Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Mastozoología neotropical
versión impresa ISSN 0327-9383
Mastozool. neotrop. vol.20 no.1 Mendoza jun. 2013
ARTÍCULO
Ecology of small terrestrial mammals in an isolated Cerrado patch, eastern Paraguay: communities, species, and effects of enso, precipitation, and fire
Robert D. Owen
Department of Biological Sciences, Texas Tech University, Lubbock, TX 79409 USA, and Raúl Casal 2230 c/ Pizarro, Barrio Republicano, Asunción, Paraguay [Correspondence: <rowen@tigo.com.py>].
Recibido 27 agosto 2012.
Aceptado 30 enero 2013.
Editor asociado: UFJ Pardiñas
ABSTRACT. The Cerrado extends as islands or patches into the Upper Paraná Atlantic Forest (UPAF) of Brazil and Paraguay. This study evaluates the temporal dynamics of a marginal terrestrial small-mammal community in an isolated Cerrado patch within the UPAF, at the southwestern distributional limit of the Cerrado and near the western limit of the UPAF in eastern Paraguay. Because the faunal members of marginal communities are living near the limits of their capabilities in terms of their abiotic and biotic environment, the communities in such areas are likely to be a mixture of Cerrado and UPAF species, sensitive to extrinsic variables such as climate change and anthropogenic changes in land use. In this 23-month study, temporal dynamics of a terrestrial small-mammal community were evaluated with respect to El Niño/Southern Oscillation, precipitation, and fre. Densities of some species were quite variable. Generally, responses to precipitation paterns became evident only several months after burning, or (on the frequently-burned grid) were not observed. Some species more characteristic of forest or second-growth vegetation were encountered only seasonally or sporadically. Taken together, these observations provide strong support for the importance of long-term population studies in marginal or transitional locations, where two or more ecoregions are represented, and the fauna characteristic of each ecoregion will be sensitive to fluctuations in its biotic and abiotic environment.
RESUMEN. Ecología de pequeños mamíferos terrestres en un parche aislado de Cerrado, Paraguay oriental: comunidades, especies y los efectos del ENSO, precipitación y fuego. El Cerrado se extiende en forma de islas o parches en el Bosque Atlántico del Alto Paraná (BAAPA) de Brasil y Paraguay. Esta investigación evalúa la dinámica temporal de una comunidad marginal de los mamíferos pequeños terrestres en un parche aislado de Cerrado dentro del BAAPA, en el límite de distribución suroeste del Cerrado y cerca del límite occidental del BAAPA en Paraguay oriental. Debido a que los miembros faunísticos de las comunidades marginales se encuentran viviendo cerca de los límites de sus capacidades en términos de sus ambientes abióticos y bióticos, las comunidades en dichas áreas posiblemente deben contener una combinación de especies de Cerrado y BAAPA, y ser sensibles a variables extrínsecas como cambios de clima, y de uso de suelo antropogénicos. Durante esta investigación de 23 meses, se evaluó la dinámica temporal con respecto a la Oscilación Sur / El Niño, la precipitación y el incendio. Las densidades de algunas especies fueron bastante variables. En general, las respuestas a los patrones de precipitación se hicieron evidentes solamente varios meses después de las quemas, o (en la parcela frecuentemente quemada) no fueron observadas. Algunas especies, más características de bosques o de vegetación secundaria, fueron encontradas solamente en forma esporádica o estacionalmente. En conjunto, estas observaciones proporcionan una fuerte evidencia sobre la importancia de los estudios poblacionales a largo plazo en áreas marginales o transicionales, en donde dos o más ecorregiones están representadas, y la fauna característica de cada ecorregión será susceptible a fuctuaciones de su ambiente biótico y abiótico.
Key words: Cricetidae; Didelphidae; Echimyidae; Fire; ENSO; Precipitation.
Palabras claves: Cricetidae; Didelphidae; Echimyidae; Fuego; ENSO; Precipitación.
INTRODUCTION
The composition and dynamics of terrestrial small-mammal communities in the Neotropics are to date poorly documented (Mares, 1982; Lacher and Mares, 1986; Mares and Ernest, 1995; Willig et al., 2000). Accordingly, studies of terrestrial small-mammal communities are increasingly the focus of intensive studies; however, the majority of these studies are either broad-scale comparisons of faunal communities (Ojeda et al., 2000; Willig et al., 2000), or are evaluations of a community situated well within the geographic and ecologic boundaries of the ecoregion being evaluated (e.g., Ribeiro and Marinho-Filho, 2005; Bernardes, 2006; Becker et al., 2007; Magnusson et al., 2010).
Nevertheless, it may be of equal interest and value to assess community composition and dynamics in marginal or even isolated patches of a particular ecoregion. Faunal members of such communities are presumed to be living near the limits of their capabilities in terms of their abiotic (e.g., soils, weather, climate) and biotic (vegetation composition and structure, predators, competitors) environment. Moreover, such communities are likely to be mixed (i.e., with components of both the "target" ecoregion and the nearby ecoregion, or matrix in which the "target" patch is embedded), and sensitive to extrinsic variables such as climate change and anthropogenic changes in land use (Willig et al., 2000).
The Cerrado is one of the largest ecoregions of South America, centered on the Planalto Central of central Brazil and extending as islands or patches into the Amazon and Caatinga biomes in Brazil, the Pantanal of Bolivia and Brazil, and the Upper Paraná Atlantic Forest (UPAF) of Brazil and Paraguay (Eiten, 1972; Coutinho, 1990; Carmignotto et al., 2012). This ecoregion is actually a mosaic of habitats (Eiten, 1972; Ab'Saber, 1977; Coutinho, 1990; Alho, 2005), although a unifying factor in all of these is that fire is an important determinant of foral species composition and vegetation structure (Borchert and Hansen 1983; Pivello and Coutinho, 1996; Vieira and Marinho-Filho, 1998; Henriques et al., 2000; Briani et al., 2004; Simon et al., 2009; Werneck, 2011).
The Cerrado exhibits high levels of biodiversity and endemism (Mares, 1992; Bonvicino et al., 1996; Werneck, 2011; Carmignotto et al., 2012). Fire is important, directly and indirectly, to the faunal community (Pivello and Coutinho, 1996; Vieira and Marinho-Filho, 1998; Briani et al., 2004), although detailed studies regarding its role are few (e.g., Countinho, 1990; Gettinger and Ernest, 1995; Mistry, 1998). Several studies have examined fire-related small mammal ecology (Vieira and Marinho-Filho 1998; Vieira, 1999; Briani et al., 2004), reporting that responses of communities and species depend on several factors including extent and intensity of the fire. In this context, timing of droughts may be important, both in their direct effects on available resources, and by their effects on the frequency and intensity of fires (Pivello and Coutinho, 1996; Craine et al., 2012).
