Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Mastozoología neotropical
versión impresa ISSN 0327-9383
Mastozool. neotrop. vol.18 no.1 Mendoza ene./jun. 2011
ARTICULOS Y NOTAS
The karyotype of a rare south american marsupial, the bushy-tailed opossum genus Glironia (Didelphimorphia: Didelphidae)
Cleiton Fantin1 and Maria Nazareth F. da Silva2
1 Universidade do Estado do Amazonas, Departamento de Biologia. Av. Djalma Batista, 2470, 69050-010, Manaus, AM, Brazil.
2 Instituto Nacional de Pesquisas da Amazônia, Coleção de Mamíferos, Caixa Postal 478, Manaus-AM, CEP 69060-000, Brazil. [Correspondence: Maria Nazareth F. da Silva <nazareth@inpa.gov.br>]
ABSTRACT: We describe for the first time the karyotype of Glironia venusta (Didelphidae, Didelphimorphia), a rare South American marsupial. G. venusta has a diploid number 2n=18 and a number of autosomal arms NA=22. The diploid number of 18 chromosomes is somewhat unexpected, considering prior assignment of Glironia to the subfamily Caluromyinae (2n=14 in other species of the subfamily). The only other didelphid genus known to have 2n=18 chromosomes is Monodelphis. Chromosome banding, however, revealed a pattern closer to that observed for the 2n=14 species. Considering recent studies in didelphid phylogeny, wherein Glironia is sister to the remaining members of the family, the diploid number observed for Glironia may represent the ancestral state of the family, or may result from convergent evolution.
RESUMEN: Cariotipo de un marsupial suramericano raro, zarigüeya de cola peluda, del género Glironia (Didelphidae, Didelphimorphia). Se describe aquí por primera vez el cariotipo de Glironia venusta. El número diploide de G. venusta es 2n=18 y el número de brazos de autosómicos NA=22. El número diploide de 18 es un tanto inesperado, considerando la inclusión anterior de Glironia a la subfamilia Caluromyinae (2n=14 para las otras especies conocidas). El único otro género conocido de Didelphidae presentando 2n=18 es Monodelphis. Sin embargo, estudios de bandas de cromosomas muestran un patrón mas parecido al observado para las especies 2n=14. Considerando estudios recientes en la filogenia de los Didelphidae, donde se sugiere la hipótesis de que Glironia sea el grupo hermano de los restantes miembros existentes de esta familia, el número diploide observado puede representar el estado ancestral de la familia, o puede haber resultado de evolución convergente.
Key words: Ag-NOR; Amazonia; C-banding; Conventional staining.
Palabras clave: Ag-NOR; Amazonia; Bandas C; Coloración convencional.
The karyotypes of didelphid marsupials are characterized by a relatively low number of chromosomes, with diploid numbers of 2n = 22 in the larger species of opossums (Didelphis, Philander, Chironectes and Lutreolina) and one species of "mouse-opossum" Tlacuatzin canescens (Engstrom and Gardner, 1988; Zarza et al., 2003), 2n = 18 in the short-tailed opossums of the genus Monodelphis and 2n = 14 (by far the most common diploid number) in Metachirus, Caluromys and five genera of small-bodied "mouse opos sums" (Gracilinanus, Marmosa, Marmosops, Micoureus and Thylamys; Carvalho et al., 2002, and references cited therein).
The bushy-tailed opossum Glironia venusta (Didelphidae, Didelphimorphia) is considered one of the rarest South American marsupials and is listed as "Vulnerable" in the 2006 IUCN Red List of Threatened Species (IUCN, 2006). Very little is known about its habits and natural history. It is considered to be arboreal and has a highly developed hallux (Marshall, 1978; Emmons and Feer, 1997). In addition to its abilities for arboreal locomotion, the postcranial morphology of Glironia venusta also indicates some capacities for terrestrial habits (Flores and Díaz, 2009). Indeed, G. venusta has also been observed to use the lower strata of the forest (MNF da Silva, pers. obs.), has been captured with live traps in the forest understory (Díaz and Willig, 2004; Santos-Filho et al., 2007) and has also been captured in pitfall traps on the ground (Bernarde and Rocha, 2003).
