Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Mastozoología neotropical
versão impressa ISSN 0327-9383
Mastozool. neotrop. vol.19 no.1 Mendoza jun. 2012
ARTÍCULOS Y NOTAS
The agouti Dasyprocta leporina (Rodentia: Dasyproctidae) as seed disperser of the palm Astrocaryum aculeatissimum
Alexandra S. Pires1 and Mauro Galetti1,2
1 Programa de Pós-Graduação em Biologia Vegetal, Instituto de Biologia, Universidade Estadual Paulista, CP 199, 13506-900 Rio Claro, SP, Brasil; present adress: Departamento de Ciências Ambientais, Universidade Federal Rural do Rio de Janeiro, Rodovia BR 465, Km 07, Seropédica, RJ 23890-000, Brasil [Correspondence: <aspires@ufrrj.br>].
2 Laboratório de Biologia da Conservação, Departamento de Ecologia, Universidade Estadual Paulista, CP 199, 13506-900 Rio Claro, SP, Brasil.
Recibido 3 junio 2011.
Aceptado 27 octubre 2011.
Editor asociado: N Cáceres
ABSTRACT: Some large-seeded plants depend heavily on agoutis for seedling recruitment. The importance of Dasyprocta leporina as seed disperser of the Atlantic Forest palm Astrocaryum aculeatissimum was evaluated using camera-traps and seed removal experiments. Agoutis were registered at 67% of the records obtained through the monitoring of mature fruits; palms were visited from 07:00 to 18:45 h. Dispersal distances ranged from 0.5 to 48.7 m (mean ± sd = 6.8 ± 9.1 m) and most of the removed seeds were buried (57.8%). These results corroborate the importance of agoutis for the seed dispersal of Astrocaryum palms.
RESUMO: A cutia Dasyprocta leporina (RODENTIA: DASYPROCTIDAE) como dispersora de sementes da palmeira Astrocaryum aculeatissimum. Algumas plantas de sementes grandes são fortemente dependentes de cutias para o recrutamento de suas plântulas. A importância de Dasyprocta leporina como dispersora de sementes da palmeira da Mata Atlântica Astrocaryum aculeatissimum foi avaliado com o uso de armadilhas fotográficas e experimentos de remoção de sementes. Cutias foram observadas em 67% dos registros obtidos durante o monitoramento de frutos maduros; as palmeiras foram visitadas entre 07:00 e 18:45 h. As distâncias de dispersão variaram de 0.5 a 48.7 m (media ± dp = 6.8 ± 9.1 m) e a maioria das sementes removidas foram enterradas (57.8%). Estes resultados confirmam a importância de cutias para a dispersão de sementes de palmeiras do gênero Astrocaryum.
Key words: Activity patterns; Atlantic Forest; Rodents; Seed dispersal; Seed predation.
Palavras-chave: Dispersão de sementes; Mata Atlântica; Padrões de atividade; Predação de sementes; Roedores.
Agoutis (Dasyprocta spp.) are known to eat a variety of fruits and seeds in Neotropical forests and savannas (Henry, 1999; Silvius and Fragoso, 2003; Dubost and Henry, 2006). Due to its use of large seeds and its habit of hoarding food to later consumption-a behavior found in a small group of mammals like acouchies (Forget, 1991; Jansen et al., 2006), squirrels (Glanz, 1984; Paschoal and Galetti, 1995; Bordignon and Monteiro-Filho, 1999), some small rodents (Brewer and Rejmánek, 1999; Yasuda et al., 2000; Theimer, 2001; Cheng et al., 2005; Grenha et al., 2010) and a few Australian marsupials (Dennis, 2003; Murphy et al., 2005)-agoutis act as important seed disperses (Smythe, 1978, 1989; Asquith et al., 1999; Silvius and Fragoso, 2003; Galetti et al., 2006; Donatti et al., 2009). Some largeseeded plant species may depend entirely on agoutis' scatter-hoarding behavior for seedling recruitment (Smythe, 1989; Vander Wall, 1990). Seeds from the families Arecaceae, Chrysobalanaceae, Fabaceae, Lecythidaceae, Meliaceae, Myristicaceae, Moraceae and Sapotaceae are among the most consumed and dispersed by agoutis (Forget and Milleron, 1991; Peres and Baider, 1997; Brewer and Rejmánek, 1999; Silvius and Fragoso, 2003; Gorchov et al., 2004; Andreazzi et al., 2009).