Precipitation levels in Neotropical savannas are at least partially characterized by fluctuations due to El Niño / Southern Oscillation (ENSO), with drought, and consequent increased frequency and extent of wildfres, typical of El Niño periods (Roberts, 2000; Marengo, 2004). Precipitation levels in the Cerrado correlate at least generally with the ENSO patterns, as measured by the Southern Oscillation Index (SOI). The SOI and the previous year's fire extent were found to be associated with population fluctuations of Necromys lasiurus, a characteristic Cerrado rodent species, over a 22-year period (Magnusson et al., 2010). Thus, SOI, precipitation, and fire all appear to be determinants of terrestrial small-mammal populations and communities in the Cerrado, but in a complex fashion not yet well understood.
Although most of eastern Paraguay is considered to be within the UPAF, isolated patches of Cerrado also occur within several departments of northeastern Paraguay (Simon et al., 2009). Both the Cerrado and the UPAF have experienced extensive land-use conversion for ranching and agriculture (Klink and Moreira, 2002; Klink and Machado, 2005; Silva et al., 2006; Huang et al., 2007, 2009), and both are considered to be Biodiversity Hotspots and Conservation Priority Areas (Myers et al., 2000; Willig et al., 2000).
This study evaluates the temporal dynamics- including effects of ENSO, precipitation, and fire-of a marginal terrestrial small-mammal community in an isolated Cerrado patch within the UPAF, at the southwestern distributional limit of the Cerrado and near the western limit of the UPAF in eastern Paraguay. An expected result of the study was that the community is species-rich, with elements of both ecoregions present (Bezerra et al., 2009), and that population densities are generally low, as many of the species are near their ecological limits. Similarly, substantial fluctuations in both species richness and population density, as well as community composition, could be expected to result from the factors mentioned above.
MATERIALS AND METHODS
Study site, abiotic data, and small-mammal sampling
The study was conducted in the Reserva Natural de Bosque Mbaracayú (RNBM) in Depto. Canindeyú, eastern Paraguay. The RNBM is located within the Upper Paraná Atlantic Forest (UPAF) ecoregion in an area where patches of Cerrado are also present. The RNBM covers 644 km2 and is divided into a roughly rectangular core, which is dominated by a large continuous forest fragment (one of the largest extant UPAF forest fragments in Paraguay), to the east of which is a large patch of Cerrado, known locally as Aguara Ñu (Fig. 1).
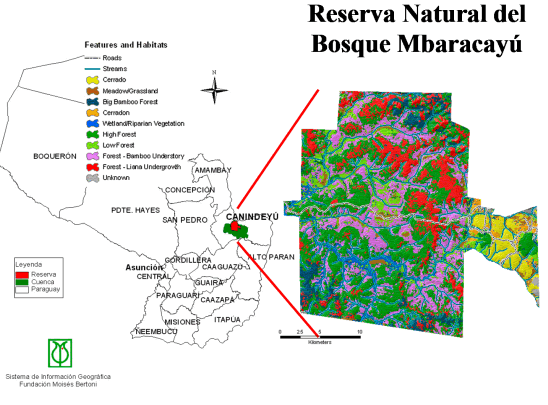
Fig. 1. Map of Paraguay, showing location of Reserva Natural del Bosque Mbaracayú (RNBM) in Depto. Canindeyú. Aguara Ñu (an isolated Cerrado patch in Paraguay, and site of this study) is the eastern extension of the RNBM.
The Southern Oscillation Index (SOI) is defined as the sea-level pressure difference between the eastern and western Pacific, and is used widely as an indicator of the strength and phase of the ENSO cycle (Rasmusson, 1985; Manobavan et al., 2003). SOI data were obtained from http://www.esrl.noaa.gov/psd/enso, accessed 10 October 2011. Monthly precipitation data, expressed as average daily precipitation, were from the station in Salto del Guairá, Canindeyú (ca. 100 km E of Aguara Ñu), a locality with similar weather patterns to the study site. These data were accessed on 10 October 2011 from http://climexp.knmi.nl//getprcpall.cgi?id=someone@somewhere&WMO=86210&STATION=SALTOS_DEL_GUAIRA.
Two mark-recapture grids were established to collect demographic and ecological information on terrestrial small-mammal communities. Each grid consisted of a 10 x 10 array of trap stations spaced 10 m apart over an area of ≈10,000 m². Each trapping station had one standard Sherman live-trap (7.5 x 9.0 x 23.0 cm; H. B. Sherman Traps, Tallahassee, Florida) placed on the ground. The original study design involved Grid A being subjected to periodic controlled burns, with Grid B as a control not subject to burning. Grid A was subject to a controlled burn in July 2001, as planned. Shortly thereafter, an uncontrolled burn (from a neighboring ranch) occurred across large areas of Aguara Ñu, including Grid B and much of the area surrounding Grid A. Sampling for small mammals began in August 2001. Additional unplanned burns which included Grid B occurred in November 2001, and May 2002; both of these burns were extensive, including much of the area around Grid A. However, Grid A itself was protected from burning after the initial burn of July 2001 (Pople, 2003). Thus, the single burn on Grid A was a "local" burn (only the grid, not the surrounding matrix), whereas the burns that included Grid B were "general" burns, including the matrix around both grids.
The two sampling grids were monitored periodically from August 2001 through June 2003 (Table 1). The 12 sampling sessions were distributed throughout the 23-month study so that each month of the year was sampled at least once, except for January and July, which were not sampled. To enable robust estimates of population sizes, most trapping campaigns consisted of 6 consecutive nights. Due to a variety of logistical issues, some sessions included fewer sampling nights (Table 2). No sampling was conducted on Grid B in November 2001 because an unplanned burn occurred as the sample period was beginning. The February 2002 session included only two sampling nights; data from this session were not included in quantitative analyses. Traps were baited each evening with a mixture of rolled oats and peanut butter. Traps were checked each morning and animals processed and released at the site of capture. For each capture, the animal's specific identity, sex, age class, reproductive condition, and mass were noted, along with the date, grid, and trap station number. Upon first capture, a Passive Integrated Transponder (PIT) tag (Biomark Inc., Boise, ID, USA) was implanted in each animal and the number recorded. A PIT tag is a glass-encapsulated (ca. 12.5 x 2.0 mm) microchip which is inserted subdermally between the shoulders of the animal, and enables permanent individual identification by use of a hand-held electronic reader held within 10 cm of the animal. Upon each recapture, the PIT tag number was recorded along with the other information noted above. All field protocols followed American Society of Mammalogists guidelines for the use of wild mammals in research (Animal Care and Use Committee, 1998), and were approved by the Texas Tech University Animal Care and Use Committee; 220 voucher specimens, including examples of each species encountered, were collected during this study. The majority were collected from trapping transects > 0.5 km from the mark-recapture grids, in similar habitat. Several were collected from the grids, either as inadvertent trap deaths or due to particular interest (e.g., Monodelphis kunsi, a new species record for Paraguay; De la Sancha et al., 2007). All vouchers have been, or will be, deposited in the Natural Science Research Laboratory, Texas Tech University, or the Museo Nacional de Historia Natural del Paraguay.
