Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Mastozoología neotropical
versión On-line ISSN 1666-0536
Mastozool. neotrop. vol.20 no.2 Mendoza dic. 2013
NOTA
Demographic responses of akodon azarae (rodentia: cricetidae) enclosed populations to rogenhofera bonaerensis bot fly parasitism
María L. Gelin Spessot, María D. Gomez, and José W. Priotto
Grupo de Investigación en Ecología Poblacional y Comportamental (GIEPCO), CONICET, Departamento de Ciencias Naturales, Universidad Nacional de Río Cuarto, Agencia Postal N°3, 5800 Río Cuarto, Córdoba, Argentina [Correspondencia: María L. Gelin Spessot <mlgelin.s@gmail.com>].
Recibido 22 abril 2013.
Aceptado 29 agosto 2013.
Editor asociado: M Lareschi.
ABSTRACT.
We assessed the effect of Rogenhofera bonaerensis parasitism on demographic parameters of Akodon azarae enclosure populations using CMR models. Survival (S), transition (?) and encounter (p) probabilities were modelled using program MARK, restricted by time, sex, abundance and infection state (non-infected and infected individuals). A low rate of infection and a high recovery rate of infected individuals were observed. Survival only showed temporal variation with decreasing values by June. The parasitosis caused by the fly seems to have no influence on the demography of A. azarae populations. The observed pattern in demography of enclosure populations is in agreement with natural populations of this species.
RESUMEN.
Análisis demográfico de la respuesta poblacional de Akodon azarae al parasitismo de la mosca Rogenhofera bonaerensis.
Se evaluó el efecto del parasitismo de la larva de R. bonaerensis sobre parámetros demográficos de poblaciones de clausura de Akodon azarae utilizando modelos de CMR. Las probabilidades de sobrevida (S), transición (?) y encuentro (p) fueron modelados utilizando el programa MARK restringidos por tiempo, sexo y estado de infección (individuos infectados y no infectados). Se observó una baja tasa de infección y una alta tasa de recuperación de los individuos infectados. La sobrevida solo mostró variación temporal con valores decrecientes hacia junio. Según nuestras observaciones, la parasitosis causada por la mosca no tiene influencia en la demografía de las poblaciones de A. azarae. El patrón observado en la demografía de poblaciones de clausura está en concordancia con las poblaciones naturales de esta especie.
Key words: Capture-mark-recapture; Multistate; Survival; Transition.
Palabras clave: Captura-marcado-recaptura; Multiestado; Sobrevida; Transición.
Demographic parameters may have different impact on animal populations and may be influenced by endogenous factors such as intraand interspecific competition, predation and parasitism (Boonstra et al., 1980; Lebreton et al., 1992; Eccard et al., 2002). Parasites can have important effects on host population dynamics (Gulland, 1995; Hudson et al., 1998), some investigations of host-parasite interactions have revealed a negative impact of parasitism on the host species.
Fly larvae of the Cuterebridae family are sub-cutaneous parasites of mammals of the New World (Sabrovsky, 1986) that cause myiasis in dermal and sub-dermal tissues. Cuterebrid larvae may affect a host in a variety of ways, altering its physiology and behavior, and directly or indirectly causing its death. In some situations hosts may obtain certain benefits from being parasitized. Cuterebra-infested mice may live longer, although this longevity comes at the expense of decreased reproduction (Slansky, 2007). On the other hand, bot fly parasitism has been shown to negatively impact gonadal development, and to decrease both survival and reproduction in voles (Timm and Cook, 1979; Boonstra et al., 1980).
Data analysis of animals that are marked, released and recaptured (CMR) provides a powerful and robust tool to estimate and model unbiased demographic parameters (Williams et al., 2002). Multistate models offer a broad family of models with which to estimate survival and transition probability for a variety of situations, providing a convenient framework to model spatial and individual variation of population dynamics (Lebreton and Pradel, 2002). In disease ecology, it is key to know the status of infection, infected and uninfected, and the hazard ratio of mortality in these two states (Telfer et al., 2002; Oli et al., 2006).
