds above 120 m (values closer to 1, Fig. 3a) and signs of clustering for short distances between 10 and 120 m. In contrast, dry season function shows a clearly aggregated distribution at all scales above 10 m, e.g., g(r) > 1 (Fig. 3b).
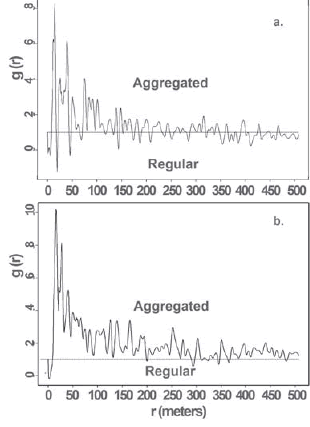
Fig. 3. Univariate analyses using the paircorrelation function g(r) (PCF). Black solid line, oberved g(r) values; dotted line g(r) = 1, indicates randomness. Values above and below the dotted line indicate aggregation and regularity; a. PCF for the rainy season distribution; b. PCF function for the rainy season distribution.
DISCUSSION
As expected, the capybara population under study displays significant changes in abundance and spatial distribution in response to seasonal variations of habitat resources. In this study we suggest that seasonal fluctuations in population size may reflect life cycle adaptations of capybaras to the influence of factors such as resource availability, predation pressure or disease, as well as environmental factors such as rainy cycles (Odum, 2005).
The fact that herd intensity remained constant during the study period in the study area, while population size and herd size increased during the dry season, suggests the presence of a behavioral mechanism that allows for the existence of crowded populations during the dry season. Increases in population size as well as in herd sizes should be related to birth pulses occurring mainly during October through November, at the end of the rainy season. Unfavorable habitat conditions during the dry season favor the formation of larger groups based on two advantages: 1) increment in group size probably reduces predation risk, as occurs in other rodents (Ebensperger and Wallem, 2002) and 2) in habitats or periods with limited water resource, sociability and grouping may increase to reduce water and energy stress, as has been evidenced in the mole rat (Spalax ehrenbergi) (Ebensperger, 2003).
Clumped patterns describe the main spatial structure in both seasons. Aggregation may occur in response to behavioral and ecological factors, including limited dispersal (Rees et al., 1996), predation avoidance and philopatry (Stein and Georgiadis, 2006) and environmental heterogeneity (Mokany, 2008), playing a fundamental role during the dry season when the spatial structure shows a higher degree of clumpness. Behavioral responses during the dry season may support the idea that individuals in the studied population may aggregate as a result of local habitat and seasonal climate changes, since flooding processes and features of the savanna are mainly determined by rainfall annual regime (Jongman et al., 2008). This behavior supports the idea that capybaras establish a "refuge", a favorable site that could be assumed as remnant swamps and water bodies, from which individuals disperse and return regularly to satisfy their metabolic and behavioral requirements (Odum, 2005). Grassland productivity, measured as aerial biomass, is higher during the rainy season (Ricaurte, 2007; Scholte, 2007), therefore food and water resources are not considered limiting factors. Thus, herd spatial distribution might be influenced by landscape characteristics, i.e., habitat homogeneity in regard to land refuges for resting (dry savannas) and escape coverage (tall grasses).
Spatial dynamics plays a different role at short scales. Since regular patterns were identified at this scale in both seasons it is probably correct to assume that territoriality (a characteristic behavior in capybaras; Macdonald, 1981; Herrera and Macdonald, 1989) may be influencing spatiation among herds. Regular patterns mainly occur because of intraspecific competition (Campbell, 1992). When the number of individuals within a group increases the per capita food income decreases, resulting in stronger intraspecific competition (Chapman et al., 1995). Although intraspecific competition for space has not been measured in capybaras, social behaviors (i.e., social tolerance) displayed in response to abiotic habitat conditions may lead herds to overlap home ranges and defend a territory with a common key resource (i.e., water). Such competition for space may result in a regular distribution at a local scale (Campbell, 1992), as was identified in our analyses.
Even though competition may create a density-dependent mechanism, this competition effect is compensated with a different behavioral and population mechanism that results in increased survival. Behavioral mechanism assumes an increase in effectiveness of individuals in seeking resources and an ability to modify the microclimatic and microhabitat conditions. Perhaps, in response to water scarcity, capybaras in seasonally flooded savannas dig ditches and bathing ponds that hold water during the dry season (Ojasti, 1973). On the other hand, population growth rate may be favored by population dynamics, since the maximum birthrate peak coincides with the end of the rainy season (Ojasti, 1973).