Known originally from Bolivia, Ecuador and Peru (Marshall, 1978; Díaz and Willig, 2004), its occurrence in Brazil has now been documented at nine widely separated localities in the Amazon and Paraguay basins (da Silva and Langguth, 1989; Patton et al., 1996; Nogueira et al., 1999; Bernarde and Rocha, 2003; Santos-Filho et al., 2007; Calzada et al., 2008, Rossi et al., 2010). Voucher specimens from Brazil are deposited in the mammal collection at the National Institute for Amazonian Research (INPA 699, 2570, 4577, 5237), in the Museu de Zoologia da Universidade de São Paulo (MZUSP 34664), and in the mammal collection of the Universidade Federal de Mato Grosso (UFMT 696) (Santos-Filho et al., 2007; Rossi et al., 2010). The sites in Rondônia (Bernarde and Rocha, 2003), Manaus (Calzada et al., 2008) and in southeastern Pará (Rossi et al., 2010) do not have voucher specimen. According to Barkley (2007), the total number of known specimens of G. venusta is under 25.
In this report, the description of the karyotype of the bushy-tailed opossum Glironia venusta is presented for the first time.
The single specimen available for cytogenetic analyses (INPA 4577; skull and body in fluid) represents a young female from Teotônio, on the right bank of the Rio Madeira, 19 km SW of Porto Velho, Rondônia, Brazil (8°52'27"S 64°0'28" W).
A suspension of mitotic metaphase bone-marrow cells was obtained in the field from this single available specimen employing a slightly modified version of Patton's (1967) colchicine-hypotonic salt and Carnoy's fixative protocol using a hand-operated centrifuge. Air-dried slides were subsequently prepared and stained with Wright's stain; a total of 30 cells were examined and five of those were photographed under oil immersion using bright-field optics. Chromosomes were sorted by decreasing size and classified according to Levan et al. (1964). C-banding (in a total of 10 cells) and silver staining of the nucleolar organizer regions (Ag-NORs - in 20 cells) were performed following protocols described by Sumner (1972) and Howell and Black (1980), respectively.
The diploid number of chromosomes in the female bushy-tailed opossum Glironia venusta is 18. The karyotype (Fig. 1) consists of 2 large pairs of metacentric/submetacentric (pairs 1 and 2), 1 pair of subtelocentric (pair 5) and 5 pairs of acrocentric autosomes (pairs 3, 4 and 6 to 9). We presume that the smallest pair of acrocentrics represents the X chromosomes, as this pattern has been found for many didelphid species (Palma and Yates, 1996; Svartman and Vianna-Morgante, 1999, 2003; Carvalho et al., 2002). The number of autosomal chromosomal arms (FN) is 22.

Fig. 1. Glironia venusta karyotype (2n = 18, FN = 22; INPA 4577); the presumptive sex chromosomes are indicated.
Silver-staining analysis revealed the activity of nucleolar organizer regions (NORs) on the short arms of both acrocentric chromosomes of autosomal pair number 6; the presumptive sex chromosomes did not show NOR activity (Fig. 2A and B).

Fig. 2. (A) Metaphase and (B) karyotype showing the NOR-bearing chromosomes and (C) metaphase showing the C-band pattern of Glironia venusta.
Constitutive heterochromatin was not observed in the autosomes of G. venusta. C-bands are restricted to sex chromosomes where they are revealed as diffusely stained blocks in the pericentromeric region of both presumptive X chromosomes (Fig. 2C; Table 1).
Table 1
Karyotype and C-banding patterns of didelphid marsupials.