Akodon azarae (Fischer, 1829) is one of the most abundant species of the rodent community inhabiting the agro-ecosystems in Córdoba province (Gomez et al., 2011). This species is parasitized by Rogenhofera bonaerensis (Del Ponte, 1939) (Diptera, Cuterebridae) fly larvae. In this work, demographic analyses of A. azarae enclosure populations were made using multistate CMR models in order to characterize the demography and assess the effect of R. bonaerensis parasitism on demographic parameters of these populations.
This study was carried out in an urban natural reserve in Córdoba province, Argentina (33º 07´ S, 33º 14´ W). The study area consists of natural pasture interspersed with brush and weed species, similar to A. azarae natural habitats.
The study was performed in three enclosures of 0.25 ha located in the reserve between December 2008 and June 2009. To assemble the study populations, A. azarae individuals were captured at the beginning of the reproductive period (September 2008) from an area located 30 km away from the enclosures. The captured individuals were mated in the laboratory, each couple was housed in an opaque polycarbonate cage. Both, sex and birth date of the offspring of the first litter were recorded. Parents and their offspring were ear-tagged for permanent identification. The initial population of each enclosure was made up by 6 families (each consisting of mother, father, and about 6 off-spring) that were transferred to the enclosures in early November 2008. Individuals of initial population were uninfected animals.
Each enclosure had a grid of 6 x 10 Sherman-live traps placed at 6 m intervals. Traps were checked each morning. Fortnightly CMR trapping sessions were conducted for three consecutive nights during December and January, and monthly trapping sessions of three nights in February and four nights from April to June; there was not a trapping session in March. Trap check schedule was changed in order to obtain reliable data for a study of space use of this species. For each captured individual, sex and body measurements (weight and length of body and tail) were recorded. All new individuals were marked with numbered ear-tags and released at their sites of capture. Sex, reproductive state (for males, scrotal or abdominal testicles; for females, perforate or imperforate vagina, nipples visible or not, and evidence of pregnancy) were recorded. Animals that weighed more than or equal to 16 g (females) and 18 g (males) were considered adults (Dalby, 1975; De Villafañe, 1981). Individuals were characterized as infected (presence of myiasis) and non-infected (absence of myiasis produced by the fly larvae). The latter group served as control in the subsequent analyses.
Following Hoset et al. (2009) and considering that habitat conditions and trapping schedule were similar among enclosures, we did not expect enclosure effects in recapture and demographic parameters, thus we pooled data of individuals captured in the three enclosures (a total of 721 individuals). Data were analyzed using multistate models and MARK program. Field data were transformed into capture histories showing the particular state in which the individual was captured (I, to indicate that the animal was infected; N, to indicate that the captured animal did not show signs of infection, or zero [0] indicating the sampling occasion when an animal is not captured). Population abundances were estimated using the program CAPTURE (White et al., 1982). Multistate models define transition (?), survival (S) and encounter probabilities (p). The first represents the probability of transition from uninfected to infected (in order to find out the infection rate) or from infected to uninfected (in order to find out the recovery rate); (S) represents the probability that an individual present in a population at time t survives to t + 1 and (p) is a parameter associated with the sampling and represents the probability that an individual alive and present in the study population at time t is captured during time t. Different models were built with the studied parameters restricted by time (t), sex (s) and / or infection status (i). Besides, abundance (a) was used to analyze its possible effects on transition parameter. U-CARE program (Choquet et al., 2003) was used to test goodness-of-fit of multistate models. Models were ranked according to Akaike's information criterion, corrected for small sample size (AICc), a relative measure of fit (Burnham and Anderson, 1998). Model comparison was based on ?AICc values, so when ?AICc was greater than two, the model with the lowest AICc could be considered a statistically better description of the process that generated the data.
From December 2008 to June 2009, we re-corded 3003 captures of A. azarae, with 721 individuals being caught in the three enclosures, 353 males and 368 females. Out of the total captured individuals, 114 were infected (60 males and 54 females). The number of infected individuals was increasing towards the end of the reproductive period; the highest rate of infection (number of infected individuals / total number of individuals) was recorded in April (25%). Besides, 65.11% of the individuals were infected by one larva, whereas the 22.09%, 9.30% and 3.48% by two, three and four larvae respectively.
