Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Mastozoología neotropical
versión impresa ISSN 0327-9383
Mastozool. neotrop. vol.18 no.2 Mendoza jul./dic. 2011
ARTÍCULOS Y NOTAS
Key to the genera of the Tribe Oryzomyini (Rodentia: Cricetidae: Sigmodontinae)
Marcelo Weksler1 and Alexandre R. Percequillo2
1 Departamento de Vertebrados, Museu Nacional, Universidade Federal do Rio de Janeiro, Quinta da Boa Vista, s/n., São Cristóvão, CEP-20940-040 Rio de Janeiro, RJ, Brazil [Correspondência: Marcelo Weksler <mweksler@amnh.org>].
2 Departamento de Ciências Biológicas, Escola Superior de Agricultura "Luiz de Queiroz", Universidade de São Paulo, Av. Pádua Dias, 11, Caixa Postal 9, 13418-900 Piracicaba, São Paulo, Brazil.
ABSTRACT: Due to recent phylogenetic and revisionary taxonomic work, the systematics of the Tribe Oryzomyini, the largest group of sigmodontine rodents, underwent profound changes. We present here an artificial key for the genera of this group of rodents, enabling researchers to identify oryzomyines using external, cranial and dental characteristics. We also present the most up-to-date diversity assessment of the tribe, in which we recognize 33 extant and extinct genera (plus three undescribed genus-group taxa) and 130 valid species.
RESUMO: Chave para os gêneros da tribo Oryzomyini (Rodentia: Cricetidae: Sigmodontinae). Devido a esforços recentes em filogenia e revisão taxonômica, a sistemática da Tribo Oryzomyini, o grupo mais diversificado dentre os sigmodontíneos, passou por profundas mudanças. Neste trabalho, nós apresentamos uma chave artificial para os gêneros deste grupo de roedores, que permitirá a pesquisadores identificarem oryzomyineos através de características de morfologia externa, craniana e dentária. Nós também apresentamos o panorama mais recente da diversidade da tribo, na qual reconhecemos 33 gêneros (além de três táxons do grupo do gênero ainda não descritos) e 130 espécies.
Key words: Sigmodontinae; Diversity; Identification; North, Central and South America
Palavras-chave: Sigmodontinae; Diversidade; Identificação; América do Norte, Central e do Sul.
INTRODUCTION
The oryzomyines form the most diverse tribe of sigmodontine rodents: members of the tribe are distributed in the Neotropical and Nearctic (southeastern section) regions from Tierra del Fuego to the Southern and Eastern United States, in the Galapagos Archipelago, and Trinidad and Tobago; extinct forms of the tribe are also found in several Caribbean islands, including Jamaica, Martinique, Curacao, Nevis, and Barbuda, among others (Turvey et al., 2010). Oryzomyines are found in almost all major biomes in South America, including forests, savannas, swamps, scrublands, and semi-arid environments; in many of these habitats they are among the most speciose and abundant small mammals (Voss and Emmons, 1996; Eisenberg, 1999). Most oryzomyines are predominantly cursorial, but some species display marked arboreal (e.g., Oecomys) or semiaquatic (e.g., Nectomys, Holochilus, and Lundomys) specializations. Oryzomyines serve as primary or secondary hosts to a wide range of disease-carrying organisms, including hantavirus, arenavirus, digeneans (Schistosoma), and trypanosomids (Mello, 1979; Rodrigues and Ferraz Filho, 1984; Picot, 1992; Bharadwaj et al., 1997; Fulhorst et al., 1997; Ribeiro et al., 1998; Calderon et al., 1999; Powers et al., 1999; D'Andrea et al., 2000).
The composition of the tribe has been stable since the early 1990's, and has been corroborated by phylogenetic analyses of morphological and nuclear sequences (Voss and Carleton, 1993; Steppan, 1995; Weksler, 2003, 2006), but not mitochondrial data (Smith and Patton, 1999; Bonvicino and Moreira, 2001; but see Percequillo et al. 2011 for combined nuclear and mitochondrial analysis supporting oryzomyine monophyly). For historical shifts in Oryzomyini taxonomy, see Hershkovitz (1944, 1955, 1962), Gardner and Patton (1976), Reig (1984, 1986), Carleton and Musser (1989), Voss (1991), Voss and Carleton (1993), and Weksler (2006).
Systematic work on the tribe has been active in recent years, and work in progress indicates that current taxonomy is not stable yet (Voss et al., 2002; Weksler et al., 2006; Percequillo et al., 2011). Considering these changes in the generic diversity and the lack of one reliable comprehensive source for their identification (D'Elía and Pardiñas, 2007), our aim here is to provide a dichotomous key for the extant taxa of genus-group level to allow the correct identification of these groups.
