Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Mastozoología neotropical
versión impresa ISSN 0327-9383versión On-line ISSN 1666-0536
Mastozool. neotrop. vol.22 no.1 Mendoza jun. 2015
NOTA
Maternity roost of Eptesicus brasiliensis in a liana in the southeast peruvian Amazon
Timothy J. Divoll1, Anjali Kumar2, Cesar F. Flores-Negron3, and Cindy M. Hurtado4
1 Biodiversity Research Institute, 276 Canco Rd., Portland, ME, 04104, USA [Correspondence: Timothy J. Divoll <tim.divoll@briloon.org>]
2 Massachusetts Institute of Technology, 77 Massachusetts Ave., Cambridge, MA, 02139, USA
3 Cocha Cashu Biological Station, Manu National Park, Madre de Dios, PERÚ
4 Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Apartado 14-0434, Lima, 14, PERÚ.
Recibido 20 octubre 2014.
Aceptado 7 enero 2015.
Editor asociado: M Sandoval
ABSTRACT.
We detected a maternity roost of Eptesicus brasiliensis in a large liana at Cocha Cashu Biological Station (Peru). Our objective was to describe the roost and its surrounding habitat employing visual methods to estimate colony size and time of foraging. At least 10 adults (9♀, 1♂) and one juvenile used a cavity in a liana that hung across a small trail. We used a night vision scope and infrared camera traps to note that bats left the roost for short foraging bouts (<1 hr). We also discuss the potential importance of lianas as roost sites in tropical forests.
RESUMEN.
Refugio de una colonia de maternidad de Eptesicus brasiliensis en una liana al sudeste de la Amazonía peruana.
Detectamos el refugio de una colonia de maternidad de Eptesicus brasiliensis en una liana de la estación biológica de Cocha Cashu (Perú). Nuestro objetivo fue describir el refugio y el hábitat alrededor del mismo utilizando métodos visuales para estimar el tamaño de la colonia y el tiempo de forrajeo. Al menos 10 adultos (9♀, 1♂) y un juvenil utilizaron la cavidad de una liana que colgaba sobre un sendero angosto. Utilizamos un visor nocturno y trampas-cámara infrarrojas para advertir que los murciélagos dejaron el refugio por periodos de forrajeo cortos (<1 hr). Además discutimos la importancia potencial de las lianas como lugares de refugio en bosques tropicales.
Key words: Bignoniaceae; Camera trap; Cavity roost; Cocha Cashu; Foraging.
Palabras clave: Bignoniaceae; Cocha Cashu; Forrajeo; Refugio de cavidad; Trampas-cámara.
Studies of roosting behavior and roost selection are important for conservation of forest bats (Betts, 1998). Some bats use cavities for roosting, breeding and hibernating, relying on roosting structures to provide protection from abiotic factors and potential predators. Most bats are secondary cavity users and select cavities in mainly live, dying, or dead trees, spending over half their lives within the roost environment (Kunz, 1982; Kunz and Lumsden, 2003; Diaz and Linares Garcia, 2012; Rengifo et al., 2013). The availability of roost sites is paramount to productivity and survival in cavity-roosting species. Anthropogenic land management activities, as well as natural weather events, commonly reduce roost availability in forest systems (Jones et al., 2001; Bennet et al., 2013; Tournant et al., 2013). There is a lower density of available snags for potential roosts in low latitude forests when compared to high latitude forests (Gibbs et al., 1993) potentially caused by lower rates of tree mortality, which would not favor snag creation, and higher rates of tree decomposition (Gibbs et al., 1993; Cornelius et al., 2008). Therefore, tree cavities may be a potentially unstable resource for bats in tropical systems. While some tree species have been identified as preferable for certain bat species (Evelyn and Stiles, 2003), lianas have been overlooked as potential roost sites for bats at critical life stages until now.