The Goodness-of-Fit test for multistate models showed that the most general model fitted the data satisfactorily. From a model set of 32 models, the most parsimonious candidate model was [S (t), p (.), ? (i)]. While the ?AIC between this model and the models [S (t), P (t), ? (i)] and [S (t), p (.), ? (i+a)] was lower than two, the first model was selected due to its lower number of parameters (Table 1). For the selected model, there was not infection effect on survival probabilities (S); it only varied in relation to time with decreasing values to-wards the end of the reproductive period. Due to the fact that the best model did not show differences between survival of infected and uninfected individuals, Fig. 1 shows parameter values for each period of time independently of the infection status of individuals. The model indicated that the probability of recapture was constant (p = 0.941). There was an effect of infection for the transition parameter; the results showed a notable difference between the probability of transition from infected to uninfected individuals and vice versa. There was a lower rate of transition from non-infected to infected state (? NI= 0.183) than the reverse (? IN= 0.774), i.e. there was a high recovery rate when individuals were infected.
Table 1. Multistate models of the effect of infection by Rogenhofera bonaerensis bot fly on Akodon azarae populations in three enclosures located in an urban natural reserve. Symbols: Survival (S), transition (?) and recapture (p), time (t), sex (s), infection (i), constant (.) and abundance (a). Only models with ?AICc = 4 are shown. The most parsimonious model is highlighted in bold.
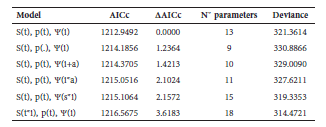

Fig. 1. Survival probabilities (mean ± SE) of Akodon azarae populations by monthly intervals. The values were obtained from the selected model: Survival [by time; S (t)], recapture [constant; p (.)] and transition probability [by state of infection; ? (i)]. DEC: December, EA JAN: Early January, LA JAN: Late January, FEB: February, APR: April, MAY: May, JUN: June.
Due to the fact that recapture probability was constant over time, NULL estimator was selected to obtain a reliable estimate of population abundance. Fig. 2 shows abundance values by sampling session for the three enclosures. The highest average abundance value was obtained in early fall (April). This value was the result of the recruitment of juveniles born during the peak of reproductive rate (in the middle of reproductive period). Population numbers started to decline thereafter.
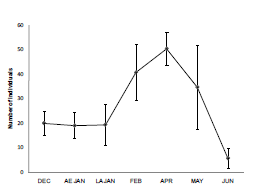
Fig. 2. Abundance values (mean ± SE ) of Akodon azarae populations by trapping session for the 3 analyzed enclosures. DEC: December, EA JAN: Early January, LA JAN: Late January, FEB: Febru-ary, APR: April, MAY: May, JUN: June.
Our study used data from CMR and multi-state models to model the consequences of infection of R. bonaerensis fly on enclosure populations of A. azarae. This analysis accounted for variation in capture probabilities providing unbiased estimates of the likelihood of infection and recovery from infection and the survival probabilities of healthy and infected individuals. Besides, the use of enclosure populations allows us to estimate demographic parameters that in natural populations would be difficult to estimate.
The results obtained in this study show that parasitosis caused by the larvae of R. bonaerensis fly seems to have no influence on the analyzed demographic parameters of A. azarae populations. The survival of individuals did not show differences between infected and uninfected individuals. Besides, transition probability showed that infected individuals had high recovery rates, which means that individuals recover the healthy status promptly. Bergallo et al. (2000) reached similar conclusions for Euryoryzomys russatus which is parasitized by Metacuterebra apicalis fly. This tolerance of rodents that have been parasitized could indicate that there has been a co-evolution between parasites and hosts resulting in a more favorable and stable relationship (Guimarães et al., 1983). This is possibly due to the lack of secondary complications and rapid recovery from injury, development of resistance to multiple injuries, property of the larvae in non-intrusive areas of the body and / or development of immunity (Catts, 1982). This result does not rule out the possibility that parasitism influence other demographic parameters of A. azarae. Parasitism could affect movement rates of individuals decreasing their ability to obtain resources (Hudson and Dobson, 1995). A. azarae has a polygynous mating system with female defense (Bonatto et al., 2013). Thus, infected males and females could have a reduced ability to compete for mates or resources (cover and food) respectively, and consequently a lower reproductive success. In this study the effect of parasitism on movement and reproductive behavior was not analyzed. Future studies should be developed to test parasitism effects on these parameters.