MATERIAL AND METHODS
The generic organization of the key follows Weksler et al. (2006), with the addition of taxa described thereafter. The position of the following taxa should be regarded as provisory: Microakodontomys is considered here as a valid and distinct genus (despite the previous Hershkovitzean footnote taxonomic decision of Weksler et al., 2006), based on preliminary molecular data (R. Paresque, pers. comm.; J. Hanson, pers. comm.) and on the distinctive morphology, including carotid circulation (pattern 3 of Voss, 1988), presence of alisphenoid strut, developed jugal, and very large orbicular apophisis of malleus (MZUSP specimens from Brasília, Brazil, collected by A. P. Carmignotto: APC 799, 813, 848, 856). The ex-"Oryzomys alfaroi" group was previously included in Handleyomys (Weksler et al., 2006), but at least one new genus should be erected to contain the alfaroi-chapmani-melanotis clade (alfaroi and chapmani groups in the present key; Weksler, 2006; Weksler et al., 2006). Sigmodontomys, presently including S. aphrastus and S. alfari, is not monophyletic (Weksler, 2006; McCain et al., 2007) and a new genus is being described for S. aphrastus (aphrastus group in the following key; Pine et al, submitted). Thus, despite the lack of formal generic names for these groups, they are included here as separate entries on the key. As a key is only an artificial tool for taxa identification, we are confident that no taxonomic imprudence is being committed here. On the contrary, for practical purposes, our procedure aims to avoid misidentifications.
The collections and specimens examined to elaborate this key were listed elsewhere (Percequillo, 1998, 2003; Weksler, 2006; Weksler et al., 2006; Percequillo et al., 2008, 2011). The nomenclature of anatomic features and figures depicting them can be found in Carleton (1973, 1980), Reig (1977), Voss and Linzey (1981), Voss (1988, 1991, 1993), Carleton and Musser (1989), Weksler (2006). We employed preferentially external traits, in order to make the key useful for field researchers; however, some generic taxa can only be confidently identified with cranial characters. Therefore, the present key combines both external and cranial features to allow the recognition of generic forms.
RESULTS
Diagnosis of Tribe Oryzomyini
The tribe can be diagnosed by seven putative synapomorphies (Voss and Carleton, 1993; Steppan, 1995; Weksler, 2006; Weksler et al., 2006): presence of long palate with prominent posterolateral pits, absence of alisphenoid strut, absence of posterior suspensory process of the squamosal attached to tegmen tympani, absence of gall bladder, 12 thoracic vertebrae, absence of hemal arches on first caudal vertebrae, and fewer than 36 caudal vertebrae; the last two synapomorphies are reversed in several oryzomyine taxa. Members of the tribe can also be recognized by the soft fur (except in Neacomys and Scolomys, which have spiny fur), small, unkeeled manual claws (except in Lundomys, with long, ventrally keeled manual claws); mammary complement of eight teats in inguinal, abdominal, postaxial, and pectoral pairs (except in Handleyomys and Scolomys, which have six mammae because they lack pectoral teats); sparsely haired tail covered with more or less conspicuous epidermal scales and lacking a terminal tuft of long hairs (the well-haired tail of Nesoryzomys does not appear scaly, and Drymoreomys and some species of Oecomys have prominently tufted tails); zygomatic plate without anterodorsal spinous process (except in Pseudoryzomys, Lundomys, and Holochilus, which exhibit a spinous process); nasal bones with rounded or squared posterior margins (except in Nectomys, Scolomys, and Sigmodontomys, which have acutely angled posterior nasal margins); smooth posterior wall of the orbit (except in Holochilus, which have a well-developed postorbital ridge); bony palate between the molar rows smooth or weakly sculpted (except in Holochilus and Lundomys, which have a well-developed median keel flanked by deep lateral gutters); alisphenoid canal with a large anterior opening (the anterior opening of the alisphenoid canal is absent or very small in Scolomys); upper incisors with smoothly rounded enamel bands (the upper incisor enamel is distinctly faceted in Holochilus); low-crowned or terraced molars (except in Holochilus, which has high-crowned, planar molars); labial flexi enclosed by a cingulum (the labial flexi are unenclosed in Holochilus and Lundomys); parallel maxillary toothrows (Holochilus and Lundomys have anteriorly convergent toothrows); median mure connected to the protocone on M1 (except in Holochilus, which have the median mure connected to the paracone); unilocular-hemiglandular stomach; male accessory reproductive gland complements that include one pair each of bulbourethral, dorsal prostate, anterior prostate, vesicular, and ampullary glands, and two pairs of ventral prostate glands (except Nesoryzomys).
Generic contents of Tribe Oryzomyini
The tribe presently comprehends 36 extant and recently extinct taxa of the genus-group (including three undescribed genera) and approximately 130 taxa of the species group (including one species to be described); the updated taxonomic arrangement is provided on Table 1. Although we present in this table the recently extinct oryzomyine taxa (†Agathaeromys, †Carletonomys, †Megalomys, †Noronhomys, †Pennatomys,), for practical purposes we include on the key only extant groups.