Nest site selection and placement is an important aspect of the natural history of a species and important for reproductive success (Goodall, 1962; Jessen et al., 2013). Persistent roost sites that are close to food sources and additionally promote a warm microclimate are particularly important for maternity roosts, which are used to suit the added demands of pregnancy and lactation and to bear and raise young (Kunz, 1982; Brigham and Fenton, 1986; Betts, 1998; Sedgeley, 2001). Since traditional tree cavities may be ephemeral, alternative and more stable vegetation types may also be used by bats as roosting sites, specifically for high cost activities such as those associated with reproduction and foraging (Sedgeley, 2001). Roost sites must also provide protection from potential predators that actively search for bats as prey, such as carnivorous bats, primates, certain birds, and specifically those that develop a search image for roost structures (Lima and O'Keefe, 2013). It has been shown that primates are able to recognize leaves modified by tent-making bats (Boinski and Timm, 1985). The ease at which bats can leave and enter roosts will also determine the length of time of exposure to potential predators (Barclay et al., 1982; Fenton et al., 1994; VonHof and Barclay, 1996). The vertical location of roost sites within a forest may also be highly variable among and within species (Kalko and Handley, 2001), potentially hampering predator search image creation. Some species may also alleviate predation and potential roost decay through fission-fusion maternity roost systems, where many roost sites are used in the same area, with frequent switching among sites within a reproductive period (Brigham et al., 1997; Popa-Lisseanu et al., 2007).
The objective of this study was to describe the physical characteristics of a liana being used as an active maternity roost. We describe the physical characteristics and plant community surrounding the liana. We then report morphometric and reproductive state of the Eptesicus brasiliensis colony roosting within the liana and supply notes about their nocturnal activity patterns.
The E. brasiliensis roost was located at Cocha Cashu Biological Station (11°53'S, 71°26'W) along the Rio Manu, in the Madre de Dios region of southeastern Peru (Foster et al., 1986). This tropical floodplain forest is an example of a pristine successional plant community with disturbance caused by the rerouting of a meandering river and seasonal flooding, rather than anthropogenically (Terborgh, 1983; Foster et al., 1986). The area around Cocha Cashu Biological Station, within Manu National Park, has also remained free from organized hunting, thus high mammal diversity has maintained in this pristine forest (Janson and Emmons, 1990). An expedition directed at surveying mammal and bird species along the elevational gradient in Manu National Park detected 222 species of mammals, of which 92 were bats (Patterson et al., 2006).
The roost was situated in a late successional floodplain forest in a canopy liana (Bignoniaceae: Arribidaea florida; Vasquez and Rojas, 2004; C.F.F-N., pers. comm.) on a levee, 430 m from the start of trail KS (Fig. 1). The elevation at the roost site is 345 m above sea level and it is approximately 510 m from the Rio Manu and 950 m from Cocha Cashu, two permanent water sources. The forest is characterized by a short canopy (~25 m high), with successional trees, understory plants, and woody shrubs commonly associated with late regenerating forest. There were no standing hardwood trees approximately 200 years old, which are characteristic of a lowland Amazonian forest of this area, such as Cedrela odorata, but there were several fallen and rotting C. odorata trees in the immediate vicinity of the roost location, aging the forest as greater than 200 but less than 400 years old (Vasquez and Rojas, 2004). At the roost location the levees were approximately 10 m apart and 1.5-2 m above ground level. The canopy plants located on the same levee as the liana roost included: Annonaceae: Unonopsis floribunda; Arecaceae: Iriartea deltoidea and Socratea exorrhiza; and Euphorbiaceae: Sapium sp. The understory plants located on the levee around the roost were: Piperaceae: Piper spp.; Marantaceae: Calathea sp.; Annonaceae: Oxandra sp.; Myristicaceae: Otoba parvifolia; Arecaceae: Astryocaryum spp.; and Theophrataceae: Clavija tarapotana. Canopy trees located in the depressions immediately surrounding the roost were: Rubiaceae: Calycophyllum spruceanum; Combretaceae: Terminalia oblonga; Moraceae: Soracea sp.; and Meliaceae: Trichilia sp. All of these woody tree species are characteristic of a late successional forest with seasonal flooding (Vasquez and Rojas, 2004).

Fig. 1. Map of trails at the Cocha Cashu Biological Station. The liana roost is marked with a star and is situated between two permanent water sources: 0.51 km from the Rio Manu, and 0.95 km from Cocha Cashu. Digital Elevation Model was downloaded from ArcGIS Online.