Specifically on palm species, seed dispersal by Dasyprocta spp. was observed in the genus Acrocomia (Scariot, 1998), Astrocaryum (Smythe, 1989; Brewer and Rejmánek, 1999; Galetti et al., 2006; Donatti et al., 2009; Jorge and Howe, 2009), Attalea (Silvius, 2002; Pimentel and Tabarelli, 2004; Almeida and Galetti, 2007), Bactris (Silva and Tabarelli, 2001), Phytelephas (Dalling et al., 1996) and Syagrus (Guimarães et al., 2005). Despite the variety of studies reporting palm seed dispersal and predation by Dasyprocta spp., little is known about seed dispersal distances, especially in the Atlantic Forest. In this study we described the importance of the red-rumped agouti Dasyprocta leporina Linnaeus, 1758 in the seed dispersal of the palm Astrocaryum aculeatissimum (Schott) Burret, 1934 (Arecaceae) in the lowland forests of Rio de Janeiro state, southeastern Brazil. More specifically, we assessed fruit removal, seed fate following removal and dispersal distances. Besides that, the activity patterns of D. leporina while feeding on A. aculeatissimum seeds were also described.
The palm A. aculetassimum, locally known as iri or brejaúva, is endemic to the Brazilian Atlantic Forest, occurring from Bahia to Santa Catarina (Henderson et al., 1995). Stems are solitary or aggregated, spiny, and four to eight meters tall. Infructescences may have from 23 to 116 (n = 9, unpublished data) single-seeded spiny fruits with a tiny mesocarp and a hard endocarp. Fruit length and diameter are 4.5 ± 0.6 and 3.2 ± 0.2 mm, respectively (data are mean ± sd, n = 242, unpublished data). Such dimensions limit the range of vertebrates that can handle and consume Astrocaryum fruits, which are considered typical megafauna dispersed-fruits, whose primary seed dispersers were extinct in the end of the Pleistocene (Guimarães et al., 2008). Nowadays, besides rodents-which are the main seed dispersers (Kiltie, 1981; Smythe, 1989; Forget, 1991; Brewer and Rejmánek, 1999; Brewer, 2001; Donatti et al., 2009)-primates, peccaries and tapirs have also been reported as consumers of Astrocaryum fruits (Hladik and Hadlik, 1969; Terborgh, 1986; Henry et al., 2000; Beck, 2006; Andreazzi et al., 2009). Non dispersed seeds of Astrocaryum usually suffer high levels of predation by scolytid and bruchid beetles, reducing seedling recruitment near parents (Smythe, 1989; Delobel et al., 1995; Galetti et al., 2006; Dracxler et al., 2011).
Dasyprocta leporina occurs from Central Amazon to Southeast Brazil. Animals weight from 3.0 to 6.0 kg, are territorial and their home ranges varied from 3.0 to 8.5 ha (Emmons and Feer, 1990; Silvius and Fragoso, 2003; Jorge and Peres, 2005). The species is categorized as Vulnerable in the red list of the threatened fauna of Rio de Janeiro municipality (Vera y Conde et al., 2000) due to the small size of remaining forest fragments and illegal hunting.
This study was carried out in the main forest tracts of the two largest remnants (3500 and 6300 ha) of the threatened lowland Atlantic Forest at the state of Rio de Janeiro, respectively the União (22°27'S, 42°02'15 W; UN-3500) and Poço das Antas (22°32' S, 42°18' W; PA-6300) Biological Reserves. The climate of the region is tropical warm and humid with average annual temperatures about 24 ºC and average annual precipitation of c. 2100 mm (Programa Mata Atlântica, unpublished data). These areas differ in the abundance of A. aculeatissimum palms which is almost four times higher at União (94 versus 24 reproductive stems/ha; Pires, 2006).
During the fruiting seasons of 2003 and 2004 (from August to December) eight fruit individuals were monitored using camera-traps (DeerCam® and Trapacâmera®) aiming to identify the main consumers of A. aculeatissimum fruits. Cameras worked all day long until the end of film or batteries, when they were replaced, resulting in a sampling effort of 1950 h. The selected time interval from one photographic record to another was one minute. For each picture obtained, the species and the day and hour of the visit were recorded.