Table 1
Numbers of individuals encountered, and numbers of captures (following the slash) of each species on each grid in each sampling session. When subadults were encountered, the number of individuals is included in parentheses. Dashed line indicates no individuals encountered; NS (November 2001, Grid B) indicates no sampling conducted. Total number of individuals encountered may be fewer than the sum of individuals encountered across all sessions, because some individuals were encountered in more than one sampling session.
Table 2
Species accumulation within sampling sessions, and among sessions. Cumulative species richness (cumulative number of species encountered) by sampling night for each sampling session, on each grid. Cumulative richness among sessions follows within-session richness.
Analyses
The total number of individuals on each grid was estimated for each species for each sampling session. Population abundance was estimated as the Minimum Number Known Alive (MNKA; Krebs, 1966), adjusted for the number of nights in the sampling session. For each species separately, this adjustment was calculated based on the pooled data for all new captures (non-recaptures for each trapping night in a session) across all sampling sessions. From these pooled samples, the average proportional increase in MNKA was calculated across all sampling nights five and six, and the resulting adjustment was applied for each species captured at least once during nights one-five (for five-night sessions) or nights one-four (for four-night sessions) to provide comparable sixnight MNKA estimates for all sampling sessions. This number was then rounded to the nearest integral value (i.e., the best estimate, based on probabilities of new captures on nights five and six of the number of individuals that would have been encountered if sampling had continued for six nights).
Mean Maximum Distance Moved (MMDM) was calculated as the hypotenuse of a right triangle with the other two sides being the differences between smallest and largest row value and column value of stations where the individual was encountered (Owen et al., 2010). To ensure that the number of captures was suficient for the MMDM estimate to approach the asymptotic (parametric) value for the individual, MMDM values were tested for significant correlation with number of captures upon which the MMDM values were based, and only MMDM values based on a number of captures large enough to show nonsignificant correlations were used as estimates of MMDM for that species, grid, and sampling session.
Based on the results from the tests of variation in MMDM, all classes (sexes, ages, grids) for a species were pooled to calculate the average MMDM for each species and then to calculate the effective grid size for that species. The effective grid size for a particular species is calculated by adding one-half of the MMDM for that species to each side of the core grid, and assuming the corners to be rounded (Schnell et al., 2008; Owen et al., 2010). Population density (individuals/ha) was calculated for each species as the estimated abundance divided by the effective grid size.
Species diversity (Simpson's Index) was calculated using population densities for each grid, for each sampling session. Species diversity values were compared between the two grids across all sampling sessions, using Univariate Analysis of Variance (ANOVA), to evaluate whether the small-mammal communities on the two grids responded differently to biotic and abiotic factors being evaluated.
Several demographic characteristics (population densities, presence of subadults, and longevity) are reported for the species encountered in the study. MMDM values for each species were tested against sex, age, and grid, using one-way ANOVAs. For individuals captured during more than one sampling session, longevity was estimated as the length of time known to be alive on the grid.
An initial evaluation tested for significant correlations among monthly Southern Oscillation Index (SOI) and precipitation values, and time since the last burn (TSB, in months), on each grid. These tests included evaluation both of SOI and a delayed effect of SOI (SOI-D, i.e., relationship between value of SOI for the previous month and either precipitation or TSB); monthly precipitation (mm/day) and cumulative precipitation over periods of two, three, four, six, and twelve months; and precipitation anomaly values (percent of normal precipitation), and cumulative precipitation anomaly over periods of two, three, four, six, and twelve months. Correlations were calculated between both SOI variables and all 6 precipitation and 6 precipitation anomaly values, and between these values and TSB on both Grids A and B. The effects of these three suites of abiotic variables on community richness and diversity were evaluated using regression analysis. Also, the effects of ENSO, precipitation, and TSB on individual species characteristics (density and mean maximum distance moved) were assessed, again using regression analysis.
No adjustment was made for experiment-wide error; this is clearly an exploratory study, and α = 0.05 was utilized not for rejection of a null hypothesis, but for indication of a trend or tendency in associations among abiotic factors, and community and species characteristics. Thus, values of P < 0.05 are referred to as "important", rather than "significant". All statistical analyses were conducted using SAS 9.2 for Windows (SAS Institute, 2002-2008).
RESULTS
Community characteristics
Field sampling resulted in 420 captures from 12 000 trap-nights (3.5% overall trap success). The 420 captures were of 219 individuals, representing two orders (Didelphimorphia, Rodentia), three families (Didelphidae, Cricetidae, Echimyidae), eight genera, and ten species. Nine species were captured on each grid, eight of which were common to both grids. Monodelphis kunsi was captured only on Grid A, and Oxymycterus delator only on Grid B (Table 1).
Species richness varied from 2-6 on Grid A, and 0-7 on Grid B. Within sessions, no new species were encountered after five nights (N=13 for six-night sessions, Table 2). Among sessions, 13 months (seven sampling sessions) were needed to arrive at final species richness on Grid A, and 22 months (ten sessions) on Grid B (Table 2). Species diversity in this study varied from 0.18 to 1.53 (Grid A) and 0.00 to 1.38 (Grid B). Diversity values in the two grids tended to vary concordantly, and no significant difference was detected between the two grids across all sessions, suggesting that they were responding similarly to extrinsic factors, in terms of overall diversity values.
Species varied widely in accumulation of new captures in sampling nights two-six (Table 3). The second sampling night showed average increases of between 27% (Monodelphis domestica) and 117% (Clyomys laticeps). Tree species (M. domestica, Calomys tener, C. laticeps) exhibited non-monotonic changes in average rates of increase. Average proportional increases ranged from 7-44% on night five, and 0-16% on night six. These values were applied to the results from four- and five-night sampling sessions, respectively, to derive a comparable six-night abundance estimate for each species on each grid. This adjustment affected abundance estimate values only for the single four-night session (August 2002). For this session, abundance estimates were increased from the four-night totals by two individuals of N. lasiurus on Grid A, and by one individual for three species on both Grids A and B.
Table 3
Proportional increase of new captures over cumulative total, by species, with both grids and all sampling sessions pooled. The average increase in population abundance (MNKA) for each sampling night is shown for each species, as a proportion of the previous cumulative abundance. No individuals of Akodon montensis were caught on night 1 of any sampling session; thus no value for the proportional increase on night 2 can be calculated.