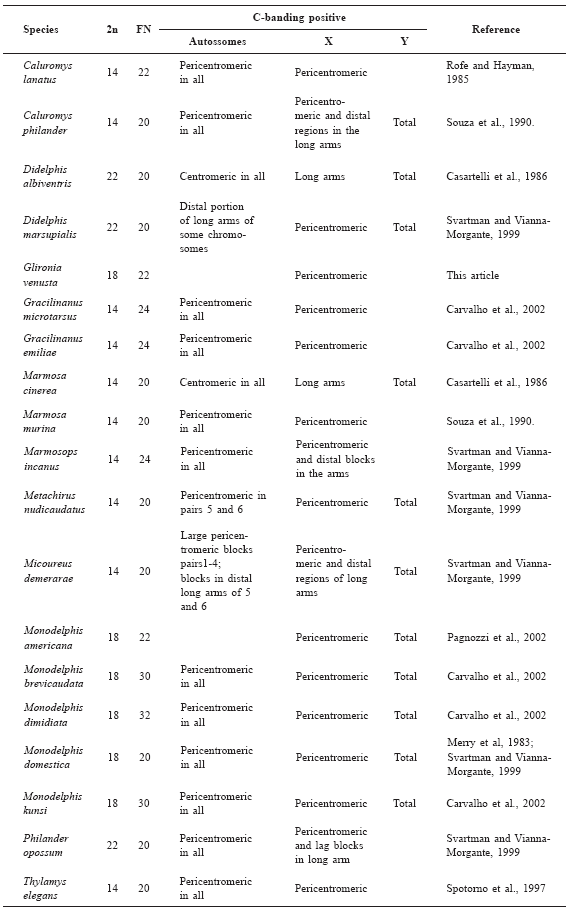
G-banding and treatment with CMA3 were performed but we did not yield reliable results. These trials were probably hampered by the high condensation of the chromosomes and by limited suitable material in the cell suspensions. When additional materials become available, a new trial of both sets of analyses should be performed.
In general, the distribution and expression of NORs in marsupials has been reported on one or more autosomal pairs, alone or in association with the sex chromosomes; in the latter case, NORs are either restricted to the X chromosome or they are found on both X and Y chromosomes (Svartman and Vianna-Morgante, 2003). According to Svartman and Vianna-Morgante (1999, 2003), pair 7 in species with 2n = 22, corresponds to pair 5 in species with 2n = 18 and pair 6 in species with 2n = 14 and these homeologous pairs are NOR-bearing chromosomes. Although pairs 5 and 6 are of similar size in the Glironia karyotype, pair 5 is approximately 9.0% larger then the NOR-bearing pair 6. This pattern contrasts to the pattern found in the 2n = 18 karyotype but fits with that of the 2n = 14 species, including its presence on the short arms, reinforcing the idea that the presence of NORs on this chromosome is a conserved condition in Didelphidae.
The only other didelphid genus previously reported to have a diploid number of 18 is Monodelphis. The fundamental number among species of Monodelphis varies considerably from 22 to 32 (Carvalho et al., 2002). Among these karyotypes (e.g., by Palma and Yates, 1996; Carvalho et al., 2002; Svartman and Vianna-Morgante, 2003), that of M. glirina from Bolivia (reported as M. brevicaudata; see Voss et al., 2001) by Palma and Yates (1996) is very similar to that of Glironia, despite differences between our respective classifications of chromosomes (compare op. cit.: Figure 4 and Table 1 with our Fig. 1 and the karyotypic description above). Additional work such as chromosome banding and in-situ hybridization may determine the degree of similarity between these two karyotypes.
The similarity in diploid number and karyotype between these two genera is somewhat surprising since Glironia is often classified with Caluromys (2n=14) and Caluromysiops (diploid number unknown) in a distinct subfamily or family (Hershkovitz, 1992; Kirsch et al., 1995; Kirsch and Palma, 1995; McKenna and Bell, 1997; Gardner, 2005). However, the phylogenetic work of Voss and Jansa (2009), based on cladistic analyses of both molecular sequences and morphological characters, supports an alternative hypothesis, namely that Glironia is sister to all other opossums and is not the sister group of a Caluromys+Caluromysiops clade. The separation of Glironia from Caluromys and Caluromysiops is also reinforced by the distinctiveness of the skeletal morphology of Glironia when compared to these two genera (Flores and Díaz, 2009). If Glironia is sister to all other opossums, then the 2n=18 karyotype could either be ancestral for didelphimorphs, or the similarity in diploid number of Glironia and Monodelphis resulted from convergent evolution. The validity of these hypotheses can be examined in future analyses of cytogenetic and molecular markers once such are available.