In relation to time dependence in survival probability, there is no information about CMR data in natural populations, but a winter declination in numbers associated with breeding in-terruption and mortality increase was observed (Gomez et al., 2011). This is in agreement with the decrease of survival probabilities and the low population numbers at the beginning of winter observed in this study. The pattern of population abundance observed in enclosure populations, with low population numbers at the beginning and the end of breeding period and the highest numbers in early autumn, matches those values observed in A. azarae natural populations (Gomez et al., 2011).
The selected multi-state model did not show any effects of gender on the transition probability. Zuleta and Vignau (1990) obtained similar results for A. azarae. Meanwhile, A. molinae females appear to be more frequently infected than males (Brigada et al., 1992).
In relation to time variation in the occurrence of bot fly parasitism in Akodon species, Zuleta and Vignau (1990) recorded that the highest infection rate occurred in late spring and fall. In our study, the spring peak in infection rate could not be observed due to the fact that the rodents of the initial populations were free of bot fly infection. The greatest average infection rate recorded in early autumn was in agreement with the previous results. Although the best model did not show a strong association between transition probabilities and abundance, the greatest infection rate was registered together with the abundance peak. The lack of dependence between abundance and infection prevalence was registered by Zuleta and Vignau (1990) using regression analysis.
In conclusion, the demographic parameters of enclosure populations of A. azarae were not affected by the parasitism caused by R. bonaerensis fly larvae. The observed pattern in survival and abundance is in agreement with natural populations of this species. However, more intensive and longer term field studies would be necessary to elucidate the dynamics of the association between R. bonaerensis and A. azarae.
Acknowledgments.
We are very grateful to Florencia Bonatto for field assistance during this study. This research was made possible by grants from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Secretaría de Ciencia y Técnica (SECyT), Universidad Nacional de Río Cuarto.
LITERATURE CITED
1. BERGALLO HG, F MARTINS-HATANO, N JUCÁ, and D GETTINGER. 2000. The effect of botfly parasitism of Metacuterebra apicalis (Diptera) on reproduction, survival and general health of Oryzomys russatus (Rodentia), in Southeastern Brazil. Mammalia 64:439-446. [ Links ]
2. BONATTO MF, J CODA, D GOMEZ, J PRIOTTO, and A STEINMANN. 2013. Inter-male aggression with regard to polygynous mating system in Pampean grassland mouse, Akodon azarae (Cricetidae: Sigmodontinae). Journal of Ethology. DOI 10.1007/s10164-013-0370-4. [ Links ]
3. BOONSTRA R, C KREBS, and T BEACHAM. 1980. Impact of botfly parasitism on Microtus townsendii populations. Canadian Journal of Zoology 58:1683- 1692. [ Links ]
4. BRIGADA AM, ES TRIPOLE, and GA ZULETA. 1992. Cuterebrid parasitism (Rogenhofera bonariensis) on the Shrubland Mouse (Akodon molinae), in Argentina. Journal of Wildlife Diseases 28:646-650. [ Links ]
5. BURNHAM K and D ANDERSON. 1998. Model selection and inference: A practical information-theoretic approach. Springer-Verlag, New York. [ Links ]
6. CATTS EP. 1982. Biology of new world bot flies: Cuterebridae. Annual Review of Entomology 27:313- 338. [ Links ]
