Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Mastozoología neotropical
versión impresa ISSN 0327-9383
Mastozool. neotrop. vol.18 no.2 Mendoza jul./dic. 2011
ARTÍCULOS Y NOTAS
Abundance and activity patterns of the margay (Leopardus wiedii) at a mid-elevation site in the Eastern Andes of Ecuador
E. Natasha Vanderhoff1, Anne-Marie Hodge2, Brian S. Arbogast2, Jonas Nilsson3, and Travis W. Knowles4
1 Department of Biology and Marine Science, Jacksonville University, 2800 University Blvd N., Jacksonville, FL 32211, USA [Correspondence: Natasha Vanderhoff <nvander4@ju.edu>].
2 Department of Biology and Marine Biology, University of North Carolina-Wilmington, 601 S. College Rd., Wilmington, NC 28403, USA.
3 Wildsumaco Wildlife Sanctuary S.A., Pacto Sumaco, Ecuador.
4 Department of Biology, Francis Marion University, Florence, SC 29502, USA.
ABSTRACT: We investigated abundance and activity patterns of the margay (Leopardus wiedii) via remote camera-trapping at Wildsumaco Wildlife Sanctuary, a new preserve located near Sumaco National Park, in the eastern Andean foothills of Ecuador. We recorded 85 capture events and ten individuals, including one juvenile, over a total of 3220 camera-trap nights. Activity was mostly nocturnal and we found no difference in abundance between primary and secondary forest. Our capture rate (2.64 captures/100 camera-trap nights) indicates a high abundance of margays in the region. The site lies within a matrix of agricultural lands and the high abundance and seasonal occurrence data seem to suggest that while one or two females may occupy the area permanently, most margays likely use the site as a corridor. Continued deforestation and habitat alteration pose a serious threat to the margays of the region. The data we present here suggest that an unusually high abundance of margays occur at the site, making it an important area for continued research and conservation efforts.
RESUMEN: Abundancia y patrones de actividad del margay (Leopardus wiedii) en un sitio de elevación media en los Andes Orientales de Ecuador. Hemos investigado la abundancia y los patrones de actividad del margay o tigrillo (Leopardus wiedii) a través de cámaras-trampa remotas en el Santuario de Vida Silvestre Wildsumaco, una nueva reserva situada cerca del Parque Nacional Sumaco, en las estribaciones orientales de los Andes del Ecuador. Se registraron 85 capturas de diez individuos, incluyendo un individuo juvenil, tras un total de 3.220 trampas noche. La actividad registrada fue principalmente nocturna y no encontramos diferencias en la abundancia entre bosque primario y secundario. Nuestra tasa de captura (2.64 capturas/100 trampas noche) indica una gran abundancia de tigrillos en la región. El sitio se encuentra dentro de una matriz de tierras agrícolas, y la frecuencia de registros y la ocurrencia estacional parecen sugerir que, si bien una o dos hembras pueden ocupar el área de forma permanente, es probable que la mayoría de los tigrillos utilice el sitio como un corredor. La persistente deforestación y la alteración del hábitat representan una amenaza grave para los tigrillos en la región. Los datos que aquí presentamos indican una abundancia anormalmente alta de tigrillos en esta zona, por lo que esta constituye un área importante para continuar con la investigación y los esfuerzos de conservación.