Species characteristics
The six commonest species (M. domestica, Akodon montensis, Calomys callosus, C. tener, N. lasiurus, and C. laticeps) were evaluated with respect to population densities, seasonal variation, reproductive activity, and longevity. Data for the other four species encountered (M. kunsi, Oligoryzomys nigripes, O. delator, and Sooretamys angouya) were used only in calculating community species richness and diversity.
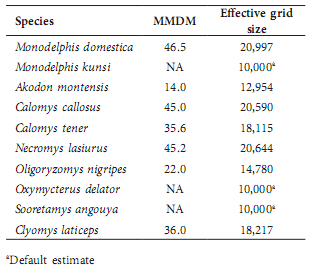
Mean Maximum Distance Moved (MMDM) values based on three or more captures showed correlation coeficients > 0.05 with the number of captures on which they were based; thus, MMDM values were used in analyses only if based on individuals with three or more captures during a sampling session. MMDM values for each species were evaluated by regression as a function of sex, age, and grid. All of these tests showed P > 0.05, except for the effect of sex in C. callosus (F(1)= 4.61, P = 0.040), with males having a higher average MMDM (51.5 +/- 24.7 m, N=20) than females (34.2 +/- 16.7 m, N=12). Consequently, all individual MMDM values were pooled for each species to calculate an average MMDM for that species, which was then used to calculate an effective grid size for that species. Data were insuficient to calculate MMDM for three species; for those, the actual grid size (10 000 m2) was used as the effective grid size (Table 4).
Table 4
Mean maximum distance moved (MMDM, in meters) and effective grid size (square meters) for each species. MMDM based on individuals with > three captures in a sampling session.
Of the six common species, four (M. domestica, C. callosus, N. lasiurus, and C. laticeps) occurred in all seasons (Table 1). Akodon montensis was trapped only during May-September, and C. tener was most abundant in June, with lower numbers noted from August-December. Subadult individuals of four species (M. domestica, C. callosus, C. tener, and N. lasiurus) were encountered, indicating reproductive activity for these species (Table 1). Of these four, only C. callosus apparently reproduces throughout the year. M. domestica, C. tener, and N. lasiurus exhibited reproductive seasonality. No subadults of A. montensis or C. laticeps were captured.
Longevity (MKA) was recorded for each of the four species for which one or more individuals was captured during two or more sampling sessions. Highest MKA values for these species were three months for M. domestica, five for C. callosus, eight for N. lasiurus, and 12 months for C. laticeps.
El Niño / Southern Oscillation
No important relationships were encountered when species richness and diversity were regressed against ENSO (SOI and SOI-D) values. C. callosus was the only species associated with ENSO, with population densities on Grid B positively associated with SOI from the previous month (SOI-D: F(1) = 10.22, r = 5.502, P = 0.019).
Precipitation
On Grid A, monthly precipitation (Precip: F(1) = 8.98, r = -1.695, P = 0.024) and 2-month cumulative precipitation (Precip2: F(1) = 9.25, r = -0.739, P = 0.023) were negatively associated with species richness, as well as with species diversity (Precip: F(1) = 6.06, r = -0.394, P = 0.049; Precip2: F(1) = 8.72, r = -0.185, P = 0.026). Grid B showed no association of any precipitation value with either species richness or diversity.
Five species showed important associations with precipitation or precipitation-anomaly values on one or both grids. On Grid A, densities of M. domestica were positively associated with 12-month cumulative precipitation levels (Precip12: F(1) = 6.66, r = 1.872, P = 0.042), and C. laticeps was negatively associated with the same variable (Precip12: F(1) = 6.98, r = -3.201, P = 0.038). With data from both grids pooled, two species were negatively associated with precipitation levels: A. montensis with monthly precipitation (Precip: F(1) = 6.87, r = -1.323, P = 0.040), and C. tener with four- and 6-month cumulative precipitation (Precip4: F(1) = 7.67, r = -0.348, P = 0.032; Precip6: F(1) = 13.65, r = -0.293, P = 0.010). Densities of the both Calomys species were positively associated with precipitation anomaly values. C. callosus on Grid A was associated with monthly, two- and six-month anomaly levels (Anom: F(1) = 18.33, r = 13.395, P = 0.005; Anom2: F(1) = 6.06, r = 6.627, P = 0.049; Anom6: F(1) = 15.61, r = 7.117, P = 0.008, and C. tener with twomonth (Grid B, Anom2: F(1) = 7.49, r = 1.717, P = 0.034) and six-month (grids pooled, Anom6: F(1) = 7.62 r = 2.617, P = 0.033) levels.
Fire
No important association was identified of either species richness or diversity with time since last burn (TSB) for either Grid A or Grid B. On Grid A (burned only once, at the beginning of the study), C. callosus was negatively associated with TSB (F(1) = 29.35, r = -0.363, P = 0.002), and N. lasiurus was positively associated (F(1) = 10.86, r = 0.652, P = 0.016). There were no important associations of TSB with MMDM for any species.
DISCUSSION
This study, of a marginal Cerrado patch within an Upper Paraná Atlantic Forest matrix, experienced an overall trapping success of 3.5%. In other Cerrado ecotone studies, Rocha et al. (2011) reported trapping success of < 2%, whereas Bezerra et al. (2009) reported a capture rate of 7.6%, although the rate was 2.1% when excluding a high number of captures of two species in three of their trap lines.
Community characteristics
Species diversities in this study varied from 0.00 to 1.53, somewhat lower than those reported by Bonvicino et al. (2002), who recorded overall species diversity values of 2.63 (undisturbed site) and 2.16 (disturbed), in Goiás state (central Cerrado), Brazil. Few studies have been made of terrestrial smallmammal communities in marginal Cerrado localities. This study recorded two didelphid species, seven cricetids, and one echimyid, a community composition similar to those found in Cerrado-Amazon transition ecotones by Bezerra et al. (2009) and Rocha et al. (2011). In a marginal Cerrado locality in northern Bolivia, Emmons (2009) recorded seven species of rodents and no marsupials in seven years of sampling.
Within sampling sessions no new species was encountered after the fifth sampling night of any session, on either grid. Across sampling sessions, maximum richness was attained about one year after most recent burn, on both grids. In contrast, in the Bolivian Cerrado final richness was reached during the first sampling session, and richness declined thereafter (Emmons, 2009). Similarly, in a study in the central Brazilian Cerrado, richness generally declined from year one through 15, although one marsupial species (Gracilinanus agilis) was encountered only in sites at least six years post-burn (Briani et al., 2004). Despite contrasting patterns of species richness in these three studies, each argues for mid- or long-term sampling in Cerrado, especially in areas subject to burning.