7. CHOQUET R, AM REBOULET, R PRADEL, O GIMENEZ, and JD LEBRETON. 2003. User´s Manual for U-CARE, Version 2.0. [ Links ]
8. DALBY PL. 1975. Biology of pampa rodents: Balcarce Area, Argentina. Publications of the Museum, Michigan State University Biological Series 5:149-272. [ Links ]
9. DE VILLAFAÑE G. 1981. Reproducción y crecimiento de Akodon azarae azarae. Revista Chilena de Historia Natural 1:193-204. [ Links ]
10. ECCARD J, I KLEMME, T HORNE, and H YLONEN. 2002. Effects of competition and season on survival and maturation of young bank vole females. Evolutionary Ecology 16:85-99. [ Links ]
11. GOMEZ MD, L SOMMARO, A STEINMANN, M CHIAPERO, and J PRIOTTO. 2011. Movement distances of two species of sympatric rodents in linear habitats of Central Argentine agro-ecosystems. Mammalian Biology 76:58-63. [ Links ]
12. GUIMARAES JH, N PAPAVERO, and AP PRADO. 1983. As miases na regiao neotropical (Identificao, biología, bibliografía). Revista Brasilera de Zoologia 1:239-416. [ Links ]
13. GULLAND FMD. 1995. The impact of infectious diseases on wild animal populations-A review. Pp. 20-51, in: Ecology of infectious diseases in natural populations. Broad patterns and processes (BT Grenfell and AP Dobson, eds.). Cambridge University Press. [ Links ]
14. HOSET KS, JF LE GALLIARD, and G GUNDERSEN. 2009. Demographic responses to a mild winter in enclosed vole populations. Population Ecology 51:279-288. [ Links ]
15. HUDSON PJ and AP DOBSON. 1995. Macroparasites: observer patterns. Pp. 52-89, in: Ecology of infectious diseases in natural populations (BT Grenfell and AP Dobson, eds.). Publication of the Newton Institute. [ Links ]
16. HUDSON PJ, AP DOBSON, and D NEWBORN. 1998. Prevention of population cycles by parasite removal. Science 282(5397):2256-2258. [ Links ]
17. LEBRETON JD and R PRADEL. 2002. Multistate recapture models: Modelling incomplete individual histories. Journal of Applied Statistics 29(1-4):353- 369 [ Links ]
18. LEBRETON JD, K BURNHAM, J CLOBERT, and D ANDERSON. 1992. Modeling survival and testing biological hypotheses using marked animals: A unified approach with case studies. Ecological Monographs. 62:67-118. [ Links ]
19. OLI MK, M VENKATARAMAN, PA KLEIN, LD WENDLAND, and MB BROWN. 2006. Population dynamics of infectious diseases: A discrete time model. Ecological Modelling 198:83-194. [ Links ]
20. SABROVSKY CW. 1986. North American species of Cuterebra, the rabbit and rodent bot flies (Diptera: Cuterebridae). Entomological Society of America, College Park, Maryland. pp 240. [ Links ]
21. SLANSKY F. 2007. Insect/mammal associations: Effects of cuterebrid bot fly parasites on their hosts. Annual Review of Entomology 52:17-36. [ Links ]
22. TELFER S, M BENNETT, K BOWN, R CAVANAGH, L CRESPIN, S HAZEL, T JONES, and M BEGON. 2002. The effects of cowpox virus on survival in natural rodent populations: increases and decreases. Journal of Animal Ecology 71:558-568. [ Links ]
23. TIMM RM and EF COOK. 1979. The effect of bot fly larvae on reproduction in white-footed mice, Peromyscus leucopus. American Midland Naturalist 101:211-217. [ Links ]
24. WHITE GC, DR ANDERSON, KP BURNHAM, and DL OTIS. 1982. Capture-recapture and removal methods for sampling closed populations. Los Alamos National Laboratory Report LA-8787-NERP, Los Alamos, New Mexico, USA. [ Links ]
25. WILLIAMS B, J NICHOLS, and M CONROY. 2002. Analysis and management of animal populations. Academic Press. pp. 817. [ Links ]
26. ZULETA GA and ML VIGNAU. 1990. Bot f ly parasitism (Rogenhofera bonariensis) (Diptera, Cuterebridae) in the Pampean grassland mouse (Akodon azarae) in Argentina. Journal Wildlife of Diseases 26:11-17. [ Links ]