Key words: Camera-trap; Carnivores; Felidae; Sumaco Napo-Galeras National Park; Wildsumaco
Palabras clave: Cámaras-trampa; Carnívoros; Felidae; Parque Nacional Sumaco Napo-Galeras; Wildsumaco
INTRODUCTION
The margay (Leopardus weidii), one of the least known Neotropical felids, is listed as Globally Near Threatened by the International Union for Conservation of Nature (IUCN) and recent findings suggest that margays may be less abundant than previously thought (Payan et al., 2008). Despite their large geographic distribution (central Mexico to Uruguay; Fig. 1), the margay often occurs at low densities (i.e., ≤ 5 individuals/100 km2; de Oliveira, 1998). This species is primarily associated with dense forest habitats; however, margay populations have also been observed to extend into more disturbed forests and near human settlements (de Oliveira, 1998). Margays are capable of hunting prey in the trees and possess morphological adaptations associated with this foraging strategy, such as their distinctive long tail and hind foot that can both pronate and supinate, allowing them to climb down trees head first (Eisenberg, 1989; de Oliveira, 1998; Calleia et al., 2009). Despite these specializations, margays also move along the ground frequently, and, accordingly, their diet consists of both arboreal and terrestrial animals (Emmons and Feer, 1990; de Oliveira, 1998). The margay has a more generalized diet than that of other small Central and South American cats, such as the ocelot (Leopardus pardalis) and oncilla (Leopardus tigrinus); it consumes a wide variety of small vertebrates, including arboreal mammals and birds, as well as fruit (Konecny, 1989; Wang, 2002). The margay's ability to move and hunt efficiently both throughout the forest canopy and along the ground allows this species to occupy an unusual and important niche within Neotropical communities (Di Bitetti et al., 2010). Deforestation, habitat fragmentation, and poaching all pose threats to remaining margay populations (Payan et al., 2008), yet it is difficult to construct effective management plans when so little is known about the natural history of this species.
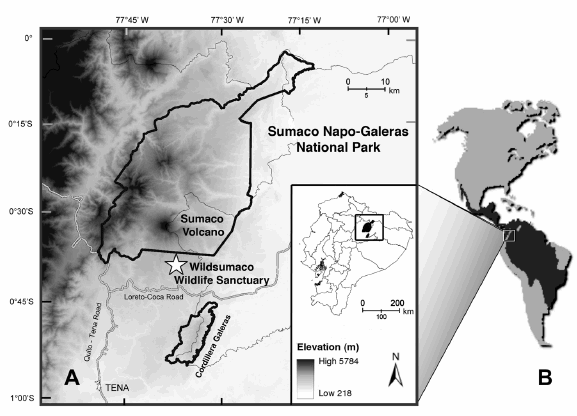
Fig. 1. Location of Wildsumaco Wildlife Sanctuary and Sumaco Napo-Galeras National Park, Ecuador (A), and geographic distribution of the margay (B). The sanctuary (indicated by star) is located at an elevation of approximately 1400 m in the eastern foothills of the Andes, southeast of Sumaco Volcano.
The margay's elusive behavior, sparse occurrence throughout its distribution, and apparent preference for dense primary forest habitats make it a challenging species to study, and as a result, many aspects of its activity and movement patterns remain poorly documented. Traditional studies of carnivore activity patterns typically involved highly invasive procedures, including capture, sedation, and attachment of detection devices to individuals. These procedures often place significant stress on the animals, and may potentially change the very behaviors researchers are attempting to study (Powell and Proulx, 2003). Alternatively, non-invasive sampling methods have also been used to study carnivores. In particular, scat and track surveys, which minimize disruption to both the environment and natural behavior patterns of the focal species, have been widely used (Heinemeyer et al., 2008). These sampling regimes involve significant limitations, however, because both scat and tracks are often difficult to locate and are quickly degraded in dense, wet habitats, such as tropical forest (Foresman and Pearson, 1998). Recently, major advances in technology have allowed researchers to survey wildlife populations using remote cameras (Karanth 1995; Trolle and Kery, 2003; Kelly and Holub, 2008). These "camera-trapping" surveys have been used to successfully study a variety of Neotropical felids, including jaguars (Panthera onca; Wallace et al., 2003; Silver et al., 2004), ocelots (Leopardus pardalis; Di Bitetti et al. 2006; Maffei and Noss, 2008), pumas (Puma concolor; Kelly et al., 2008), and Geoffroy's cats (Leopardus geoffroyi; Cuellar et al., 2006). Camera-trapping methods are especially useful for studying species with prominent diagnostic features, such as the spotted coat of the margay, because each individual bears distinctive and unique markings. As such, individuals can be identified and be monitored without the need to artificially tag or mark the subject animals.