Species characteristics
This study found that the encounter pattern for new captures is highly variable among species. The echimyid rodent C. laticeps averaged more new captures on night two than night one, the didelphid M. domestica had a higher proportion of new captures on night three than on night two, and the sigmodontine C. tener had a higher proportion on night five than on nights four or six. Other species' new capture proportions tended to decline monotonically during sampling sessions, from beginning (night two) values varying from 0.50 (C. callosus) to 1.10 (N. lasiurus). This wide variability of new-capture probability patterns, along with the within-session species accumulation patterns discussed above, indicate strongly that sampling sessions of at least five nights are necessary to adequately sample small mammal richness and abundance in Cerrado habitat.
Although M. domestica was encountered in every month sampled except June, subadult individuals were captured only during November-May, with highest numbers in December, indicating a seasonal (wet season) reproductive period for the species in this marginal Cerrado location. Similarly, Bergallo and Cerquiera (1994) reported breeding to coincide with the wet season in Bahia state, Brazil. In contrast, in a study in the Caatinga, Streilen (1982) indicated that the species is a year-round polyestrous breeder.
In the present study A. montensis, a sigmodontine rodent typically associated with Atlantic Forest, appeared only sporadically on the Cerrado grids, and only in the dry season in the months of May (Grid B), August, and September (Grid A). None of the individuals encountered were subadult, and no evidence of reproductive activity was observed in the samples. All but one of the individuals encountered were males. These observation suggest that A. montensis moves into Cerrado habitat only sporadically, perhaps as resources become scarce in their preferred forest habitat. Talamoni and Dias (1999), working in southeastern Brazil, found A. montensis both in forested and Campo Cerrado habitats, suggesting that the species is a habitat generalist. No individual of A. montensis in this study was captured during more than one sampling session. Bernardes (2006) reported that A. cursor (a closely-related species) occurring in her Cerrado grids in Minas Gerais state, Brazil, persisted on the grid for no longer than one-two months.
Calomys callosus was generally present on both grids, with sporadic population surges. Subadults were encountered in April, August, and October-December, indicating that members of this species are reproductively capable throughout much of the year. Mello (1980) and Alho and Pereira (1985), both working in the central Cerrado, stated that populations of C. callosus (probably actually C. expulsus, a closely-related congener previously considered conspecific with C. callosus) increased from March through August (dry season) and decreased in September and October (late dry season).
Calomys tener was generally uncommon or absent on both grids, but with occasional extreme spikes in population density. Subadults were observed only in October and December (late dry and early wet seasons), whereas Bernardes (2006) reported year-round reproductive activity in a central Cerrado locality. No C. tener were encountered in more than one sampling session, in accord with Bernardes' (2006) finding that individuals of this species did not persist on the grids for more than one or two months.
Necromys lasiurus was encountered on both grids, but was absent or rare (one individual encountered) during the first year of this study, after which population densities rose quickly and substantially on both grids. Subsequently, the species was present for nearly a year (with highest densities in August and again in the following May), and then fell to near zero. Vieira et al. (2005), in the central Cerrado, reported N. lasiurus to be absent on their grids from January through June, and present from July through December. Becker et al. (2007), in a study in São Paulo state, Brazil, reported higher abundances in October than in January and February. Also in agreement with a pattern of dry-season population peak, Bernardes (2006) found populations initially low, after which they rose quickly and remained high, with peaks in the dry season (June-July) for two consecutive years.
Subadults of N. lasiurus were encountered in the present study only in May and August, indicating a dry-season reproductive period for this species. Mares et al. (1989), based on specimens collected in Distrito Federal and Minas Gerais state, Brazil, reported males with scrotal testes, pregnant or lactating females, and subadult individuals in various months throughout the year. Bernardes (2006) also found year-round reproduction in N. lasiurus in Minas Gerais. In contrast, Vieira (1997) encountered juveniles of the species only at the end of the dry season (September-October) in São Paulo state. Becker et al. (2007) also stated that most reproduction in this species occurred during the dry period in central Brazil.
Average MMDM for N. lasiurus in this study was 45.2 m, similar to the 42 m (movement in 24 hours) reported by Vieira et al. (2005). Dos Santos Pires et al. (2010) reported lower values, reporting 31.3 m (males) and 23.1 m (females) between successive captures, and Bernardes (2006) reported distances moved between 39 to 60 m, with extent of movement varying seasonally. The longest persistence on a grid recorded for N. lasiurus in this study was eight months; Magnusson et al. (1995) reported several individuals on their grids for "longer than seven months" and Bernardes (2006) reported persistence for as long as 26 months.
El Niño / Southern Oscillation
In the present study, no significant relaionships were encountered between SOI and either species diversity or species richness, indicating either that ENSO fluctuations indeed have little or no effect on this Cerrado community, or that the various small terrestrial mammal species exhibit suficiently distinct responses, so as to be masked in community indicators such as richness and diversity. Manobavan et al. (2003) examined the relationship between SOI and terrestrial vegetation (NDVI) indices taken along the 6o S latitude from coast to coast across South America, including some Cerrado, identifying and quantifying lags between ENSO perturbations and vegetation response. They found that terrestrial vegetation lost its sensitivity to ENSO perturbations in the post 1993 period, which would further suggest that the response of faunal communities and individual species to ENSO variation might also be difficult to define over longer periods of time. Meserve et al. (1995) described the effects of ENSO on terrestrial small-mammal populations in semiarid Chile as complex, suggesting "different capabilities for small mammals to respond to an extrinsic, large-scale event".
Precipitation
Species richness and diversity were both negatively associated with one- and two-month cumulative precipitation on Grid A. Grid A was burned only once, at the beginning of the study. Thus, it appears that small-mammal species richness and diversity levels are closely tracking precipitation in the absence of frequent burning, and that more-frequent burning (as was experienced on Grid B) effectively disrupts the association of these community characteristics with precipitation for a period of at least several months. In fact, although population densities for several species were responsive to precipitation when data from the two grids were pooled, Grid B (frequent burning) showed very few associations between population levels and precipitation; most of these associations were observed on Grid A, further supporting the conclusion that precipitation is less influential in Cerrado habitats subject to frequent burning.
Fire
A considerable literature exists on the effects of fire on savanna communities, including smallmammal communities in Australia, North America, and South America (e.g., Kaufman et al., 1988, 1989; Meserve et al., 1995; Letnic and Dickman, 2005; Legge et al., 2008), together indicating that small-mammal communities, and their component species, may be affected by fire and other extrinsic factors, in complex and species-specific ways. In the present study, no important associations were encountered between TSB and either species richness or species diversity, on either grid. In contrast, Briani et al. (2004), in the central Brazilian Cerrado, observed that species diversity declined from one (or two) to three years after burning, and subsequently either increased or remained stable. Although several species encountered in this study are characteristic of the Cerrado, they may have dissimilar responses to burning. M. domestica exhibited no response to TSB. In contrast, other didelphid species may be strongly affected by fire (e.g., Gracilinanus agilis; Briani et al. 2004). C. callosus populations on Grid A were negatively associated with TSB on both Grid A (local burn) and Grid B (general, including the matrix around Grid A), indicating that populations of this species were highest soon after burns (both on the grid and in the surrounding areas), and declined with time after the burn. This finding is in accord with several other reports. Vieira and Marinho-Filho (1998) reported that C. callosus (probably C. expulsus, see above) was absent from their sites before burning, and present soon after burning. Vieira (1999) found post-fire population increase of C. callosus; Henriques et al. (2000) reported that C. callosus showed a small decline after burning, followed by strong increase; and Briani et al. (2004) and Henriques et al. (2006) indicated that C. callosus was most abundant in "young" (one-two years after burning) sites.