Fruit removal experiments were carried out from November to December 2003, during the period of the natural falling of ripe fruits. Fruits were collected in the field, taken to the laboratory for marking and placed in the field in the following morning. To be able to follow fruit fate, every seed was marked as follows: on each fruit a five millimeters metal ring was passed through a small hole made in the endocarp in each fruit. A 50 m thread was tied to the metal ring and a spool was placed inside a small container attached to a tree. Groups of five threaded endocarps were placed in 20 experimental stations spaced every 50 m, along pre-existing trails within each site. Marked fruits were left in the field for 30 consecutive days and after this time the fate of every fruit was recorded. Fruit fate was then categorized as: intact (not removed from experimental station), preyed upon by rodents or dispersed. Dispersed fruits were classified as moved away (dispersed but not buried) or scatter-hoarded (dispersed and buried) and had their distances to experimental stations measured. As adult palm density differed between areas, which could result in differences in the amount of available fruits and a consequently reduction in fruit removal at experiments, analyses were performed separately for each site.
The agouti was the main visitor of A. aculeatissimum fruits, appearing at 67% of the photographic records (n = 42). The other species detected were the nine-banded armadillo Dasypus novemcinctus Linnaeus, 1758, which was registered nine times, the paca Cuniculus paca Linnaeus, 1766, the crab-eating fox Cerdocyon thous Linnaeus, 1766, the common opossum Didelphis aurita Wied-Neuwied, 1826, the brown four eyed opossum Metachirus nudicaudatus É. Geoffroy Saint-Hilaire, 1803 and the teiu lizard Tupinambis meriane Dumeril e Bibron, 1839-all registered only once. Besides D. leporina, possibly only the paca consumes A. aculeatissimum fruits as the amount of pulp is almost insignificant and endocarp hardness makes access to seeds difficult.
The visits of D. leporina to fruiting palms occurred during the day, from 07:00 h to 18:45 h, with a peak around noon time (Fig. 1). This result contrasts with the bimodal pattern of activity generally found by other authors (e.g. Smythe, 1978; Lambert et al., 2009; Norris et al., 2010), with peaks shortly after sunrise and before sunset. Nonetheless, foraging activity of agoutis can vary in response to food availability, daytime temperatures and moon phases (Smythe, 1978; Jorge and Peres, 2005; Lambert et al., 2009). For palms visited more than once by agoutis in the same day, the interval among subsequent visits was relatively short (mean ± sd = 16.9 ± 30.6 min, n = 18), suggesting that the same individual visited the palm repeatedly. Agoutis were seen interacting directly with fruits (Fig. 2).

Fig. 1. Activity patterns of Dasyprocta leporina while feeding on fruits of the palm Astrocaryum aculeatissimum at two Atlantic Forest Biological Reserves (União and Poço das Antas), Rio de Janeiro state, Brasil.

Fig. 2. Dasyprocta leporina interacting with Astrocaryum aculeatissimum fruits at Poço das Antas Biological Reserve, Rio de Janeiro state, Brasil.
The number of removed fruits at experimental stations was higher at PA-6300ha (Fig. 3), corresponding to a removal rate of 1.47 seeds/ day in this site against 0.67 seeds/day at UN- 3500ha. At both sites most of the removed seeds were dispersed (Fig. 3). Just nine seeds at PA-6300ha were preyed upon; one at the experimental station and the others at distances varying from 10 up to 47 m from the initial place where they were set (26.75 ± 25.47 m). The majority of dispersed seeds was scatter-hoarded in both sites (Fig. 3). Buried seeds were usually cached alone and near landmarks, such as roots or fallen logs, an agouti behavior already reported by other authors (Kiltie, 1981; Smythe, 1989). Scatter-hoarded seeds had the exocarp previously removed and were buried 1-4 cm deep, with the germinative pore turned down.
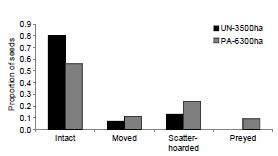
Fig. 3. Fate of Astrocaryum aculeatissimum seeds in controlled experiments carried out at two Atlantic Forest Biological Reserves (União and Poço das Antas), Rio de Janeiro state, Brasil.