In order to obtain basic data on margays in the eastern foothills of the Andes, we conducted camera-trapping surveys at the Wildsumaco Wildlife Sanctuary in Ecuador from December 2008 to July 2010. We used digital photographs obtained from camera-traps to (1) identify individual margays; (2) estimate relative abundance of margays; and (3) document habitat usage, activity patterns and residence time.
METHODS
Study Site
Wildsumaco Wildlife Sanctuary (hereafter WWS) is located on the eastern slopes of the Andes in Ecuador (1400 m elevation; 00° 41.250' S, 77° 36.049' W; Fig. 1). The sanctuary and its associated non-profit Río Pucuno Foundation were established in 2007 to protect high-quality forested habitats in the area and discourage further deforestation. As part of the mission of the foundation, several parcels of land have been purchased. The sanctuary itself is approximately 400 hectares in size and comprises eight parcels of land, all of which are near to, although not always contiguous with, one another. The sanctuary intermingles with agricultural lands consisting of fields of naranjilla (Solanum quitoense), a popular fruit used for juice, and pasture land for domestic cattle, as well as both primary and secondary forests.
Camera Sampling Method
We used 12 Reconyx (Holmen, Wisconsin) Rapidfire RC55 cameras with an infrared sensor set to run continuously at each station. Each image was individually time stamped and dated. As this was an initial survey, we established a sampling regime aimed at maximizing the probability of capture. Cameras were placed throughout a 5 km2 sampling area, typically near human or game trails, and moved approximately every 12-16 weeks in order to cover both primary and secondary forest. Distance among cameras varied, but was always greater than 200 m. In addition we used only one camera per trapping station and cameras were positioned one meter or less off the ground. Our study consisted of a total of 3220 camera-trap nights and two main sampling periods: December 2008 through July 2009 (Period 1) and November 2009 through July 2010 (Period 2; see Table 1 for sampling details). Each camera remained at a station for a minimum of 22 nights and a maximum of 113 nights (average of 73 nights).
Table 1
Camera sampling dates and habitat types for investigating margays at Wildsumaco Wildlife Sanctuary, Ecuador.

Analyses
Margays were easily distinguished from other felids, including ocelots, by their proportionately long tails (Eisenberg, 1989); we had several images for each capture event, and we examined the ratio of the tail to hind leg length to verify that each capture was indeed a margay. Two of the authors (ENV and AMH) separately examined images for individual identifications; we independently identified individuals using distinguishing markings, such as stripes, spots and tail bands (Fig. 2). This method has been used for other patterned Neotropical felids like ocelots and jaguars (see Trolle and Kery, 2003; Silver et al., 2004). In all but one case (a juvenile) we were able to determine the sex of an individual based on the presence or absence of testes. Juveniles were determined based on size and if they were photographed following an adult female on camera. Despite using a single camera per station, we typically obtained multiple photographs of each individual during a single capture event (i.e., margays would turn in front of the camera allowing for analysis of both sides of the animal, but see results for more details). This allowed us to determine the number of unique margays observed during the study, as well as the sex of all adult individuals.

Fig. 2. Margay coat patterns are unique for individuals and can be used to identify recapture events. (A) Four different individuals. B) The same individual (Female 2) photographed on three separate occasions. Yellow ovals in B highlight a unique Y-shaped marking running laterally on the left side of Female 2.
We used temporal data to reconstruct individual residence time at the sanctuary and overall margay activity patterns. For activity patterns we calculated the absolute number of captures occurring during hourly intervals. Sunrise and sunset occur approximately 12 hours apart, at 06:00 and 18:00 hours.
We estimated abundance indirectly by calculating capture rates (number of margay captures/ 100 camera-trap nights). A "capture" was defined as an independent photographic event (typically containing a series of photographs), occurring at least 0.5 hours apart from any other such event. We calculated capture rates for each sampling period and compared margay counts (number of captures) with a Poisson test to see if captures differed significantly between sampling periods. We also calculated the capture rate for the entire study period. To ascertain habitat usage by margays, we compared captures between the two habitats using a Poisson test. All Poisson tests were done with ProcGenmod in SAS (SAS Institute, 2003).