With grid data pooled, C. tener was negatively associated with TSB on Grid B (widespread burn). This indicates that C. tener experienced a population decline with time after burning in the surrounding matrix. On Grid B, C. tener populations were positively associated with two-month cumulative precipitation anomaly, indicating greater activity or trapability during periods of greater than normal precipitation, in a habitat which experiences frequent burning (not seen in Grid A, which did not experience frequent burning). Other authors have reported similar responses by C. tener to fre. Vieira and Marinho-Filho (1998) reported that C. tener (and its congener C. callosus-probably C. expulsus) were absent before burning, then appeared soon after burning, whereas Vieira (1999) indicated that C. tener showed no marked changes in population levels due to fire. Henriques et al. (2000) reported that C. tener populations declined immediately after fire, then increased three months after fire, with recruitment of juveniles. They also noted that microhabitat use by C. tener changed after fire, away from marginal areas of the habitat patch. Similarly, Briani et al. (2004) and Henriques et al. (2006) found C. tener to be most abundant in "young" (one-two years after burning) sites.
Necromys lasiurus densities on Grid A were positively associated with TSB on Grid B (burns which included the Cerrado matrix surrounding Grid A). Thus, it appears that the population of this species was much reduced following a burn, and then began to increase with time. The lack of this association on Grid B is suggestive that N. lasiurus populations do not exhibit this recovery for some time (several months, at least) after a burn. In a study by Vieira and Marinho-Filho (1998), N. lasiurus disappeared until the fifth week after burning and remained at low population densities at least through the eigth week. Similarly, Vieira (1999) reported populations of N. lasiurus to be substantially reduced after fire. Briani et al. (2004) and Henriques et al. (2006) encountered N. lasiurus rarely in the first year post-fire, and more commonly in two-three year sites. Magnusson et al. (2010), in an Amazonian savanna, reported that densities of this species were positively associated with SOI and fire extent (during the previous year), over several years, thus indicating that although the immediate effect of burning on N. lasiurus populations is negative, fire is necessary or at least beneficial to the species over longer periods. These authors indicated that ENSO variability, precipitation, and fire interact complexly in their effects on N. lasiurus populations, with path analysis indicating SOI to be the basic driver of N. lasiurus densities, through three distinct paths: (1) directly, (2) through precipitation, and (3) through precipitation and fire extent.
Contrast between frequent and infrequent burning
For Grid A, which was burned only once at the beginning of the study, species richness and diversity were both negatively associated with one- and two-month cumulative precipitation. Thus, it appears that species richness and diversity levels were closely tracking precipitation in the absence of frequent burning, with activity or trappability levels increased during periods of lower precipitation. However, burning effectively disrupted the response of these community characteristics to precipitation, for a period of several months.
Moreover, many individual species exhibited different responses to abiotic variables on Grid A, suggesting distinct adaptations to the abiotic characteristics of the Cerrado. The marsupial M. domestica responded positively to sustained periods of high precipitation; in contrast, the echimyid rodent C. laticeps responded negatively to the same variable. Two common cricetid species also exhibited distinct responses on Grid A to abiotic factors, with C. callosus responding positively to both short-term and mid-term cumulative precipitation anomaly, and negatively to TSB (i.e., positively to burning), both on the grid and in the surrounding matrix. In contrast, N. lasiurus was not affected by precipitation variables, and responded positively to TSB (i.e., negatively to burning). Akodon montensis, a species characteristic of forest or second-growth vegetation, was found to use the Cerrado habitat only seasonally or sporadically. Taken together, these observations provide strong support for the importance of long-term population studies in marginal or transitional locations, where two or more ecoregions are represented, and the fauna characteristic of each ecoregion will be sensitive to fluctuations in their biotic and abiotic environment.
ACKNOWLEDGMENTS
I thank the Fundación Moises Bertoni (FMB) for access to field sites and field laboratory and housing facilities, and for permission to use Fig. 1. Within the FMB, I thank Rosalía Fariña, Sixto Fernández, Freddy Ramírez, René Palacios, Danilo Salas, Myriam Velásquez, and Osvaldo Fernández Pintos, all of whom helped in numerous ways with field work or with other logistical assistance. Ismael Mora, Cesar Manchini, Cesar Benítez, Guillermo Santacruz, and Robert Owen-Miller all contributed as part of the field crew. Doug Goodin and David Koch both provided advice and guidance toward locating the precipitation and ENSO data used in these analyses. Margarita Mieres provided a translation of the abstract. Paul Smith, Gary Schnell, Phil Myers, an anonymous reviewer, and Ulyses Pardiñas thoroughly and thoughtfully reviewed various versions of this manuscript, and are gratefully acknowledged. And finally, thanks go to the people at the TTU Library Document Delivery Service, without whose help it would not have been possible to locate and obtain much of the literature cited in this article.