On September 4, 2013, while walking on Trail KS (2 m wide), we heard audible squeaking sounds coming from a liana stretched across the trail. Upon inspection, we noticed a crack in the liana where apparently two separate vines may have grafted together and never fully closed, offering a small hollow as refuge for several bats, which were situated in the farthest corner of the hollow. The bats appeared to be of the genus Eptesicus and one appeared very small and dull colored, as if a juvenile. The roost opening was 1.59 m above the ground; the crevice opening was 1.75 cm wide by 24.3 cm long. The diameter at the approximate centroid of the roost opening was 17.2 cm (Fig. 2).

Fig. 2. Liana roost with trail KS in the background. There was only one apparent roost opening, indicated by the white arrow. The roost opening was 1.59 m above ground, the crevice opening was 1.75 cm wide by 24.3 cm long, and the diameter of the liana at the approximate centroid of the roost opening was 17.2 cm.
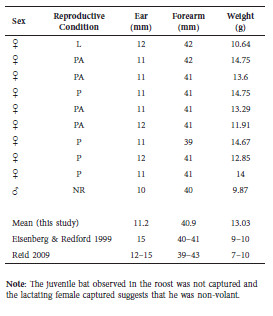
On September 5, 2013, we set an Austbat© (Australia) style harp trap in front of the roost in the afternoon with factory strings re-tied every 2 cm to target small Vespertillionidae. We checked the trap 110 minutes after sundown to find ten individual E. brasiliensis roosting under the plastic roost baffle in the catch bag of the trap. All were adults (9♀, 1♂), identified using two field guides (Eisenberg and Redford, 1993; Reid, 2009), and no other bats were present in the trap. One adult female was lactating and we did not catch any juvenile bats, so the apparent juvenile may not have been volant. Forearm, ear, and weight measurements were taken on all bats using a metal ruler and digital pocket balance. All individuals were assessed for age by ossification of wing joints and reproductive condition by inspection of genitals and nipples (Kunz and Anthony, 1982), with stomach palpation for females (Table 1).
Table 1 Data collected on individual bats from the harp trap survey. Ten adult Eptesicus brasiliensis were captured by harp trap. For comparison, mean values from this study were compared to measurements presented in two field guides. Reproductive condition codes are as follows: L = lactating, P = pregnant, PA = parous, NR = non-reproductive.
Exit counts were conducted on two separate nights with two methodologies to assess how effective our initial harp trapping method was in quantifying roost size. The first was on September 20, 2013, using a 4x Yukon NVMT3 night vision scope with a 50 mm lens, positioned 10 m away and pointing directly at the roost opening. Sunset at the roost location on September 20 was at 17:40. The total time of the exit count was 58 minutes and 44.07 seconds and six bats were observed leaving the roost and returning within the observation time (Table 2). We do not know if the first bat to leave was the first bat to return. However, all six bats exited the roost within a 13 minute time span and at least one bat foraged for at least 36 minutes before returning to the roost. It was observed that bats circled around the roost several times before re-entering it. The second method was 2 infrared (IR) motion video camera traps, Bushnell HD Trophy Camera Model 119537, which were placed facing the roost exit at 15:30 on September 28, 2013, and retrieved at 07:30 on September 29, 2013. One was placed 1 m away directly in front of the entrance and the other was positioned 2.5 m to the west but also facing the roost exit. Cameras were set at the finest settings with one minute video recording upon detection of movement as an experimental effort to determine their use for unmanned exit counts at known roost sites. The camera placed 1 m in front of the roost detected two bats entering the roost: one at 18:18:56 and one at 18:31:13. The camera 2.5 m to the side detected one bat leaving the roost at 20:26:08 and one bat returning to the roost at 23:27:30. We suspect that the cameras missed many bats exiting and entering the roost site as these activities may happen faster than the cameras can be triggered to record (trigger speed = 0.6 sec). IR cameras that can continuously record for an entire night may be better suited for unmanned exit counts. However, the extra battery power required may make this unfeasible at remote field sites.
Table 2 Times of bat departure and return during the exit count using a night vision scope. Times reported here only represent one bat leaving or returning to the roost and do not refer to individual bats.

Considering the night vision survey and the IR camera survey, it appears that E. brasiliensis leaves the roost to forage shortly after sunset and returns within an hour. Though there are missing data from the IR camera survey, it appears that bats left the roost later in the night again for a foraging bout and were active until almost midnight, when one bat returned.