RESULTS
A total of 85 separate captures were recorded over the entire study period. From these photographs, we identified 10 individuals (2 males, 7 females, and one juvenile of undetermined sex; Table 2). On average, an individual margay was captured 7.5 times (range 1-17 captures). Monthly data suggest a high turnover rate at the site (Fig. 3); for example, only one female (F2) was photographed throughout the study period, and one of the two males (M2) was only captured twice over the whole duration of the study, both times within the same month and on the same camera. Temporal activity patterns were almost exclusively nocturnal, with peaks of activity between 01:00-03:00 and 22:00-00:00 hours (Fig. 4). Only 3 capture events were between 07:00 and 17:00 hours.
Table 2
Camera-trap capture data for individual margays at Wildsumaco Wildlife Sanctuary, Ecuador.


Fig. 3. Monthly occurrence of individual margays (grey bars) at Wildsumaco Wildlife Sanctuary, Ecuador over a two year period (Dark bars indicate periods in which no sampling was conducted).

Fig. 4. Margay activity patterns based on number of camera-trap captures at Wildsumaco Wildlife Sanctuary, Ecuador.
Capture rates did not significantly differ between the two survey periods (Period 1 = 2.29 captures/100 camera-trap nights, Period 2 = 3.52 captures/100 camera-trap nights; Poisson Test χ2 = 3.54, p = 0.0599). Therefore, we combined the data and estimated an overall capture rate for the study of 2.64 captures/100 camera-trap nights. In total, primary and secondary forests were surveyed for 2146 and 1074 camera-trap nights, respectively. Capture rates were not significantly different between the two habitats (primary forest = 2.52 captures/100 camera-trap nights, secondary forest = 2.70 captures/100 camera-trap nights; Poisson Test, χ2 = 0.37, p = 0.545).
DISCUSSION
Our survey of margays resulted in 85 separate capture events of 10 individuals over the course of 3220 trap nights. These are the first demographic and temporal data reported for margays in the eastern foothills of the Andes. Our data suggest that a few males and several females typically occupy the reserve at any given time. Males at our site likely hold territories from which same sex individuals are excluded; yet both males and females appear to tolerate overlapping spatial occupancy with additional females. In most felids males will occupy a territory in which several females reside (Emmons, 1988; Di Bitetti et al., 2006); this also may be the case with the margays at our site. Of all of the margays recorded, Females 1 and 2 appeared to share the site for the longest period of time, with sightings of each of them made at the site for at least 5 consecutive months. The temporal dominance of the two females at the site indicates that this population may consist of a mix of resident and transient individuals, or that it may overlap only slightly with the territorial borders of the less-frequently captured individuals. Telemetry data or GPS tracking would be required to elucidate detailed information on movement patterns and spatial relationships among individuals at Wildsumaco.
The total abundance of margays detected within a given month appeared to vary seasonally, peaking in the spring and summer rainy season (June-July). The highest number of margays detected at WWS within a given month was four, during June 2010. This is the height of the rainy season at WWS. Furthermore, during November, one of the driest months of 2009 (unpubl. data), only one individual was recorded. Although slightly speculative at this stage, our data suggest that resource abundance related to rainfall may be an important factor influencing margay density at WWS. Alternatively, studies in captive populations have shown that margay sperm production increases in summer months (Morais et al., 2002); therefore, it also might be possible that courtship activity increases during the months of December, January and February resulting in more frequent camera detections. Continued sampling throughout the year will be needed to verify the possible correlation between margay abundance, rainfall and reproductive cycle.