LITERATURE CITED
1. AB'SABER AN. 1977. Os domínios morfoclimáticos na América do Sul-primeira aproximação. Geomorfologia 52:1-22. [ Links ]
2. ALHO CJR. 2005. Intergradation of habitats of non-volant small mammals in the patchy Cerrado landscape. Arquivos do Museu Nacional, Rio de Janeiro 63:41-48. [ Links ]
3. ALHO CJR and LA PEREIRA. 1985. Population ecology of a cerrado rodent community in central Brazil. Revista Brasileira de Biologia 45:597-607. [ Links ]
4. ANIMAL CARE AND USE COMMITTEE. 1998. Guidelines for the capture, handling, and care of mammals as approved by the American Society of Mammalogists. Journal of Mammalogy 79:1416-1431. [ Links ]
5. BECKER RG, G PAISE, LC BAUMGARTEN, and EM VIEIRA. 2007. Estrutura de comunidades de pequenos mamíferos e densidade de Necromys lasiurus (Rodentia, Sigmodontinae) em áreas abertas de cerrado no Brasil central. Mastozoología Neotropical 14:157-168. [ Links ]
6. BERGALLO HG and R CERQUIERA. 1994. Reproduction and growth of the opossum Monodelphis domestica (Mammalia: Didelphidae) in northeastern Brazil. Journal of the Zoological Society of London 232:551-563. [ Links ]
7. BERNARDES ML. 2006. Estudo de uma comunidade de pequenos mamíferos, com enfoque na variação da população de Bolomys lasiurus (Lund, 1841), em uma área de cerrado no estado de Minas Gerais. Unpubl. M.S. thesis, Pontifícia Universidade Católica de Minas Gerais, Belo Horizonte, 83 pp. [ Links ]
8. BEZERRA AMR, AP CARMIGNOTTO, and FHG RODRIGUES. 2009. Small non-volant mammals of an ecotone region between the cerrado hotspot and the Amazonian rainforest, with comments on their taxonomy and distribution. Zoological Studies 48:861-874. [ Links ]
9. BONVICINO CR, R CERQUEIRA, and V DE ARAÚJO SOARES. 1996. Habitat use by small mammals of upper Araguaia River. Revista Brasileira de Biologia 56:761-767. [ Links ]
10. BONVICINO CR, SM LINDBERGH, and LS MAROJA. 2002. Small non-flying mammals from conserved and altered areas of Atlantic Forest and Cerrado: comments on their potential use for monitoring environment. Brazilian Journal of Biology 62:765-774. [ Links ]
11. BORCHERT M and RL HANSEN. 1983. Effects of fooding and wildfire on valley side wet campo rodents in central Brazil. Revista Brasileira de Biologia 43:229-240. [ Links ]
12. BRIANI DC, ART PALMA, EM VIEIRA and RPB HENRIQUES. 2004. Post-fire succession of small mammals in the Cerrado of central Brazil. Biodiversity and Conservation 13:1023-1037. [ Links ]
13. CARMIGNOTTO AP, M DE VIVO, and A LANGGUTH. 2012. Mammals of the Cerrado and Caatinga: Distribution patterns of the tropical open biomes of central South America. Pp. 307-350, in: Bones, Clones, and Biomes: The history and geography of Recent Neotropical mammals (BD Patterson and LP Costa, eds.). The University of Chicago Press. [ Links ]
14. COUTINHO LM. 1990. Fire in the ecology of the Brazilian cerrado. Ecological Studies: Analysis and Synthesis 84:82-105. [ Links ]
15. CRAINE JM, JB NIPPERT, AJ ELMORE, AM SKIBBE, SL HUTCHINSON, and NA BRUNSELL. 2012. Timing of climate variability and grassland productivity. Proceedings of the National Academy of Sciences USA, 2012:1118438109v1-201118438. [ Links ]
16. DE LA SANCHA N, S SOLARI, and RD OWEN. 2007. First records of Monodelphis kunsi Pine (Didelphimorphia, Didelphidae) from Paraguay, with an evaluation of its distribution. Mastozoología Neotropical 14:241-247. [ Links ]
17. DOS SANTOS PIRES A, FA DOS SANTOS FERNANDEZ, BR FELICIANO, and D DE FREITAS. 2010. Use of space by Necromys lasiurus (Rodentia, Sigmodontinae) in a grassland among Atlantic Forest fragments. Mammalian Biology 75:270-276. [ Links ]
18. EITEN G. 1972. The Cerrado vegetation of Brazil. The Botanical Review 38:201-341. [ Links ]
19. EMMONS LH. 2009. Long-term variation in small mammal abundances in forest and savanna of Bolivian Cerrado. Biotropica 41:493-502. [ Links ]
20. GETTINGER D and KA ERNEST. 1995. Small-mammal community structure and the specificity of ectoparasite associations in central Brazil. Revista Brasileira de Biologia 55:331-341. [ Links ]
21. HENRIQUES RPB, MXA BIZERRIL, and T KOHLSDORF. 1997. Abundância, riqueza e seleção de habitat de pequenos mamíferos dos cerrados do Brasil central. Pp. 127-130, in: Contribuçã ao conhecimento ecológico de cerrado (LL Leite and CH Saito, eds.). Universidade de Brasília, Brasília. [ Links ]
22. HENRIQUES RPB, MXA BIZERRIA, and ART PALMA. 2000. Changes in small populations after fire in a patch of unburned cerrado in Central Brazil. Mammalia 64:173-185. [ Links ]
23. HENRIQUES RPB, DC BRIANI, ART PALMA, and EM VIEIRA. 2006. A simple graphical model of small mammal succession after fire in the Brazilian Cerrado. Mammalia 2006:226-230. [ Links ]
24. HUANG C et al. 2007. Rapid loss of Paraguay's Atlantic forest and the status of protected areas - A Landsat assessment. Remote Sensing of Environment 106:460-466. [ Links ]
25. HUANG C et al. 2009. Assessment of Paraguay's forest cover change using Landsat observations. Global and Planetary Change 67:1-12. [ Links ]
26. KAUFMAN GA, DW KAUFMAN, and EJ FINCK. 1988. Influence of fire and topography on habitat selection by Peromyscus maniculatus and Reithrodontomys megalotis in ungrazed tallgrass prairie. Journal of Mammalogy 69:342-352. [ Links ]
27. KAUFMAN DW, GA KAUFMAN, and EJ FINCK. 1989. Rodents and shrews in ungrazed tallgrass prairie manipulated by fire. Proceedings of the Eleventh North American Prairie Conference 1989:173-178. [ Links ]
28. KLINK CA and AG MOREIRA. 2002. Past and current human occupation, and land use. Pp. 69-88, in: The Cerrados of Brazil (PS Oliveira and RJ Marquis, eds.). Columbia University Press, New York. [ Links ]
29. KLINK CA and RB MACHADO. 2005. Conservation of the Brazilian Cerrado. Conservation Biology 19:707-713. [ Links ]
30. KREBS CJ. 1966. Demographic changes in fluctuating populations of Microtus californicus. Ecological Monographs 36:239-273. [ Links ]
31. LACHER TE Jr. and MA MARES. 1986. The structure of Neotropical mammal communities: an appraisal of current knowledge. Revista Chilena de Historia Natural 59:121-134. [ Links ]
32. LEGGE S, S MURPHY, J HEATHCOTE, E FLAXMAN, J AUGUSTEYN, and M CROSSMAN. 2008. The shortterm effects of an extensive and high-intensity fire on vertebrates in the tropical savannas of the central Kimberley, northern Australia. Wildlife Research 35:33-43. [ Links ]