Seed dispersal distances ranged from 0.5 to 48.7 m and differed among scatter-hoarded and non-buried seeds. At both sites non-buried seeds were removed up to 10 m from experimental stations (Fig. 4a). Scatter-hoarded seeds follow the same pattern at UN-3500ha, while at PA-6300ha 60% of them were dispersed for more than 10 m (Fig. 4b). Removal distances are in accordance with those found by Donatti (2004) in São Paulo state, where it was found that agoutis were the only species responsible for long-distance events (> 15 m) of A. aculeatissimum seed dispersal. The author, however, did not found differences in the removal distances between buried or non-buried seeds.

Fig. 4. Dispersal distances of Astrocaryum aculeatissimum seeds in two Atlantic Forest Biological Reserves (União and Poço das Antas), Rio de Janeiro state, Brasil.
Differences between sites in the removal rates and dispersal distances could be due to the higher density of A. aculeatissimum at UN-3500ha (Pires, 2006). Removal rates were ca. 50% lower in this area, which could be a result of the competition between experimental threaded fruits and those naturally available on the ground. This result can also reflect predator satiation, as found in other studies involving seed rodents (e.g. Theimer, 2001; Romo et al., 2004; Briani and Guimarães, 2007; Vieira et al., 2011). Considering removal distances, it is expected that more valuable seeds be hidden further from parent trees to difficult their encounter by predators and other competitors (Forget et al., 1998, Jansen et al., 2002). So, the higher distances of buried seeds at PA- 6300ha could be a result of the lower abundance of reproductive palms in this area (Pires, 2006). In fact, negative relationships between fruit abundance and seed removal distances have already been reported by other authors (Haugaasen et al., 2010; Herrera et al., 2011). Nonetheless, the influence of other factors not evaluated in this study, such as agouti density or abundance of other food resources in each area, cannot be discarded.
As mentioned above, the seeds remaining below parent palms usually suffer high predation by scolytid and bruchid beetles, and the same was observed in the areas studied here (see Galetti et al., 2006). Therefore, D. leporina should play a fundamental role in the recruitment of A. aculeatissimum seedlings as observed in other studies involving agoutis and Astrocaryum palms (e.g. Smythe, 1989). In fact, in fragmented or defaunated forests where agoutis are rare or absent, A. aculeatissimum seedling recruitment was greatly reduced (Galetti et al., 2006, Donatti et al., 2009; Jorge and Howe, 2009). Considering this, management options should be planned to enhance the recruitment of Astrocaryum palms and other plant species dispersed by agoutis in areas where these animals are absent or scarce due to habitat loss or hunting. Considering the costs and failure risks associated with the manual addition of seeds and seedlings, the reintroduction or translocation of agoutis seems to be a better option.
Acknowledgments.
To ICMBio for allowing us to work at the study areas and provide many facilities there. To Fernando Fernandez for the inestimable logistical support provided by the Laboratório de Ecologia e Conservação de Populações/UFRJ during this study. To Pierre-Michel Forget, Nilton Cáceres, and anonymous reviewers for comments in early versions of the manuscript. Cassia Lima helped in field work. Idea Wild donated part of field equipment. Financial support was provided by FAPESP. ASP received a scholarship from FAPESP and MG receives a fellowship from CNPq.