Acknowledgements. The specimen used for this study was obtained during a consulting project funded by Furnas Centrais Elétricas S.A. We are grateful to Dr. Antônia Franco, project coordinator, and to the project field biologists Bárbara A. Costa, Carla G. Bantel, Manoel dos Santos Filho, Dionei José da Silva and Carlos Leonardo G. Vieira who collected the Glironia specimen and prepared the cell suspension in the field. We thank Drs. James L. Patton and Robert S. Voss for reviewing the manuscript and Dr. Marta Svartman for helpful criticisms, but any errors of fact or interpretation are our responsibility. We also thank Dr. Eliana Feldberg for providing support and permission to work on her laboratory at INPA. Thanks also to Glenn H. Shepard Jr for editorial assistance on the final draft and figures. MNFS research is partly funded by CNPq and FAPEAM, Brazil.
LITERATURE CITED
1. BARKLEY LJ. 2007. Genus Glironia O. Thomas, 1912. Pp 12-14, in: Mammals of South America (AL Gardner, ed). The University of Chicago Press, Chicago. [ Links ]
2. BERNARDE PS and VJ ROCHA. 2003. New Record of Glironia venusta (Bushy-tailed opossum) (Mammalia, Glironiidae) for the state of Rondônia, Brasil. Biociências, Porto Alegre, 11(2):183-184. [ Links ]
3. CALZADA J, M DELIBES, C KELLER, F PALOMARES,and W MAGNUSSON W. 2008. First record of the bushy-tailed opossum, Glironia venusta, Thomas, 1912, (Didelphimorphia) from Manaus, Amazonas, Brazil. Acta Amazônica 38(4):26. [ Links ]
4. CARVALHO BA, LFB OLIVEIRA, AP NUNES, and MS MATTEVI. 2002. Karyotypes of nineteen marsupial species from Brazil. Journal of Mammalogy 83(1):58-70. [ Links ]
5. CASARTELLI C, SR ROGATTO, and I FERRARI. 1986. Cytogenetic analysis of some Brazilian marsupials (Didelphidae: Marsupialia). Canadian Journal of Genetics and Cytology 28:21-29. [ Links ]
6. DA SILVA MNF and A LANGGUTH. 1989. A new record of Glironia venusta from the lower Amazon, Brazil. Journal of Mammalogy 70(4):873-875. [ Links ]
7. DÍAZ MM and MR WILLIG. 2004. Nuevos registros de Glironia venusta y Didelphis albiventris (Didelphimorphia) para Perú. Mastozoología Neotropical. [ Links ]
8. EMMONS LE and F FEER. 1997. Neotropical rainforest mammals, a field guide, 2nd edition. Chicago: Univ. Chicago Press. [ Links ]
9. ENGSTROM MD and AL GARDNER. 1988. Karyotype of Marmosa canescens (Marsupialia: Didelphidae): a mouse opossum with 22 chromosomes. Southwestern Naturalist 33:231-233. [ Links ]
10. FLORES D and M DIAZ. 2009. The postcranial skeleton of Glironia venusta (Didelphidae, Caluromynae): description and functional morphology. Zoosystematic & Evolution 85:311-339. [ Links ]
11. GARDNER AL. 2005. Order Didelphimorphia. In: Mammal Species of the World (DE Wilson and DM Reeder, eds.). Mammal Species of the World, 2; 142 pp. Johns Hopkins University Press. [ Links ]
12. GARDNER AL. 2007. Subfamily Caluromyinae Kirsch, 1977. P. 3, in: Mammals of South America (AL Gardner, ed.). 669 pp. The University of Chicago Press, Chicago. [ Links ]
13. HERSHKOVITZ P. 1992. The South American gracile mouse opossums, Genus Gracilinanus Gardner and Creighton, 1989 (Marmosidae, Marsupialia): a taxonomic review with notes on general morphology and relationships. Fieldiana Zool. 70:1-56. [ Links ]
14. HOWELL WM and DA BLACK. 1980. Controlled silver staining of nucleolus organizing regions with a protective colloidal developer: a one step method. Experientia 36:1014-1015. [ Links ]
15. IUCN 2006. New World Marsupial Specialist Group 1996. Glironia venusta. In: IUCN 2006. Red List of Threatened Species. <www.iucnredlist.org>. Downloaded on 04 November 2006. [ Links ]