CONCLUSIONS
Since clumping was detected at different scales and degrees in both seasons, it is reasonable to expect ecological and behavioral forces to play different roles during each season. Given the higher clumping degree during the dry season, we will expect a strong influence of behavioral responses (i.e., predation avoidance) and environmental influences (i.e., resource scarcity). During this period food and water are scarce and most of the swamps and rivers dry up. Consequently, individuals compete strongly for resources such as food and space, resulting in these being limiting factors for capybara distribution, predation avoidance and reproductive behavior. Since water bodies are the main refuge from predators, and play an important role in body thermoregulation and reproduction (they are the main cover used for copulation; Ojasti, 1973), capybaras may aggregate toward these favorable environmental conditions to increase the ability to detect and escape from predators (protection given by the dilution effect; Ebensperger and Cofré, 2001) and the chance to be the sire of the next generation.
ACKNOWLEDGEMENTS
This work was supported by IDEAWILD and a grant from the Universidad Nacional de Colombia. We thank M. Delgado and our field assistants for providing logistical facilities, as well as G. Alarcón-Nieto and A. Camargo for very useful comments on an early draft of this paper. We also thank D. Blumstein and J. Pinzón for further constructive comments on this manuscript.
LITERATURE CITED
1. BADDELEY A and R TURNER. 2005. Spatstat: an R package for analyzing spatial point patterns. Journal of Statistical Software 12:1-42. [ Links ]
2. BAROT S, J GIGNOUX, and J MENAUT. 1999. Demography of a Savanna Palm Tree: Predictions from Comprehensive Spatial Pattern Analyses. Ecology 80:1987-2005. [ Links ]
3. BUCKLAND ST, DR ANDERSON, KP BURNHAM, JL LAAKE, DL BORCHERS, and L THOMAS. 2001. Introduction to Distance Sampling. Oxford University Press, London. [ Links ]
4. BYERS JA. 1992. Dirichlet Tessellation of Bark Beetle Spatial Attack Points. Journal of Animal Ecology 61:759-768. [ Links ]
5. CAMPBELL DJ. 1992. Nearest-neighbour graphical analysis of spatial pattern and a test for competition in populations of singing crickets (Teleogryllus commodus). Oecologia 92:548-551. [ Links ]
6. CAMPBELL DJ. 1996. Aggregation and regularity: an inclusive one-tailed nearest-neighbour analysis of small spatially patchy populations. Oecologia 106:206-211. [ Links ]
7. CHAPMAN C, W WRANGHAM, and L CHAPMAN. 1995. Ecological constraints on group size: an analysis of spider monkey and chimpanzee subgroups. Behavioral Ecology and Sociobiology 36:59-70. [ Links ]
8. CLARK KD and F EVANS. 1954. Distance to nearest neighbor as a measure of spatial relationships in populations. Ecology 4:445-453. [ Links ]
9. CRIST TO and JA WIENS. 1996. The distribution of ant colonies in a semiarid landscape: implications for community and ecosystem processes. Oikos 76:301-311. [ Links ]
10. EBEENSPERGER LA. 2003. Restricciones fisiológicas y evolución de la sociabilidad en roedores. Pp. 463-480, in: Fisiología Ecológica & Evolutiva (F Bozinovic, ed.). Ediciones Universidad Católica de Chile, Santiago, Chile. [ Links ]
11. EBEENSPERGER LA and H COFRÉ. 2001. On the evolution of group-living in the New World cursorial hystricogath rodents. Behavioral Ecology 12:227-236. [ Links ]
12. EBEENSPERGER LA and K WALLEM. 2002. Grouping increases the ability of the social rodent, Octodon degus, to detect predators when using exposed microhabitats. Oikos 98:491-497. [ Links ]
13. GONZÁLEZ-JIMÉNEZ E. 1995. El Capybara (Hydrochoerus hydrochaeris) - Estado actual de su producción. Estudio FAO Producción y Sanidad Animal 122, Roma, Italia. [ Links ]
14. GORDON DM and AW KULIG. 1996. Founding, foraging, and fighting: Colony size and the spatial distribution of harvester ant nests. Ecology 77:2393-2409. [ Links ]