Our overall capture rate of 2.64 captures/100 camera-trap nights (capture success of 0.026%) suggests that WWS, and the eastern Andean foothills of Ecuador in general, might harbor a relatively high abundance of margays compared to other areas. Previous camera surveys of margay populations elsewhere have had lower success rates, such as Goulart et al. (2009), in which margays were captured by cameras only 15 times in the course of 4825 trap nights (0.003% success) covering a 30 km2 area in Southern Brazil. Similarly, Tobler et al. (2008) had less than one margay capture per 100 camera-trap nights in Peru. Other camera-trap investigations, especially those conducted in areas that also have ocelots, have detected either few or no margays (Trolle, 2003; Maffei et al., 2005; Di Bitetti et al., 2010). A recent capture of an ocelot at our site indicates that ocelots do occur at WWS, but do so at low abundances or very infrequently, especially relative to margays (A. M. Hodge, unpublished data). The low abundance of ocelots at our site may reduce interspecific competition and allow for a greater number of margays, as has been suggested by other researchers (Payan et al., 2008; de Oliveira et al., 2010). The unusually high density of margays at WWS is likely to have an important influence on the overall community ecology of immediate and surrounding areas. Furthermore, source-sink dynamics are critical for maintaining healthy carnivore populations in areas where habitat destruction and hunting exist (Robinson et al., 2010), and high-density sites such as WWS likely play an important role in maintaining regional populations by replenishing margay abundance in localities in which anthropogenic and environmental pressures are causing significant attrition of margay populations. Future research will seek to establish whether margay activity patterns correlate, either positive or negatively, with those of other sympatric carnivores.
The high local abundance of margays we found at WWS may also be influenced by sampling; the sanctuary's relatively small size (< 5 km2) and/ or configuration of the sanctuary within the surrounding matrix, could lead to overestimates of abundance if the area surveyed is relatively small in relation to the animal's home range (Maffei and Noss, 2008). We do not know the home range for margays in the area of WWS, and estimates of the typical home range for this species vary widely, ranging from 1-20 km2 (Payan et al., 2008). Given this uncertainty, at this moment we cannot accurately estimate margay density. Other studies of forest animals in South America have shown that protected areas can harbor elevated densities of some species, due to surrounding habitat destruction (Defler, 1981). The forested areas of the WWS exist as patches within a matrix of agricultural activities, and it appears that different individual margays concentrate their activities within these protected forested patches with intervening pasture land. Margays also may be using the sanctuary as a corridor to move from less favorable patches to more favorable ones. WWS is close to Sumaco National Park, and it is likely that margays use the forested areas that connect the sanctuary and the park as corridors. This would be consistent with our observations that most individuals captured appeared at the site for only a short period of time, suggesting a transient nature of the margay population within the sanctuary.
We found no significant difference in capture rates between primary and secondary forest. While several studies have shown that margays prefer undisturbed forested habitats and appear to be less tolerant of habitat disturbance than other Neotropical felids (Emmons and Feer, 1990; Payan et al., 2008), other studies suggest that margays are more tolerant of disturbance or that possible prey items for margays may be more abundant in degraded areas (Di Bitetti et al., 2010). At our site there was a large amount of variation among individuals; most individuals tended to be captured in either primary or secondary forest. Female 2, for example, was only captured in primary forest and Female 5 was only captured in secondary forest. While this could be an artifact of our sampling design, it may also be the case that different margays preferentially inhabit and/or move through different types of forest.
Habitat alteration, primarily deforestation for agricultural lands, is one of the primary threats to remaining margay populations (Payan et al., 2008). Due to the relatively high rate of vegetation regeneration in this area of the tropics, even land currently in use by humans could revert to acceptable secondary forest or become corridors between areas of primary forest if it were allowed to recover. In contrast, primary forest is not easily regenerated on short time scales; as such, it is of especially high conservation priority for the sanctuary.
Although our surveys are ongoing, the data we collected over a two year period suggest that there is an unusually high abundance of margays at WWS. Furthermore, the large number of margays living in and moving through the Sanctuary may have important implications for margay conservation and community dynamics in the broader Sumaco region. Our camera-trapping surveys at WWS are ongoing, and we hope to use these and additional approaches to further elucidate the abundance, density, and potential intraguild interactions of margays with sympatric carnivores in the foothills of the eastern Andes of Ecuador.