Table 1
List of genera and species currently assigned to Tribe Oryzomyini

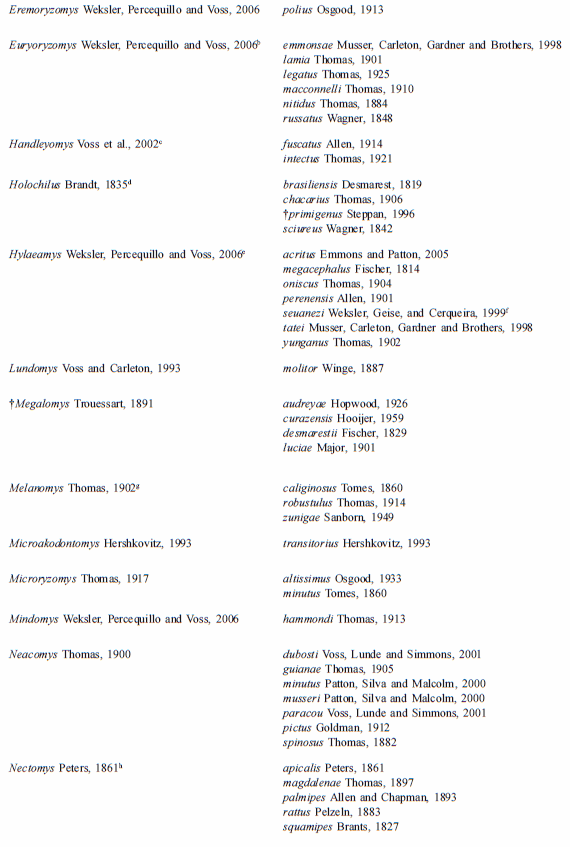
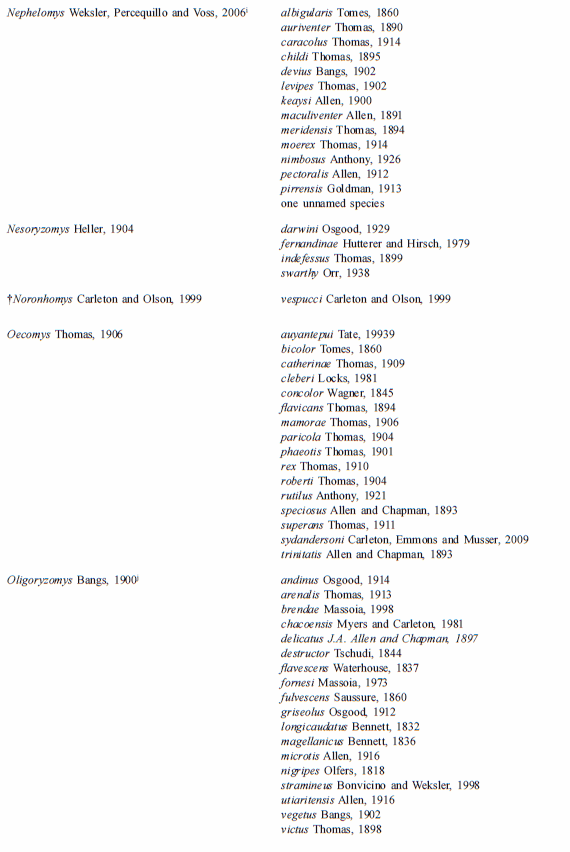
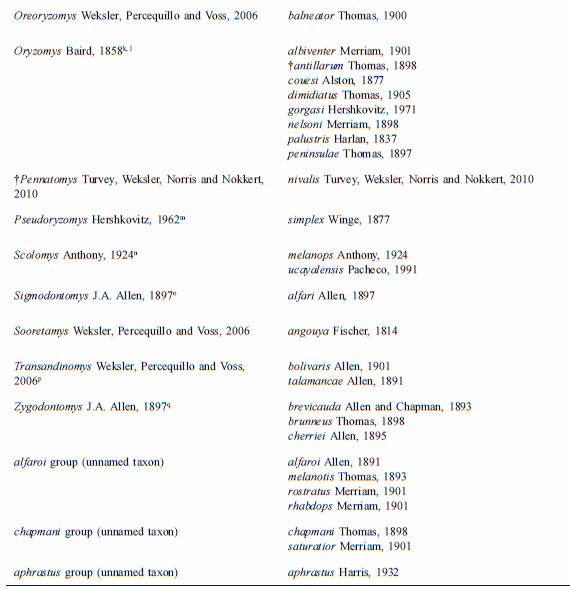
a- Percequillo et al., 2008. b- Weksler, 1996; Musser et al., 1998; Percequillo, 1998. c- Voss et al., 2002; d- Steppan, 1996 e- Musser et al., 1998; Percequillo, 1998; Emmons and Patton, 2005. f- We believe that laticeps is a junior synonym of megacephalus and that the valid name for the species occurring in the Atlantic Forest in Brazil, from Bahia to Rio de Janeiro, is H. seuanezi (Weksler, Geise, and Cerqueira, 1999); additional revisionary work is required to resolve this issue; g- Hanson and Bradley (2008) stated that genus Melanomys assembles four species, M. caliginosus, M. chrysomelas and two other forms; we are convinced that the genus is more diverse than previously known, but as more consistent analysis of morphological variation along with examination of type specimens are still lacking, we will keep the current usage; h- Bonvicino, 1994. i- Percequillo, 2003; j- Weksler and Bonvicino, pers. com.; k- Voss and Weksler, 2009; l- Carleton and Arroyo-Cabrales, 2009; m-Voss and Myers, 1991; n- Gómez-Laverde et al., 2004; o-Hanson and Bradley (2008) recovered Sigmodontomys and Melanomys as not reciprocally monophyletic employing the genus cyt-b, but with weak support; this relationship deserves a comprehensive analysis, as Melanomys is clearly one of the most distinctive groups of Oryzomyini (Weksler 2006), possessing several apomorphies related to its vole-like aspect: short tail, lack of counter-shading, dark pelage, and short pinna; p- Musser et al., 1998; q-Voss, 1991; Weksler, 2006; González et al. 2010; the latter stated that genus Zygodontomys is more diverse than previously considered, but more consistent analysis should be performed to validate these assumptions.