While some bat species use vine tangles as roost sites (Fenton et al., 2001) this is, to the best of our knowledge, the first record of roost site selection by bats in a liana cavity. Lianas (woody vines) are important components of tropical ecosystems, where they currently make up to 40% of the woody stems and more than 25% of the woody species (Schnitzer and Bongers, 2011). There is evidence that liana abundance and biomass is increasing in tropical forests which may increase the importance of lianas for bat roost sites (Schnitzer and Bongers, 2011). Since the 1970's biomass and abundance of lianas in Neotropical forests have increased with some Amazonian forests showing a doubling in liana abundance in a 20-year period (Philips et al., 2002; Schnitzer and Bongers, 2011). As liana abundance increases in Neotropical forests, tree diversity, recruitment, growth, fecundity, and survival will likely decrease (Schnitzer and Bongers, 2011), potentially making lianas a newly important roost site for bats.
Lianas may offer protection that larger tree cavities do not. Amazonian lianas may be more abundant than they were historically, therefore it is possible that predators do not have a search image for lianas as roost sites as they do for tree cavities. Neotropical lianas are smaller and less stable than paleotropical lianas and large lianas are less common in Neotropical forests than African forests (Emmons and Gentry, 1983). Smaller sized, more fragile lianas are presumably related to the evolution of prehensile tails in vertebrates that need to travel across canopy gaps (Emmons and Gentry, 1983). Neotropical lianas are used more for locomotion whereas African lianas are used more as a food source (Emmons and Gentry, 1983). Thus, it is logical that Neotropical predators may travel and search for food in the canopy rather than along the bases of lianas. Though lianas quite literally connect large trees in the canopy and provide canopy travel access for vertebrates (Charles- Dominique et al., 1981), the spatial lack of horizontal tree branches proximal to lower portions of large lianas may reduce predation potential for Neotropical bats roosting in liana cavities closer to the ground. Cavities on tree limbs or tree trunks may be more accessible to arboreal predators via horizontal branches.
The observed liana roost was located in the center of a 2 m wide trail. Therefore, it is possible that the maternity roost may be capturing a greater percentage of sunlight than other roost sites located in the dense forest understory. The retention of heat and absorption of solar radiation is particularly important for keeping pups warm when mothers leave the roost to forage (Sedgeley, 2001). The proximity of the roost site to the two permanent water sources may also be important if E. brasiliensis is foraging over water or drinking from those sources.
Lianas should not be overlooked as quality roosting sites in forests. Studies on roost selection in tropical forests should include lianas as potential roosting structures and researchers should add lianas to their search image repertoire. Similarly, forest management practices should consider the importance of lianas to bats in addition to other functions within the tropical forest system. Particularly, large Neotropical lianas should be investigated as a limiting resource on reproductive success in forest bats. As far as we know, this is the first documented report of a maternity roost of E. brasiliensis in lianas.
Acknowledgments.
Students of the 2013 San Diego Zoo Global and the Wallace Research Foundation assisted with observations in the field and provided overall enthusiasm and support for this project. Special thanks go to Nicole Mitidieri and Adrian Torres Pacuar for helping with plant identification. Great thanks to Roxana P. Arauco, Verónica Chávez, Jessica Groenendijk, Florencia Trama, Federico Rizo-Patron and CORBIDI for assisting with logistical support. Cocha Cashu Biological Station extended access to their land for data collection. Three anonymous reviewers made useful comments on the manuscript. Research was conducted under permits from the Servicio Nacional de Áreas Protegidas por el Estado (SERNANP) and Manu National Park (Permit number PNM N°15- 2013-SERNANP-PNM-JEF) and in accordance with the laws of Peru. This project was supported by in-kind support from the Singapore University of Technology and Design and Massachusetts Institute of Technology to A.K. and in-kind support from Biodiversity Research Institute to T.D.