33. LETNIC M and CR DICKMAN. 2005. The responses of small mammals to patches regenerating after fire and rainfall in the Simpson Desert, central Australia. Austral Ecology 30:24-39. [ Links ]
34. MAGNUSSON WE, A DE LIMA FRANCISCO, and TM SANAIOTTI. 1995. Home-range size and territoriality in Bolomys lasiurus (Rodentia: Muridae) in an Amazonian savanna. Journal of Tropical Ecology 11:179-188. [ Links ]
35. MAGNUSSON WE, VMG LAYME, and AP LIMA. 2010. Complex effects of climate change: population fluctuations in a tropical rodent are associated with the southern oscillation index and regional fire extent, but not directly with local rainfall. Global Change Biology 16:2401-2406. [ Links ]
36. MANOBAVAN M, NS LUCAS, DS BOYD, and N PETFORD. 2003. The sensitivity and response of terrestrial South American vegetation to interannual climatic variability induced by the ENSO. Journal of Environmental Informatics 2:1-10. [ Links ]
37. MARENGO J. 2004. Interdecadal variability and trends of rainfall across the Amazon basin. Teoretical and Applied Climatology 78:79-96. [ Links ]
38. MARES MA. 1982. The scope of South American mammalian biology: perspectives on a decade of research. Pp. 1-26, in: Mammalian Biology in South America (MA Mares and HH Genoways, eds.). Pymatuning Laboratory of Ecology Special Publications No. 6. Linesville, Pennsylvania. [ Links ]
39. MARES MA. 1992. Neotropical mammals and the myth of Amazonian biodiversity. Science 255:976-979. [ Links ]
40. MARES MA and KA ERNEST. 1995. Population and community ecology of small mammals in a gallery forest of central Brazil. Journal of Mammalogy 76:750-768. [ Links ]
41. MARES MA, JK BRAUN, and D GETTINGER. 1989. Observations on the distribution and ecology of the mammals of the Cerrado grasslands of central Brazil. Annals of the Carnegie Museum 58:1-60. [ Links ]
42. MELLO DA. 1980. Estudo populacional de algumas espécies de roedores do cerrado (norte do mumicipio de Formosa, Goiás). Revista Brasileira de Biologia 40:843-860. [ Links ]
43. MESERVE PL et al. 1995. Heterogeneous responses of small mammals to an El Niño Southern Oscillation event in northcentral semiarid Chile and the importance of ecological scale. Journal of Mammalogy 76:580-595. [ Links ]
44. MISTRY J. 1998. Fire in the Cerrado (savannas) of Brazil: an ecological review. Progress in Physical Geography 22:425-448. [ Links ]
45. MYERS N, RA MITTERMEIER, CG MITTERMEIER, GAB DE FONSECA, and J KENT. 2000. Biodiversity hotspots for conservation priorities. Nature 403:853-858. [ Links ]
46. OJEDA RA, PG BLENDINGER, and R BRANDL. 2000. Mammals in South American drylands: Faunal similarity and trophic structure. Global Ecology and Biogeography 9:115-123. [ Links ]
47. OWEN RD, DG GOODIN, DE KOCH, Y-K CHU, and CB JONSSON. 2010. Spatiotemporal variation in Akodon montensis (Cricetidae: Sigmodontinae) and hantaviral seroprevalence in a subtropical forest ecosystem. Journal of Mammalogy 91:467-481. [ Links ]
48. PIVELLO VR and LM COUTINHO. 1996. A qualitative successional model to assist in the management of Brazilian cerrados. Forest Ecology and Management 87:127-138. [ Links ]
49. POPLE RG. 2003. The ecology and conservation of the White-winged Nightjar Caprimulgus candicans. Unpubl. Ph.D. thesis, Univ. of Cambridge, Cambridge. [ Links ]
50. RASMUSSON EM. 1985. El Niño and variation in climate. American Scientist 73:68-177. [ Links ]
51. RIBEIRO R and J MARINHO-FILHO. 2005. Estrutura da comundade de pequenos mamíferos (Mammalia, Rodentia) da Estação Ecológica de Águas Emendadas, Planaltina, Distrito Federal, Brasil. Revista Brasileira de Zoologia 22:898-907. [ Links ]
52. ROBERTS SJ. 2000. Tropical fire ecology. Progress in Physical Geography 24:281-288. [ Links ]
53. ROCHA RG, E FERREIRA, BMA COSTA, ICM MARTINS, YLR LEITE, LP COSTA, and C FONSECA. 2011. Small mammals of the mid-Araguaia River in central Brazil, with the description of a new species of climbing rat. Zootaxa 2789:1-34. [ Links ]
54. SAS INSTITUTE INC. 2002-2008. Statistical Analysis Systems, ver. 9.2. Cary, North Carolina. [ Links ]
55. SCHNELL GD, ML KENNEDY, C SÁNCHEZ-HERNÁNDEZ, M DE L ROMERO-AMARAZ, BDN ESTEVEZ, JA GUERRERO, TL BEST, MC WOOTEN, and RD OWEN. 2008. Habitat preference of the endemic tawny deermouse (Peromyscus perfulvus), a species of conservation concern. Southwestern Naturalist 53:9-20. [ Links ]
56. SILVA JF, MR FARIÑAS, JM FELFILI, and CA KLINK. 2006. Spatial heterogeneity, land use and conservation in the Cerrado region of Brazil. Journal of Biogeography 33:536-548. [ Links ]
57. SIMON MF, R GRETHER, LP DE QUEIROZ, C SKEMA, RT PENNINGTON, and CE HUGHES. 2009. Recent assembly of the Cerrado, a Neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proceedings of the National Academy of Science 106:20359-20364. [ Links ]
58. STREILEIN KA. 1982. Ecology of small mammals in the semiarid Brazilian Caatinga III: Reproductive biology and population ecology. Annals of the Carnegie Museum 51:251-269. [ Links ]
59. TALAMONI SA and MM DIAS. 1999. Population and community ecology of small mammals in southesastern Brazil. Mammalia 63:167-181. [ Links ]
60. VIEIRA EM. 1999. Small mammal communities and fire in the Brazilian Cerrado. Journal of Zoology, London 249:75-81. [ Links ]
61. VIEIRA EM and J MARINHO-FILHO. 1998. Pre- and post-fire habitat utilization by rodents of Cerrado from central Brazil. Biotropica 30:491-496. [ Links ]
62. VIEIRA EM, G IOB, DC BRIANI and ART PALMA. 2005. Microhabitat selection and daily movements of two rodents (Necromys lasiurus and Oryzomys scotti) in Brazilian Cerrado, as revealed by a spool-and-line device. Mammalian Biology 70:359-365. [ Links ]
63. VIEIRA MV. 1997. Dynamics of a rodent assemblage in a cerrado of southeast Brazil. Revista Brasileira de Biologia 57:99-107. [ Links ]
64. WERNECK FP. 2011. The diversification of eastern South American open vegetation biomes: Historical biogeography and perspectives. Quaternary Science Reviews 30:1630-1648. [ Links ]
65. WILLIG MR, SJ PRESLEY, RD OWEN, and C LÓPEZ-GONZÁLEZ. 2000. Composition and structure of bat assemblages in Paraguay: A subtropical-temperate interface. Journal of Mammalogy 81:386-401. [ Links ]