ARTIFICIAL KEY TO ORYZOMYINE GENERA
1. Dorsal and ventral fur with grooved spines................................................................................2
1'. Dorsal and ventral fur without grooved spines.........................................................................3
2. Six mammae in inguinal, abdominal, and postaxial pairs (Fig. 1A)...........................Scolomys
2'. Eight mammae in inguinal, abdominal, postaxial, and pectoral pairs.......................Neacomys
3. Hindfeet with hypothenar pad absent or vestigial (Fig. 1B).....................................................4
3'. Hindfeet with developed hypothenar pad................................................................................11
4. Hindfeet without natatory fringes - continuous combs of stiff hairs along the plantar margins and sometimes between the digits (Fig. 1C)..................................................................................5
4'. Hindfeet with natatory fringes....................................................................................................8
5. Dorsal surface of hindfeet covered with dark hairs, feet appear brown...................................6
5'. Dorsal surface of hindfeet sparsely covered with short silvery hairs, feet appear grayish white or pale tan.........................................................................................................................................7
6. Hind feet black or dark brown, with interdigital webs (Fig. 1D)......................Sigmodontomys
6'. Hind feet without interdigital webs....................................................................aphrastus group
7. Interorbital region weakly beaded even in adults (Fig. 2A), mesoloph small on M1, M2, absent in M3 (Fig. 3C).........................................................................................................Pseudoryzomys
7'. Interorbital region with strongly developed supraorbital crest in adults (figure 2A), mesoloph present and well developed on M1-M3.............................................................................Oryzomys
8. Nasal with acutely pointed posterior terminus (Fig. 2B); molars brachyodont, cingula closing labial folds........................................................................................................................................9
8'. Nasal with blunt posterior terminus, molars hypsodont or planar, labial folds open...........10
9. Body pelage with strong countershading; upper incisors orthodont...................Amphinectomys
9'. Body pelage with weak countershading; upper incisors opisthodont..........................Nectomys
10. Alisphenoid strut present (Fig. 2C); postorbital ridge present; accessory labial root on M1 present................................................................................................................................Holochilus
10'. Alisphenoid strut absent; postorbital ridge absent; accessory labial root on M1 absent......................................................................................................................................Lundomys
11. Plantar pads on hindfeet highly developed, large and fleshy, interdigitals 1-4 set close together, often in contact (Fig. 1B); mystacial vibrissae long and abundant (Fig. 1E)............................12
11'. Pads smaller, interdigitals 1 and 4 displaced proximally relative to 2 and 3, mystacial vibrissae shorter and more sparse...........................................................................................................13
12. Venter without gular patch of self-colored hairs; palate long (Fig. 3A); supraorbital margins squared, strongly beaded or with distinct crests (fig. 2A) ...............................................Oecomys
12'. Venter with gular patch of self-colored hairs; dorsal surface of hindfeet with distinct dark patch; palate short; supraorbital margins lightly beaded............................................Drymoreomys
13. Tail much shorter than head and body (ca. 85% of head and body length)........................14
13'. Tail subequal or longer than head and body.........................................................................15
14. Dorsal pelage grizzly or light brown...................................................................Zygodontomys
14'. Dorsal pelage dark brown.........................................................................................Melanomys
15. Sphenofrontal foramen absent (Fig. 2D).................................................................................16
15.' Sphenofrontal foramen present...............................................................................................24
16. Small mice (HBL of adults rarely larger than 110 mm).......................................................17
16'. Medium or large rats (HBL of young rarely smaller than 110 mm)...................................19
17. Anteromedian flexus present (Fig. 3C)...................................................................................18
17'. Anteromedian flexus absent..............................................................................chapmani group
18. Jugal absent or vestigial; alisphenoid strut absent; stapedial foramen present (pattern 2; Voss, 1988; except in Oligoryzomys rupestris); mesoloph and mesolophid frequently present (Fig. 3C; absent in a few specimens of O. fornesi)....................................................................Oligoryzomys
18'. Jugal present; alisphenoid strut always present; stapedial foramen absent (pattern 3; Voss, 1988); mesoloph and mesolophid always absent...............................................Microakodontomys