LITERATURE CITED
1. ALMEIDA LB and M GALETTI. 2007. Seed dispersal and spatial distribution of Attalea geraensis (Arecaceae) in two remnants of Cerrado in southeastern Brazil. Acta Oecologica 32:180-187. [ Links ]
2. ANDREAZZI CS, AS PIRES, and FAS FERNANDEZ. 2009. Mamíferos e palmeiras neotropicais: interações em paisagens fragmentadas. Oecologia Brasiliensis 13:554-574. [ Links ]
3. ASQUITH NM, J TERBORGH, AE ARNOLD, and CM RIVEROS. 1999. The fruits the agouti ate: Hymenaea courbaril seed fate when its disperser is absent. Journal of Tropical Ecology 15:229-235. [ Links ]
4. BECK H. 2006. A review of peccary-palm interactions and their ecological ramifications across the Neotropics. Journal of Mammalogy 87:519-530. [ Links ]
5. BORDIGNON M and ELA MONTEIRO-FILHO. 1999. Seasonal food resources of the squirrel Sciurus ingrami in a secondary Araucaria Forest in southern Brazil. Studies on Neotropical Fauna and Environment 34:137-140. [ Links ]
6. BREWER SW. 2001. Predation and dispersal of large and small seeds of a tropical palm. Oikos 92:245-255. [ Links ]
7. BREWER SW and M REJMÁNEK. 1999. Small rodents as significant dispersers of tree seeds in a Neotropical forest. Journal of Vegetation Science 10:165-174. [ Links ]
8. BRIANI DC and PR GUIMARãES. 2007. Seed predation and fruit damage of Solanum lycocarpum (Solanaceae) by rodents in the cerrado of central Brazil. Acta Oecologica 31:8-12. [ Links ]
9. CHENG JR, ZS XIAO, and ZB ZHANG. 2005. Seed consumption and caching on seeds of three sympatric tree species by four sympatric rodent species in a subtropical forest, China. Forest Ecology and Management 216:331-341. [ Links ]
10. DALLING JW, KE HARMS, JR EBERHARD, and I CANDANEDO. 1996. Natural history and uses of tagua (Phytelephas seemannii) in Panamá. Principes 40:16-23. [ Links ]
11. DELOBEL A, G COUTURIER, F KAHN, and JA NILSSON. 1995. Trophic relationship between palms and bruchids (Coleoptera: Bruchidae: Pachymerini) in Peruvian Amazonia. Amazoniana 13:209-219. [ Links ]
12. DENNIS AJ. 2003. Scatter-hoarding by musky rat-kangaroos, Hypsiprymnodon moschatus, a tropical rain-forest marsupial from Australia: implications for seed dispersal. Journal of Tropical Ecology 19:619-627. [ Links ]
13. DONATTI CI. 2004. Consequências da defaunação na dispersão e predação de sementes e no recrutamento de plântulas da palmeira brejaúva (Astrocaryum aculeatissimum) na Mata Atlântica. Dissertação de Mestrado, Escola Superior de Agricultura Luiz de Queiroz, Universidade de São Paulo, Piracicaba, Brazil. [ Links ]
14. DONATTI, CI, PR GUIMARãES, and M GALETTI. 2009. Seed dispersal and predation in the endemic Atlantic rainforest palm Astrocaryum aculeatissimum across a gradient of seed disperser abundance. Ecological Research 24:1187-1195. [ Links ]
15. DRACXLER CM, AS PIRES, and FAS FERNANDEZ. 2011. Invertebrate seed predators are not all the same: seed predation by bruchine and scolytine beetles affects palm recruitment in different ways. Biotropica 43:8-11. [ Links ]
16. DUBOST G and O HENRY. 2006. Comparison of diets of the acouchy, agouti and paca, the three largest terrestrial rodents of French Guianan forests. Journal of Tropical Ecology 22:641-651. [ Links ]
17. EMMONS LH and F FEER. 1997. Neotropical rainforest mammals. The University of Chicago Press, Chicago. [ Links ]
18. FORGET PM. 1991. Scatter-hoarding of Astrocaryum paramaca by Proechimys in French Guiana: comparison with Myoprocta exilis. Tropical Ecology 32:155-167. [ Links ]
19. FORGET PM and T MILLERON. 1991. Evidence for secondary seed dispersal by rodents in Panama. Oecologia 87:596-599. [ Links ]