ACKNOWLEDGMENTS
We thank Bonnie B. and James G. Olson, founders and owners of the Wildsumaco Wildlife Sanctuary and Río Pucuno Foundation for their assistance and support to our research. Funding was provided by Francis Marion University and the University of North Carolina Wilmington. We thank two anonymous reviewers for their comments. We also thank Santiago F. Burneo of Pontificia Universidad Católica del Ecuador and Juan Manuel Guayasamín for assistance with photo identification and research support. J. Camper, R. Burger, L. Danikas, K. Floyd, A. Marlow, M. Nordgren, and M. Sokol provided logistical assistance with cameras. Numerous volunteers also assisted with camera monitoring. Linda Soetens, undergraduate intern from HAS Den Bosch (Netherlands) provided extensive assistance during the first field session. Andrea Schwandt-Arbogast provided help with the figures for the manuscript.
LITERATURE CITED
1. CALLEIA F, F ROHE, and M GORDO. 2009. Hunting strategy of the margay (Leopardus wiedii) to attract the wild pied tamarin (Saguinus bicolor). Neotropical Primates 16:32-34. [ Links ]
2. CUELLAR E, L MAFFEI, R ARISPE, and A NOSS. 2006. Geoffroy's cats at the northern limit of their range: activity patterns and density estimates from camera trapping in Bolivian dry forests. Studies on Neotropical Fauna and Environment 41:169-177. [ Links ]
3. DE OLIVEIRA TG. 1998. Leopardus wiedii. Mammalian Species 579:1-6. [ Links ]
4. DE OLIVEIRA TG, MA TORTATO, L SILVEIRA, C KASPER, FD MAZIM, M LUCHERINI, A JÁCOMO, JBG SOARES, RV MARQUES, and M SUNQUIST. 2010. Ocelot ecology and its effect on the small felid guild in the lowlands Neotropics. Pp. 559-580, in: Biology and Conservation of Wild felids (DW Macdonald and A Loveridge, eds.). Oxford University Press, UK. [ Links ]
5. DEFLER TR. 1981. The density of Alouatta seniculus in the eastern llanos of Colombia. Primates 22:564-569. [ Links ]
6. DI BITETTI MS, A PAVIOLO, and C DE ANGELO. 2006. Density, habitat use and activity patterns of ocelots (Leopardus pardalis) in the Atlantic Forest of Misiones, Argentina. Journal of Zoology 270:153-163. [ Links ]
7. DI BITETTI MS, C DE ANGELO, YE DI BLANCO, and A PAVIOLO. 2010. Niche partitioning and species coexistence in a Neotropical felid assemblage. Acta Oecologica 36:403-412. [ Links ]
8. EISENBERG JF. 1989. Mammals of the Neotropics, Volume 1: The Northern Neotropics. University of Chicago Press, Chicago, IL. [ Links ]
9. EMMONS LH. 1988. A field study of ocelots (Felis pardalis) in Peru. Review of Ecology (Terre Vie) 43:133-157. [ Links ]
10. EMMONS LH and F FEER. 1990. Neotropical rainforest mammals: a field guide. University of Chicago Press, Chicago, IL. [ Links ]
11. FORESMAN KR and DE PEARSON. 1998. Comparison of proposed survey procedures for detection of forest carnivores. Journal of Wildlife Management 62:1217-1226. [ Links ]
12. GOULART FVB, NC CACERES, ME GRAIPEL, MA TORTATO, IR GHIZONI, and LGR OLIVEIRA-SANTOS. 2009. Habitat selection by large mammals in a southern Brazilian Atlantic forest. Mammalian Biology 74:182-190. [ Links ]
13. HEINEMEYER KS, TJ ULIZIO, and RL HARRISON. 2008. Natural sign: tracks and scats. Pp. 45-74, in: Noninvasive survey methods for carnivores (RA Long, P MacKay, J Ray, and W Zielinski, eds.). Island Press, Washington, DC. [ Links ]
14. KARANTH KU. 1995. Estimating tiger Panthera tigris populations from camera capture-recapture models. Biological Conservation 71:333-338. [ Links ]