19. Stapedial foramen present (Figs. 2D, 3B)...............................................................Hylaeyamys
19'. Stapedial foramen absent or vestigial....................................................................................20
20. Tail densely furred, scales not visible....................................................................Nesoryzomys
20'. Tail sparsely furred giving naked impression, scales visible................................................21
21. Mystacial vibrissae very long (Fig. 1E); interorbital region hour-glass shaped with squared margins (Fig 2A)...............................................................................................................Sooretamys
21'. Mystacial vibrissae short; interorbital region anteriorly convergent with beaded margins...22
22. First upper and lower molars without accessory roots; mandible capsular process absent or indistinct (Fig. 2E).......................................................................................................Eremoryzomys
22'. First molars with accessory roots; capsular process well developed...................................23
23. Anterocone of M1 undivided (anteromedian flexus absent; Fig. 3C); baculum bifid.............. ........................................................................................................................................Cerradomys
23'. Anterocone of M1 divided by anteromedian flexus; baculum trifid.....................Aegialomys
24. Very small mice (HBL<100) with tail much longer than body length.................................25
24'. Medium and large rats (HBL>100) with tail as long as or longer than body length........26
25. Pelage distinctly countershaded; foramen magnum oriented caudally; anteroconid of m1 undivided (anteromedian flexid absent; Fig. 3D).........................................................Oreoryzomys
25'. Pelage not countershaded; foramen magnum oriented posteroventrally; anteroconid of m1 divided by anteromedian flexid...................................................................................Microryzomys
26. Superciliary vibrissae extending posteriorly beyond pinnae (Fig. 1E).................................27
26'. Superciliary vibrissae not extending posteriorly beyond pinnae..........................................28
27. Zygomatic notch (Fig. 2B) indistinct, small zygomatic plate..................................Mindomys
27'. Zygomatic notch deep, broad zygomatic plate................................................Transandinomys
28. Anterocone of M1 divided by anteromedian flexus (Fig. 3C)...............................Nephelomys
28'. Anterocone of M1 undivided, anteromedian flexus absent...................................................29
29. M1 without labial accessory root, m2 with 2 roots............................................Euryoryzomys
29'. M1 with labial accessory root, m2 with 3 roots...................................................................30
30. Six mammae in inguinal, abdominal, and postaxial pairs (Fig. 1A); hindfoot with hypothenar pad present; long rostrum; sphenopalatine vacuities long and wide..........................Handleyomys
30'. Eight mammae in inguinal, abdominal, postaxial, and pectoral pairs; hindfoot with hypothenar pad absent; short rostrum; sphenopalatine vacuities absent or small.........................alfaroi group
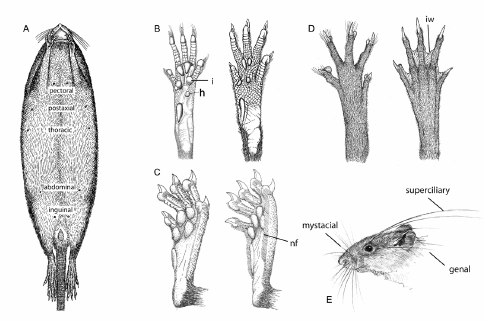
Fig. 1. Integumental characters used in the key for oryzomyine genera. A. Anatomical position of mammary pairs in sigmodontine rodents; modified from Voss and Carleton (1993: fig. 8). B. Plantar view of left hindfoot, illustrating the presence (left) and absence (right) hypothenar pad (h), and the anatomical position of the interdigital pads (i); modified from Weksler (2006: fig. 9) and Carleton and Musser (1989: fig. 9). C. Ventrolateral view of left hindfoot illustrating the absence (left) and presence (right) of natatory fringes (nf); modified from Voss (1988: fig. 6). D. Dorsal view of hindfoot left illustrating the absence (left) and presence (right) of interdigital webbing (iw); modified from Weksler (2006: fig. 10). E. Anatomical position of facial vibrissae in sigmodontine rodents; modified from Musser et al. (1998: fig. 53).

Fig. 2. Cranial characters used in the key for oryzomyine genera. A. Dorsal view of interorbital region illustrating the variations in its shape: hourglass (left), weakly beaded and convergent anteriorly (center), and strongly beaded and convergent anteriorly (right); modified from Weksler (2006: Fig. 13). B. Dorsal view of rostrum illustrating the shape of the posterior nasal terminus, pointed (left) or blunt (right), and the anatomical position of the zygomatic notch. Abbreviations are fro, frontal; max, maxillary; nas, nasal; pre, premaxillary; zn, zygomatic notch; modified from Weksler (2006: Fig. 11). C. Lateral view of the braincase illustrating the presence (left) and absence of the alisphenoid strut. Abbreviations are als, alisphenoid strut; bmf, buccinator-masticatory foramen; foa, foramen ovale accessorius; modified from Voss and Carleton (1993: Fig. 10). D. Ventral view of basicranium illustrating variation of carotid circulatory patterns. Abbreviations are cc, carotid canal; iag, groove for the infraorbital branch of stapedial artery; palc, posterior opening of the alisphenoid canal; sf, sphenofrontal foramen; stf, stapedial foramen. Modified from Carleton and Musser (1989: Fig. 17). E. Lateral view of right mandibles illustrating the anatomical position of the capsular process of the lower incisor alveolus (cap); modified from Weksler (2006: Fig. 24).