20. FORGET PM, T MILLERON, and F FEER. 1998. Patterns in post-dispersal seed removal by neotropical rodents and seed fate in relation to seed size. Pp. 25-47, in: Dynamics of tropical communities (DM Newbery, N Brown, and HHT Prins, eds.). Blackwell Science, Oxford. [ Links ]
21. GALETTI M, CI DONATTI, AS PIRES, PR GUIMARãES, and P JORDANO. 2006. Seed survival and dispersal of an endemic Atlantic forest palm: the combined effects of defaunation and forest fragmentation. Botanical Journal of the Linnean Society 151:141-150. [ Links ]
22. GLANZ WE. 1984. Food and habitat use by two sympatric Sciurus species in central Panama. Journal of Mammalogy 65:342-347. [ Links ]
23. GORCHOV DL, JM PALMEIRIM, M JARAMILLO and CF ASCORRA. 2004. Dispersal of seeds of Hymenaea courbaril (Fabaceae) in a logged rain forest in the Peruvian Amazonian. Acta Amazonica 34:251-259. [ Links ]
24. GRENHA V, MV MACEDO, AS PIRES and RF MONTEIRO. 2010. The role of Cerradomys subflavus as seed predator and disperser of the palm Allagoptera arenaria. Mastozoología Neotropical 17:61-68. [ Links ]
25. GUIMARãES PR, PFM LOPES, ML LYRA and AP MURIEL. 2005. Fleshy pulp enhances the location of Syagrus romanzoffiana (Arecaceae) fruits by seed-dispersing rodents in an Atlantic Forest in southeastern Brazil. Journal of Tropical Ecology 21:109-112. [ Links ]
26. GUIMARãES PR, M GALETTI, and P JORDANO. 2008. Seed dispersal anachronisms: rethinking the fruits extinct megafauna ate. PLOS One 3:e1745 [ Links ]
27. HAUGAASEN JMT, T HAUGAASEN, CA PERES, R GRIBEL, and P WEGGE. 2010. Seed dispersal of the Brazil nut tree (Bertholletia excelsa) by scatter-hoarding rodents in a central Amazonian forest. Journal of Tropical Ecology 26:251-262. [ Links ]
28. HLADIK A and CM HLADIK. 1969. Rapports trophiques entre vegetation et primates dans la foret de Barro Colorado (Panama). La Terre et la Vie 23:25-117. [ Links ]
29. HENDERSON A, G GALEANO, and R BERNAL. 1995. Field guide to the palms of the Americas. Princeton University Press, New Jersey. [ Links ]
30. HENRY O. 1999. Frugivory and the importance of seeds in the diet of the orange-rumped agouti (Dasyprocta leporina). Journal of Tropical Ecology 15:291-300. [ Links ]
31. HENRY O, F FEER, and D SABATIER. 2000. Diet of the lowland tapir (Tapirus terrestris L.) in French Guiana. Biotropica 32:364-368. [ Links ]
32. HERRERA JM, JM MORALES, and D GARCIA. 2011. Differential effects of fruit availability and habitat cover for frugivore-mediated seed dispersal in a heterogeneous landscape. Journal of Ecology 99:1100-1107. [ Links ]
33. JANSEN PA, M BARTHOLOMEUS, F BONGERS, JA ELZINGA, J DEN OUDEN, and SE VAN WIEREN. 2002. The role of seeds size in dispersal by a scatter-hoarding rodent. Pp. 209-225, in: Seed dispersal and frugivory: ecology, evolution and conservation (DJ Levey, WR Silva, and M Galetti, eds.). CABI Publishing,Wallingford. [ Links ]
34. JANSEN PA, F BONGERS, and HHT PRINS. 2006. Tropical rodents change rapidly germinating seeds into long-term food supplies. Oikos 113:449-458. [ Links ]
35. JORGE MLSP and CA PERES. 2005. Population density and home range size of red-rumped agoutis (Dasyprocta leporina) within and outside a natural Brazil nut stand in southeastern Amazonia. Biotropica 37:317-321. [ Links ]
36. JORGE MLSP and HF HOWE. 2009. Can forest fragmentation disrupt a conditional mutualism? A case from central Amazon. Oecologia 161:709-718. [ Links ]
37. KILTIE RA. 1981. Distribution of palm fruits on a rain forest floor: why white-lipped peccaries forage near objects. Biotropica 13:141-145. [ Links ]
38. LAMBERT TD, RW KAYS, PA JANSEN, E ALIAGA-ROSSEL, and M WIKELSKI. 2009. Nocturnal activity by the primarily diurnal Central American agouti (Dasyprocta punctata) in relation to environmental conditions, resource abundance and predation risk. Journal of Tropical Ecology 25:211-215. [ Links ]