LITERATURE CITED
1. BARCLAY RMR, CE THOMPSON, and FJS PHELAN. 1982. Screech owl, Otus asio, attempting to capture little brown bats, Myotis lucifugus, at a colony. Canadian Field Naturalist 96:205-206. [ Links ]
2. BENNETT VJ, DW SPARKS, and PA ZOLLNER. 2013. Modeling the indirect effects of road networks on the foraging activities of bats. Landscape Ecology 28:979-991. [ Links ]
3. BETTS BJ. 1998. Roosts used by maternity colonies of silver-haired bats in northeastern Oregon. Journal of Mammalogy 79:643-650. [ Links ]
4. BOINSKI S and RM TIMM. 1985. Predation by squirrel monkeys and double-toothed kites on tent-making bats. American Journal of Primatology 9:121-127. [ Links ]
5. BRIGHAM RM and MB FENTON. 1986. The influence of roost closure on the roosting and foraging behaviour of Eptesicus fuscus (Chiroptera: Vespertilionidae). Canadian Journal of Zoology 64:1128-1133. [ Links ]
6. BRIGHAM RM, MJ VONHOF, RMR BARCLAY, and JC GWILLIAM. 1997. Roosting behavior and roost-site preferences of forest-dwelling California bats (Myotis californicus). Journal of Mammalogy 78:1231-1239. [ Links ]
7. CHARLES-DOMINIQUE P, M ATRAMENTOWICZ, M CHARLES-DOMINIQUE, H GERARD, A HLADIK, CM HLADIK, and MF PREVOST. 1981. Les mammifères frugivores arboricoles nocturnes d'une forêt Guyanaise: interrelations plantes-animaux. Revue d'Ecologie (Terre et Vie) 35:341-435. [ Links ]
8. CORNELIUS C, K COCKLE, N POLITI, I BERKUNSKY, L SANDOVAL, V OJEDA, L RIVERA, M HUNTER, and K MARTIN. 2008. Cavity-nesting birds in Neotropical forests: Cavities as a potentially limiting resource. Ornithologia Neotropical 19:253-268. [ Links ]
9. DIAZ MM and VH LINARES GARCIA. 2012. Refugios naturales y artificiales de murciélagos (Mammalia; Chiroptera) en la selva baja en el noroeste de Perú. Gayana 76:117-130. [ Links ]
10. EISENBERG JF and KH REDFORD. 1993. Mammals of the Neotropics, Volume 3, The Central Neotropics: Ecuador, Perú, Bolivia, Brazil. University of Chicago Press, Chicago. [ Links ]
11. EMMONS LH and AH GENTRY. 1983. Tropical forest structure and the distribution of gliding and prehensile-tailed vertebrates. The American Naturalist 121:513-524. [ Links ]
12. EVELYN MJ and DA STILES. 2003. Roosting requirements of two frugivorous bats (Sturnira lilium and Artibeus intermedius) in fragmented Neotropical forest. Biotropica 35:405-418. [ Links ]
13. FENTON MB, IL RAUTENBACH, SE SMITH, CM SWANEPOEL, J GROSELL, and J VAN JAARSVELD. 1994. Raptors and bats: Threats and opportunities. Animal Behavior 48:9-18. [ Links ]
14. FENTON MB, E BERNARD, S BOUCHARD, L HOLLIS, DS JOHNSTON, CL LAUSEN, JM RATCLIFFE, DK RISKIN, JR TAYLOR, and J ZIGOURIS. 2001. The bat fauna of Lamanai, Belize: Roosts and trophic roles. Journal of Tropical Ecology 17:511-524. [ Links ]
15. FOSTER RB, JB ARCE, and TS WACHTER. 1986. Dispersal and the sequential plant communities in Amazonian Perú floodplain. Pp. 357-370, in: Frugivores and seed dispersal (A Estrada and TH Fleming, eds.). Dr W Junk Publishers, Dordrecht. [ Links ]
16. GIBBS JP, ML HUNTER, and SM MELVIN. 1993. Snag availability and communities of cavity nesting birds in tropical versus temperate forests. Biotropica 25:236-241. [ Links ]
17. GOODALL JC 1962. Nest building behavior in the free ranging chimpanzee. Annals of the New York Academy of Sciences 102:455-467. [ Links ]