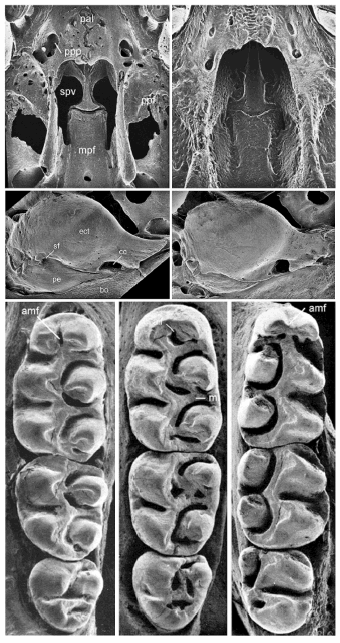
Fig. 3. Cranial and dental characters used in the key for oryzomyine genera. Above: Ventral view of the palatal and basicranial region illustrating the variations in the morphology of posterior palate. Abbreviations are mpf, mesopterygoid fossa; pal, palatine; ppf, parapterygoid fossa; ppp, posterolateral pit; spv, sphenopalatine vacuity. Middle: Medial view of auditory bulla illustrating variations in the ectotympanic morphology Abbreviations are bo, basioccipital; cc, carotid canal; ect, ectotympanic; pe, periotic; sf, stapedial foramen. Below: Anatomical position of the anteromedian flexus (amf) and mesoloph (m) in the upper molars of oryzomyines. D. Anatomical position of the anteromedian flexid (amf) in the first lower molar of oryzomyines.
ACKNOWLEDGEMENTS
We are deeply indebted to Paul Velazco, who first encouraged us to produce a key for the tribe Oryzomyini. We are also grateful to Ulyses Pardiñas, Cibele R. Bonvicino and one anonymous reviewer for comments and suggestions that greatly improved the quality of this contribution. ARP research was funded by FAPESP and CNPq fellowships.
LITERATURE CITED
1. BHARADWAJ M, J BOTTEN, N TORREZ-MARTINEZ, and B HJELLE. 1997. Rio Mamore virus: genetic characterization of a newly recognized hantavirus of the pygmy rice rat, Oligoryzomys microtis, from Bolivia. American Journal of Tropical Medicine and Hygiene 57:368-374. [ Links ]
2. BONVICINO CR. 1994. Especiação do rato d'água Nectomys. Abordagem cariológica, morfológica e geográfica. Unpublished Ph.D. Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil. [ Links ]
3. BONVICINO CR and MAM MOREIRA. 2001. Molecular phylogeny of the genus Oryzomys (Rodentia: Sigmodontinae) based on cytochrome b DNA sequences. Molecular Phylogenetics and Evolution 18:282-292. [ Links ]
4. CALDERON G, N PINI, J BOLPE, S LEVIS, J MILLS, E SEGURA, N GUTHMANN, G CANTONI, J BECKER, A FONOLLAT, C RIPOLL, M BORTMAN, R BENEDETTI, and D ENRIA. 1999. Hantavirus reservoir hosts associated with peridomestic habitats in Argentina. Emergent Infectious Diseases 5:792-797. [ Links ]
5. CARLETON MD. 1973. A survey of gross stomach morphology in new world Cricetinae (Rodentia, Muroidea), with comments on functional interpretations. Miscellaneous Publications, Museum of Zoology, University of Michigan 146:1-43. [ Links ]
6. CARLETON MD. 1980. Phylogenetic relationships in neotomine-peromyscine rodents (Muroidea) and a reappraisal of the dichotomy within New World Cricetinae. Miscellaneous Publications, Museum of Zoology, University of Michigan 157:1-146. [ Links ]
7. CARLETON MD and GG MUSSER. 1989. Systematic studies of oryzomyine rodents (Muridae, Sigmodontinae): a synopsis of Microryzomys. Bulletin of the American Museum of Natural History 191:1-83. [ Links ]
8. CARLETON MD and J. ARROYO-CABRALES. 2009. Review of the Oryzomys couesi complex (Rodentia: Cricetidae: Sigmodontinae) in Western Mexico. Bulletin of the American Museum of Natural History 331:94-127. [ Links ]
9. D'ANDREA PS, L MAROJA, R GENTILE, R CERQUEIRA, A MALDONADO JUNIOR, and L REY. 2000. The parasitism of Schistosoma mansoni (Digenea-Trematoda) in a naturally infected population of water rats, Nectomys squamipes (Rodentia-Sigmodontinae) in Brazil. Parasitology 120:573-582. [ Links ]
10. D'ELÍA G and UFJ PARDIÑAS.2007. Putting names to the phylogenetic diversity of Neotropical sigmodontine rodents: new genera for known species. Mammalia 2007:143-145. [ Links ]
11. EISENBERG JF. 1999. Biodiversity reconsidered. Pp. 527-548, in: Mammals of the Neotropics, Volume 3. The Central Neotropics: Ecuador, Peru, Bolivia, Brazil (JF Eisenberg and KH Redford, eds.). Universidade of Chicago Press, Chicago. [ Links ]
12. EMMONS LH and JL PATTON. 2005. A New Species of Oryzomys (Rodentia: Muridae) from Eastern Bolivia. American Museum Novitates 3478:1-26. [ Links ]
13. FULHORST CF, MC MONROE, RA SALAS, G DUNO, A UTRERA, TG KSIAZEK, ST NICHOL, NM DE MANZIONE, D TOVAR, and RB TESH. 1997. Isolation, characterization and geographic distribution of Cano Delgadito virus, a newly discovered South American hantavirus (family Bunyaviridae). Virus Research 51:159-71. [ Links ]