16. KIRSCH JAW and RE PALMA. 1995. DNA-DNA hybridization studies of carnivorous marsupials. V. A Further estimate of relationships among opossums (Marsupialia: Didelphidae). Mammalia 59:402-425. [ Links ]
17. KIRSCH JAW, AW DICKERMAN, and OA REIG. 1995. DNA/DNA hybridization of carnivorous marsupials.IV. Intergeneric relationships of the opossums (Didelphidae). Marmosiana 1:57-78. [ Links ]
18. LEVAN A, K FREDGA, and AA SANDBERG. 1964. Nomenclature for centromeric position on chromosomes. Hereditas 52:201-220. [ Links ]
19. MARSHALL LG. 1978. Glironia venusta. Mammalian Species 107:1-3. [ Links ]
20. MCKENNA MC and SK BELL. 1997. Classification of mammals above the species level. New York: Columbia University Press. [ Links ]
21. MERRY DE, S PATHAK, and JL VANDEBERG. 1983. Differential NOR activities in somatic and germ cells of Monodelphis domestica (Marsupialia, Mammalia). Cytogenetics and Cell Genetics 35:244-251. [ Links ]
22. NOGUEIRA JC, MNF DA SILVA, and BGO Câmara. 1999. Morphological description of the male genital system of the bushy-tailed opossum Glironia venusta Thomas, 1912 (Didelphimorphia: Didelphidae). Mammalia 63:231-236. [ Links ]
23. PATTON JL. 1967. Chromosome studies of certain pocket mice, genus Perognathus (Rodentia: Heteromyidae). Journal of Mammalogy 48:27-37. [ Links ]
24. PATTON JL, SF DOS REIS and MNF DA SILVA. 1996. A molecular view of relationships among didelphid marsupials: sequence variation in the mitochondrial cytochrome b gene. Journal of Molecular Evolution 3:3-29. [ Links ]
25. ROFE R and D HAYMAN. 1985. G-banding evidence for a conserved complement in the Marsupialia. Cytogenetics Cell Geneics 39:40-50. [ Links ]
26. ROSSI RV, CL MIRANDA, TS SANTOS JUNIOR, and TBF SEMEDO. 2010. New records and geographic distribution of the rare Glironia venusta (Didephimorphia, Didelphidae). Mammalia 74: 445-447. [ Links ]
27. SANTOS-FILHO M, MNF DA SILVA, BA COSTA, CG BANTEL, CLG VIEIRA, DJ SILVA, and AMR FRANCO. 2007. New records of Glironia venusta, Thomas, 1912 (Mammalia, Didelphidae), from the Amazon and Paraguay Basins, Brazil. Mastozoología Neotropical 14(1):103-105. [ Links ]
28. SCHMID M. 1980. Chromosome banding Amphibia, IV. Differentiation of GC- and AT- rich chromosoma regions in Anura. Chromosoma 77:83-103. [ Links ]
29. SOUZA, MJ, V MAIA, and JF SANTOS. 1990. Nucleolar organizer regions, G- and C-bands in some Brazilian species of Didelphidae. Brazilian Journal of Genetics 13:767-775. [ Links ]
30. SUMNER AT. 1972. A simple technique for demonstrating centromeric heterochromatin. Experimental Cell Research 75:304-306. [ Links ]
31. SVARTMAN M. and AM VIANNA-MORGANTE. 1999. Comparative genome analysis in American marsupials: chromosome banding and in-situ hybridization. Chromosome Research 7:267-275. [ Links ]
32. SVARTMAN M and AM VIANNA-MORGANTE. 2003. Conservation of chromosomal location of nucleolus organizer in American marsupials (Didelphidae). Genetica 118:11-16. [ Links ]
33. VOSS RS, DP LUNDE, and NB SIMMONS. 2001. The mammals of Paracou, French Guiana: a Neotropical lowland rainforest fauna. Part 2. Nonvolant species. Bulletin of the American Museum of Natural History 263. [ Links ]
34. VOSS RS and SA JANSA. 2009. Phylogenetic relationships and classification of didelphid marsupials II, an extant radiation of new world metatherian mammals. Bulletin of the American Museum of Natural History 322. [ Links ]
35. ZARZA H, G CEBALLOS, and MA STEELE. 2003. Marmosa canescens. Mammalian Species no. 725, pp. 1-4. [ Links ]
Recibido 25 enero 2010.
Aceptado 17 marzo 2011.
Editor asociado: E Lessa