18. JANSON CH and LH EMMONS. 1990. Ecological structure of the nonflying mammal community at Cocha Cashu Biological Station, Manu National Park, Perú. Pp. 314- 338, in: Four Neotropical Rainforests (AH Gentry, ed.). Yale University Press, New Haven. [ Links ]
19. JESSEN RR, GH PALMER, and JL KOPROWSKI. 2013. Maternity nest of an Amazon red squirrel in a bromeliad. Mastozoología Neotropical 20:159-161. [ Links ]
20. JONES KE, KE BARLOW, N VAUGHN, A RODRIGUEZ-DURAN, and MR GANNON. 2001. Short-term impacts of extreme environmental disturbance on the bats of Puerto Rico. Animal Conservation 4:59-66. [ Links ]
21. KALKO EKV and CO HANDLEY. 2001. Neotropical bats in the canopy: Diversity, community structure, and implications for conservation. Plant Ecology 153:319-333. [ Links ]
22. KUNZ TH. 1982. Roosting ecology of bats. Pp. 1-55, in: Ecology of Bats (TH Kunz, ed.). Plenum Press, New York. [ Links ]
23. KUNZ TH and EL ANTHONY. 1982. Age estimation and post-natal growth in the bat Myotis lucifugus. Journal of Mammalogy 63:23-32. [ Links ]
24. KUNZ TH and LF LUMSDEN. 2003. Ecology of cavity and foliage roosting bats. Pp. 3-69, in: Bat Ecology (TH Kunz and MB Fenton, eds.) The University of Chicago Press, Chicago. [ Links ]
25. LIMA SL and JM O'KEEFE. 2013. Do predators influence the behaviour of bats? Biological Reviews 88:626-644. [ Links ]
26. PATTERSON BD, DF STOTZ, and S SOLARI. 2006. Mammals and birds of the Manu Biosphere Reserve, Perú. Fieldiana Zoology 110:1-49. [ Links ]
27. PHILIPS OL, MR VASQUEZ, L ARROYO, TR BAKER, T KILLEEN, SL LEWIS, Y MALHI, AM MENDOZA, D NEILL, VP NÚÑEZ, M ALEXIADES, C CERON, AD FIORE, T ERWIN, A JARDIM, W PALACIOS, M SALIDAS, and B VINCETI. 2002. Increasing dominance of large lianas in Amazonian forests. Nature 418:770-774. [ Links ]
28. POPA-LISSEANU AG, F BONTADINA, O MORA, and C IBÁÑEZ. 2007. Highly structured fission-fusion societies in an aerial-hawking, carnivorous bat. Animal Behaviour 75:471-482. [ Links ]
29. REID F. 2009. A field guide to the mammals of Central America & southeast Mexico, 2nd ed. Oxford University Press, New York. [ Links ]
30. RENGIFO EM, W CALDERÓN, and R AQUINO. 2013. Características de refugios de algunas especies de murciélagos en la cuenca alta del rio Itaya, Loreto, Perú. Research Journal of the Costa Rican Distance Education University 5:143-150. [ Links ]
31. SCHNITZER SA and F BONGERS. 2011. Increasing liana abundance and biomass in tropical forests: Emerging patterns and putative mechanisms. Ecology Letters 14:397-406. [ Links ]
32. SEDGELEY JA. 2001. Quality of cavity microclimate as a factor influencing selection of maternity roosts by a tree-dwelling bat, Chalinolobus tuberculatus, in New Zealand. Journal of Applied Ecology 38:425-438. [ Links ]
33. TERBORGH J. 1983. Five new world primates: A study in comparative ecology. Princeton University Press, Princeton. [ Links ]
34. TOURNANT P, E AFONSO, S ROUE, P GIRAUDOUX, and J FOLTETE. 2013. Evaluating the effect of habitat connectivity on the distribution of lesser horseshoe bat maternity roosts using landscape graphs. Biological Conservation 164:39-49. [ Links ]
35. VASQUEZ R and R ROJAS. 2004. Plants of the Peruvian Amazon: Identity key to the families of Gymnospermae and Angiospermae. Arnaldoa, Special Edition. [ Links ]
36. VONHOF MK and R BARCLAY. 1996. Roost-site selection and roosting ecology of forest-dwelling bats in southern British Columbia. Canadian Journal Zoology 74:1797-1805. [ Links ]