14. GARDNER AL and JL PATTON. 1976. Karyotypic variation in oryzomyine rodents (Cricetinae) with comments on chromosomal evolution in the Neotropical cricetine complex. Occasional Papers, Museum of Zoology, Louisiana State University 49:1-48. [ Links ]
15. GÓMEZ-LAVERDE M, RP ANDERSON, and LF GARCIA. 2004. Integrated systematic reevaluation of the Amazonian genus Scolomys (Rodentia: Sigmodontinae). Mammalian Biology 69:119-139. [ Links ]
16. HANSON JD and RD BRADLEY. 2008. Molecular diversity within Melanomys caliginosus (Rodentia: Oryzomyini): evidence for multiple species. Occasional Papers, Museum of Texas Tech University 275:1-11. [ Links ]
17. HERSHKOVITZ P. 1944. Systematic review of the Neotropical water rats of the genus Nectomys (Cricetinae). Miscelaneous Publications of the Museum of Zoology, University of Michigan 58:1-101. [ Links ]
18. HERSHKOVITZ P. 1955. South American marsh rats, genus Holochilus, with a summary of sigmodont rodents. Fieldiana Zoolology 37:639-687. [ Links ]
19. HERSHKOVITZ P. 1962. Evolution of Neotropical cricetine rodents (Muridae) with special reference to the phyllotine group. Fieldiana Zoolology 46:1-524. [ Links ]
20. MCCAIN CM, RM TIMM, and M WEKSLER. 2007. Redescription of the enigmatic long-tailed rat Sigmodontomys aphrastus (Cricetidae: Sigmodontinae) with comments on taxonomy and natural history. Proceedings of the Biological Society of Washington 120:117-136. [ Links ]
21. MELLO DA. 1979. Trypanosoma (Megatrypanum) amileari n. sp., isolated from Oryzomys eliurus (Wagner, 1845) (Rodentia-Cricetidae). Annales de Parasitologie Humaine et Comparée 54:489-494. [ Links ]
22. MUSSER GG, MD CARLETON, E BROTHERS, and AL GARDNER. 1998. Systematic studies of Oryzomyine rodents (Muridae, Sigmodontinae): diagnoses and distributions of species formerly assigned to Oryzomys "capito". Bulletin of the American Museum of Natural History 236:1-376. [ Links ]
23. PERCEQUILLO AR. 1998. Sistemática de Oryzomys Baird, 1858 do Leste do Brasil (Muroidea, Sigmodontinae). Unpublished M.Sc. Thesis, Universidade de São Paulo, São Paulo, Brazil. [ Links ]
24. PERCEQUILLO AR. 2003. Sistemática de Oryzomys Baird, 1858: definição dos grupos de espécies e revisão taxonômica do grupo albigularis (Rodentia, Sigmodontinae). Unpublished Ph.D. Thesis, Univesidade de São Paulo, São Paulo, Brazil. [ Links ]
25. PERCEQUILLO AR, E HINGST-ZAHER, and CR BONVICINO. 2008. Systematic review of genus Cerradomys Weksler, Percequillo and Voss, 2006 (Rodentia: Cricetidae: Sigmodontinae: Oryzomyini), with the description of two new species from eastern Brazil. American Museum Novitates 3622:1-46 [ Links ]
26. PERCEQUILLO AR, M WEKSLER, and LP COSTA. 2011. A new genus and species of rodent from the Brazilian Atlantic Forest (Rodentia, Cricetidae, Sigmodontinae, Oryzomyini), with comments on oryzomyine biogeography. Zoological Journal of the Linnean Society 161:357-390. [ Links ]
27. PICOT H. 1992. Holochilus brasiliensis and Nectomys squamipes (Rodentia-Cricetidae) natural hosts of Schistosoma mansoni. Memorias do Instituto Oswaldo Cruz 87:255-260. [ Links ]
28. POWERS AM, DR MERCER, DM WATTS, H GUZMAN, CF FULHORST, VL POPOV, and RB TESH 1999. Isolation and genetic characterization of a hantavirus (Bunyaviridae: Hantavirus) from a rodent, Oligoryzomys microtis (Muridae), collected in northeastern Peru. American Journal of Tropical Medicine and Hygiene 61:92-98. [ Links ]
29. REIG OA. 1977. A proposed unified nomenclature for the enamelled components of the molar teeth of the Cricetidae (Rodentia). Journal of Zoology, London 181: 227-241. [ Links ]
30. REIG OA. 1984. Distribuição geográfica e história evolutiva dos roedores muroídeos sulamericanos (Cricetidae: Sigmodontinae). Revista Brasileira de Genética 7:333-365. [ Links ]
31. REIG OA. 1986. Diversity patterns and differentiation of high Andean rodents. Pp. 404-440, in: High altitude tropical biogeography (F Vuilleumier and M Monasterio, eds.). Oxford University Press, New York. [ Links ]