15. KELLY MJ and EL HOLUB. 2008. Camera trapping carnivores: trap success among camera types and across species, and habitat selection by species on Salt Pond Mountain, Giles County, Virginia. Northeastern Naturalist 15:429-262. [ Links ]
16. KELLY MJ, AJ NOSS, MS DI BITETTI, L MAFFEI, RL ARISPE, A PAVIOLO, CD DE ANGELO, and YE DI BLANCO. 2008. Estimating puma densities from camera trapping across three study sites: Bolivia, Argentina, and Belize. Journal of Mammalogy 89:408-418. [ Links ]
17. KONECNY MJ. 1989. Movement patterns and food habits of four sympatric carnivore species in Belize, Central America. Pp. 243-264, in: Advances in Neotropical Mammalogy (KH Redford and JF Eisenberg, eds). Sandhill Crane Press, Gainesville, Florida. [ Links ]
18. MAFFEI L and AJ NOSS. 2008. How small is too small? Camera trap survey areas and density estimates for ocelots in the Bolivian Chaco. Biotropica 40:71-75. [ Links ]
19. MAFFEI L, AJ NOSS, E CUELLAR, and DI RUMIZ. 2005. Ocelot (Felis pardalis) population densities, activity, and ranging behavior in the dry forests of eastern Bolivia: data from camera trapping. Tropical Ecology 21:1-6. [ Links ]
20. MORAIS RN, RG MUCCIOLO, MLF GOMES, O LACERDA, W MORAES, N MOREIRA, LH GRAHAM, WF SWANSON, and JL BROWN. 2002. Seasonal analysis of semen characteristics, serum testosterone, and fecal androgens in the ocelot (Leopardus pardalis), margay (L. wiedii), and tigrina (L. tigrinus). Theriogenology 57:2027-2041. [ Links ]
21. PAYAN E, E EIZIRIK, T DE OLIVEIRA, R LEITE-PITMAN, M KELLY, and C VALDERRAMA. 2008. Leopardus wiedii. IUCN 2010 Red List of Threatened Species. Version 2010.2. <www.iucnredlist.org.> Downloaded 1 September 2010. [ Links ]
22. POWELL RA and G PROULX. 2003. Trapping and marking terrestrial mammals for research: integrating ethics, performance criteria, techniques and common sense. ILAR Journal 44:259-276. [ Links ]
23. ROBINSON HS, RB WIELGUS, HS COOLEY, and SW COOLEY. 2010. Sink populations in carnivore management: Cougar demography and immigration in a hunted population. Ecological Applications 18:1028-1037. [ Links ]
24. SAS INSTITUTE. 2003. SAS system version 9.1 for Windows. Cary, NC: SAS Institute. [ Links ]
25. SILVER SC, LET OSTRO, LK MARSH, L MAFFEI, AJ NOSS, MJ KELLY, RB WALLACE, H GOMEZ and G AYALA. 2004. The use of camera traps for estimating jaguar Panthera onca abundance and density using capture/recapture analysis. Oryx 38:1-7. [ Links ]
26. TOBLER MW, SE CARRILLO-PERCASTEGUI, R LEITE PITMAN, R MARES, and G POWELL. 2008. An evaluation of camera traps for inventorying large- and medium-sized terrestrial rainforest mammals. Animal Conservation 11:169-178. [ Links ]
27. TROLLE M. 2003. Mammal survey in the southern Pantanal, Brazil. Biodiversity and Conservation 12:823-836. [ Links ]
28. TROLLE M and M KERY. 2003. Estimation of ocelot density in the Pantanal using capture-recapture analysis of camera-trapping data. Journal of Mammalogy 84:607-614. [ Links ]
29. WALLACE RB, H GOMEZ, G AYALA, and F ESPINOZA. 2003. Camera trapping for jaguar (Panthera onca) in the Tuichi Valley, Bolivia. Mastozoología Neotropical 10:133-139. [ Links ]
30. WANG E. 2002. Diets of ocelots (Leopardus pardalis), margays (L. wiedii), and oncillas (L. tigrina) in Southeast Brazil. Studies on Neotropical Fauna and Environment 37:207-213. [ Links ]
Recibido 10 enero 2011.
Aceptado 13 julio 2011.
Editor asociado: J Pereira