15. HERRERA EA and D MACDONALD. 1989. Resource utilization and territoriality in group-living Capybaras (Hydrochaeris hydrochaeris). Journal of Animal Ecology 58:667-679. [ Links ]
16. HERRERA EA and D MACDONALD. 1993. Aggression, dominance, and mating success among Capybara males (Hydrochaeris hydrochaeris). Behavioral Ecology 4:114-119. [ Links ]
17. JONGMAN RHG, JK SMITH, EJ CHACÓN-MORENO, and JH LOEDEMAN. 2008. Asssessing flooding patterns in llanos of the Apure region (Venezuela) using radar images. ECOTRÓPICOS 21:34-45. [ Links ]
18. MACDONALD DW. 1981. Dwindling resources and the social behavior of Capybaras Hydrochaeris hydrochaeris (Mammalia). Journal of Zoology 194:371-391. [ Links ]
19. MANE S, C MURRAY, S SHEKHAR, J SRIVASTAVA, and A PUSEY. 2005. Spatial clustering of chimpanzee locations for neighborhood identification. Proceedings of the Fifth IEEE International Conference on Data Mining. [ Links ]
20. MOKANY K, J ASH, and S ROXBURGH. 2008. Effects of spatial aggregation on competition, complementarity and resource use. Austral Ecology 33:261-270. [ Links ]
21. MONES A and J OJASTI. 1986. Hydrochoerus hydrochaeris. Mammalian Species 264:1-7. [ Links ]
22. ODUM EP and GW BARRET. 2005. Fundamentos de ecología. 5ª edición, Editorial Thompson, México. [ Links ]
23. OJASTI J. 1973. Estudio biológico del chigüire o capibara. Fondo Nacional de Investigaciones Agropecuarias (FONAIAP). Editorial Sucre, Caracas. [ Links ]
24. OJASTI J. 1990. Ecology of Capybara raising on inundated savannas of Venezuela. Tropical Ecology and Development 1980:287-293. [ Links ]
25. OJASTI J. 1991. Human exploitation of Capybara. Neotropical wildlife use and conservation. Pp. 236-253, in: Neotropical Wildlife Use and Conservation (JG Robinson and KH Redford, eds.). Chicago University Press, Chicago. [ Links ]
26. PERRY JN. 1995. Spatial analysis by distance indices. Journal of Animal Ecology 64:303-314. [ Links ]
27. PIELOU EC. 1960. A single mechanism to account for regular, random and aggregated populations. Journal of Ecology 48:575-584. [ Links ]
28. PINTO GRM, KMPMB FERRAZ, HTZ COUTO, and LM VERDADE. 2006. Detectability of Capybaras in forested habitats. Biota Neotropica [online] 6:0-0. Available from: www.scielo.br/scielo. [ Links ]
29. REES M, P GRUBB, and D KELLY. 1996. Quantifying the impact of competition and spatial heterogeneity on the structure and dynamics of a four-species guild of winter annuals. American Naturalist 147:1-32. [ Links ]
30. RICAURTE J, IM RAO, and JC MENJIVAR. 2007. Rooting strategies of Brachiaria genotypes in acid and low fertility soils of Colombia. Acta Agronomica 56:107-115. [ Links ]
31. RIPLEY BD. 1976. The secondorder analysis of stationary point process. Journal of Applied Probability 13:255-266. [ Links ]
32. SCHABENNERGER O and AA GOTWAY. 2005. Statistical methods for spatial data analysis. Text in statistical science. Chapman and Hall/CRC Press, USA. [ Links ]
33. SCHOLTE P. 2007. Maximum flood depth characterizes aboveground biomass in African seasonally shallowly flooded grasslands. Journal of Tropical Ecology 23:63-72. [ Links ]
34. STATPOINT, INC. Statgraphics Centurion XV software. Version 15.0.04. http://www.statgraphics.com/ [ Links ]
35. STEIN A and N GEORGIADIS. 2006. Spatial marked point patterns for herd dispersion in a savanna wildlife herbivore community in Kenya. Pp. 261-273, in: Case Studies in Spatial Point Process Modeling (A Baddeley, P Gregori, J Mateu, R Stoica, and D Stoyan, eds.). Springer Berlin Heidelberg publisher. [ Links ]
36. STOYAN D and A PENTTINEN. 2000. Recent applications of point process methods in forestry. Statistics Statistical Science 15:61-78 [ Links ]
37. WALLER LA and CC GOTWAY. 2004. Applied spatial statistics for public health data. John Wiley and Sons Inc., New Jersey. [ Links ]
38. WIEGAND T and KA MOLONEY. 2004. Rings, circles, and null-models for point pattern analysis in ecology. Oikos 104:209-229. [ Links ]