32. RIBEIRO AC, A MALDONADO JUNIOR, PS D'ANDREA, GO VIEIRA, and L REY. 1998. Susceptibility of Nectomys rattus (Pelzen, 1883) to experimental infection with Schistosoma mansoni (Sambon, 1907): a potential reservoir in Brazil. Memórias do Instituto Oswaldo Cruz 93:295-299. [ Links ]
33. RODRIGUES VL and ADN FERRAZ FILHO. 1984. Trypanosoma (Megatrypanum) rochasilvai, sp. n., encontrada no estado de São Paulo, Brasil, parasitando Oryzomys laticeps (Leche, 1886) (Rodentia-Cricetidae). Revista Brasileria de Biologia 44:299-304. [ Links ]
34. SMITH MF and JL PATTON. 1999. Phylogenetic relationships and the radiation of sigmodontine rodents in South America: evidence from cytochrome b. Journal of Mammalian Evolution 6:89-128. [ Links ]
35. STEPPAN SJ. 1995. Revision of the tribe Phyllotini (Rodentia: Sigmodontinae), with a phylogenetic hypothesis for the Sigmodontinae. Fieldiana Zoology, New Series 80:1-112. [ Links ]
36. STEPPAN SJ. 1996. A new species of Holochilus (Rodentia: Sigmodontinae) from the Middle Pleistocene of Bolivia and its phylogenetic significance. Journal of Vertebrate Paleontology 16:522-530. [ Links ]
37. TURVEY ST, M WEKSLER, EL MORRIS, and M NOKKERT. 2010. Taxonomy, phylogeny and diversity of the extinct Lesser Antillean rice rats (Sigmodontinae: Oryzomyini), with description of a new genus and species. Zoological Journal of the Linnean Society 160:748-772. [ Links ]
38. VOSS RS. 1988. Systematics and ecology of Ichthyomyine rodents (Muroidea): patterns of morphological evolution in a small adaptive radiation. Bulletin of the American Museum of Natural History 188:259-493. [ Links ]
39. VOSS RS. 1991. An introduction to the Neotropical muroid rodent genus Zygodontomys. Bulletin of the American Museum of Natural History 210:1-113. [ Links ]
40. VOSS RS and AV LINZEY. 1981. Comparative gross morphology of male acessory glands among Neotropical Muridae (Mammalia: Rodentia) with comments on systematic implications. Miscellaneous Publications, Museum of Zoology, University of Michigan 159: 1-41. [ Links ]
41. VOSS RS and LH EMMONS. 1996. Mammalian diversity in Neotropical lowland rainforest: a preliminary assessment. Bulletin of the American Museum of Natural History 230:1-155. [ Links ]
42. VOSS RS and MD CARLETON. 1993. A new genus for Hesperomys molitor Winge and Holochilus magnus Hershkovitz (Mammalia, Muridae) with an analysis of its phylogenetic relationships. American Museum Novitates 3085:1-39. [ Links ]
43. VOSS RS and P MYERS. 1991. Pseudoryzomys simplex (Rodentia: Muridae) and the significance of Lund's collections from the caves of Lagoa Santa, Brazil. Bulletin of the American Museum of Natural History 206:414-432. [ Links ]
44. VOSS RS and M WEKSLER. 2009. On the taxonomic status of Oryzomys curasoae McFarlane and Debrot, 2001, with remarks on the phylogenetic relationships of O. gorgasi Hershkovitz, 1971. Caribbean Journal of Science 45:73-79. [ Links ]
45. VOSS RS, M GÓMEZ-LAVERDE, and V PACHECO. 2002. A new genus for Aepeomys fuscatus Allen, 1912, and Oryzomys intectus Thomas, 1921: enigmatic murid rodents from Andean cloud forests. American Museum Novitates 3373:1-42. [ Links ]
46. WEKSLER M. 1996. Revisão sistemática do grupo de espécies nitidus do gênero Oryzomys (Rodentia, Sigmodontinae). Unpublished M.Sc. Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro. [ Links ]
47. WEKSLER M. 2003. Phylogeny of Neotropical oryzomyine rodents (Muridae: Sigmodontinae) based on the nuclear IRBP exon. Molecular Phylogenetics and Evolution 29:331-349. [ Links ]
48. WEKSLER M. 2006. Phylogenetic relationships of oryzomyine rodents (Muridae: Sigmodontinae): separate and combined analyses of morphological and molecular data. Bulletin of the American Museum of Natural History 296:1-149. [ Links ]
49. WEKSLER M, L GEISE, and R CERQUEIRA. 1999. A new species of Oryzomys (Rodentia, Sigmodontinae) from southeast Brazil, with comments on the classification of the O. capito species group. Zoological Journal of the Linnean Society 125: 445-462. [ Links ]
50. WEKSLER M, AR PERCEQUILLO, and RS VOSS. 2006. Ten new genera of oryzomyine rodents (Cricetidae: Sigmodontinae). American Museum Novitates 3537:1-29. [ Links ]
Recibido 26 enero 2011.
Aceptado 27 julio 2011.
Editor asociado: U Pardiñas