39. MURPHY MT, MJ GARKAKLIS, and GESJ HARDY. 2005. Seed caching by woylies (Bettongia penicillata) can increase sandalwood (Santalum spicatum) regeneration in Western Australia. Austral Ecology 30:747-755. [ Links ]
40. NORRIS D, F MICHALSKI, and CA PERES. 2010. Habitat patch size modulates terrestrial mammal activity patterns in Amazonian forest fragments. Journal of Mammalogy 91:551-560. [ Links ]
41. PASCHOAL MG and M GALETTI. 1995. Seasonal food use by the neotropical squirrel Sciurus ingrami in southeastern Brazil. Biotropica 27:268-273. [ Links ]
42. PERES CA and C BAIDER. 1997. Seed dispersal, spatial distribution and population structure of Brazil nut tree (Bertholletia excelsa) in southeastern Amazonia. Journal of Tropical Ecology 13:595-616. [ Links ]
43. PIMENTEL DS and M TABARELLI. 2004. Seed dispersal of the palm Attalea oleifera in a remnant of the Brazilian Atlantic Forest. Biotropica 36:74-84. [ Links ]
44. PIRES AS. 2006. Perda de diversidade de palmeiras em fragmentos florestais: padrões e processos. Tese de Doutorado. Instituto de Biociências, Universidade Estadual Paulista, Rio Claro, Brazil. [ Links ]
45. ROMO R, H TUOMISTO and BA LOISELLE. 2004. On the density-dependence of seed predation in Dipteryx micrantha, a bat-dispersed rain forest tree. Oecologia 140:76-85. [ Links ]
46. SCARIOT A. 1998. Seed dispersal and predation of the palm Acrocomia aculeata. Principes 42:5-8. [ Links ]
47. SILVA MG and M TABARELLI. 2001. Seed dispersal, plant recruitment and spatial distribution of Bactris acanthocarpa Martius (Arecaceae) in a remnant of Atlantic forest in northeast Brazil. Acta Oecologica 22:259-268. [ Links ]
48. SILVIUS KM. 2002. Spatio-temporal patterns of palm endocarp use by three Amazonian forest mammals: granivory or'grubivory'? Journal of Tropical Ecology 18:707-723. [ Links ]
49. SILVIUS KM and JV FRAGOSO. 2003. Red-rumped agouti (Dasyprocta leporina) home range use in an Amazonian forest: implications for the aggregated distribution of forest trees. Biotropica 35:74-83. [ Links ]
50. SMYTHE, N. 1978. The natural history of the Central American agouti (Dasyprocta punctata). Smithsonian Contributions to Zoology 257:1-52. [ Links ]
51. SMYTHE N. 1989. Seed survival in the palm Astrocaryum standleyanum: evidence for dependence upon its seed dispersers. Biotropica 21:50-56. [ Links ]
52. TERBORGH J. 1986. Keystone plant resources in the tropical forest. Pp. 330-340, in: Conservation biology: the science of scarcity and diversity (ME Soulé, ed.). Sinauer, Sunderland. [ Links ]
53. THEIMER TC. 2001. Seed scatterhoarding by white-tailed rats: consequences for seedling recruitment by an Australian rain forest tree. Journal of Tropical Ecology 17:177-189. [ Links ]
54. VANDER WALL SB. 1990. Food hoarding in animals. University of Chicago Press, Chicago. [ Links ]
55. VERA Y CONDE CF, C BUENO, CR BONVICINO, CEL ESBÉRARD, S MAIA-VAZ, and S SICILIANO. 2000. Mamíferos. Pp. 1-65, in: As espécies ameaçadas do município do Rio de Janeiro: fauna e flora (FR Di Maio and MBR Silva, eds.). Secretaria Municipal de Meio Ambiente do Rio de Janeiro, Rio de Janeiro. [ Links ]
56. VIEIRA EM, JF RIBEIRO, and G IOB. 2011. Seed predation of Araucaria angustifolia (Araucariaceae) by small rodents in two areas with contrasting seed densities in the Brazilian Araucaria forest. Journal of Natural History 45:843-854. [ Links ]
57. YASUDA M, S MIURA, and NA HUSSEIN. 2000. Evidence of food hoarding behaviour in terrestrial rodents in Pasoh Forest Reserve, a Malaysian lowland rain forest. Journal of Tropical Forest Science 12:164-173. [ Links ]














