Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Mastozoología neotropical
Print version ISSN 0327-9383On-line version ISSN 1666-0536
Mastozool. neotrop. vol.22 no.2 Mendoza Dec. 2015
ARTÍCULO
Chromosomal variability in tuco-tucos (Ctenomys, Rodentia) from the argentinean northeastern wetlands
Diego A. Caraballo1, Paola C. Jablonski2, Pablo J. Rebagliati2, and María Susana Rossi1
1 IFIBYNE-CONICET. Laboratorio de Fisiología y Biología Molecular, Dep. Fisiología, Biología Molecular y Celular. Facultad de Ciencias Exactas y Naturales. Universidad de Buenos Aires, Ciudad Universitaria, Pabellón II, 2do piso, EHA1428, Buenos Aires, Argentina. Tel. +54 11 4576 3386/68. [Correspondence: María Susana Rossi <srossi@fbmc.fcen.uba.ar>]
2 Laboratorio de Citogenética y Evolución, Departamento de Ecología Genética y Evolución, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Ciudad Universitaria, Pabellón II, 2do piso, EHA1428, Buenos Aires, Argentina.
ABSTRACT.
Chromosomal diversity is a key feature for understanding the evolution of mammalian species. Rodents, the most diverse taxon among mammals, exhibit a wide chromosomal diversity; the study of their karyomorphs has contributed greatly to understand the evolution in this group. South American subterranean rodents of the genus Ctenomys (tuco-tucos) are one of the most speciose mammalian genera. Chromosomal variability in several lineages of Ctenomys has been characterized; however, the description of the karyomorphs of the tuco-tucos that inhabit the broad area under the influence of the Iberá marsh in the northeast of Argentina (the Corrientes group) is incomplete. This work provides new chromosomal information of the Ctenomys Corrientes group that includes the description of 3 new and 5 previously undescribed karyomorphs. We found four fundamental numbers (FNs): 76, 78, 80 and 84 and chromosomal numbers (2n) that ranged between 41-44, 44-46, 48 and 50-70, respectively. In addition, we found a new sampling site of C. roigi, a critically endangered species.
RESUMEN.
Diversidad cromosómica en tuco-tucos (Ctenomys, Rodentia) de los humedales del noreste argentino.
La diversidad cromosómica es clave para entender la evolución de las especies de mamíferos. Los roedores, el taxón más diversificado de los mamíferos, presentan una gran diversidad cromosómica; el estudio de sus cariomorfos ha contribuido mucho a la comprensión de su evolución. Los roedores subterráneos del género Ctenomys (tuco-tucos) son uno de los géneros de mamíferos con mayor número de especies. En muchos linajes de Ctenomys la variabilidad cromosómica ha sido caracterizada; sin embargo, la descripción de los cariomorfos de los tuco-tucos que habitan en una amplia área bajo la influencia de los esteros del Iberá en el noreste de Argentina (grupo Corrientes) está incompleta. El presente trabajo brinda nueva información cromosómica del grupo Ctenomys de Corrientes, que incluye la descripción de 3 cariomorfos nuevos y 5 no descriptos previamente. Encontramos cuatro números fundamentales (NFs): 76, 78, 80 y 84 y números cromosómicos (2n) que varían entre 41-44, 44- 46, 48 y 50-70, respectivamente. Además, identificamos un nuevo sitio de muestreo de C. roigi, especie en peligro de extinción.
Key words: Chromosomal variability; Ctenomys; Iberá marsh; Tuco-tucos.
Palabras clave: Ctenomys; Esteros del Iberá; Tuco-tucos; Variabilidad cromosómica.
Recibido 2 diciembre 2014.
Aceptado 21 abril 2015.
Editor asociado: E Palma
INTRODUCTION
Subterranean rodents of the genus Ctenomys (commonly named tuco-tucos) have been considered a clear example of chromosomal evolution since the seminal work of Reig and Kiblisky (1969) because of their broad range of chromosomal numbers (from 2n = 10 to 70) (Cook et al., 1990; Ortells et al., 1990). Among mammals, the genus Ctenomys is characterized by having one of the highest numbers of living species, about 60 as registered by Woods y Kilpatrick (2005). Most of the species present quite distinctive karyotypes, a characteristic that was called to be an example of chromosomal speciation (Reig y Kiblisky, 1969). However, chromosomal polymorphisms or polytypisms also occur in Ctenomys. For instance, C. pearsoni is considered a single species although its karyotypes range between 2n = 56 to 2n = 70 as reviewed by Tomasco and Lessa (2007). Similar cases have been recently reported in the Brazilian tuco-tuco species C. lami (2n = 54 to 58) and C. minutus (2n = 42 to 50) that also depict large intraspecific karyotypical variability, mainly due to centric fusions/fissions and pericentric inversions (Freitas, 2007; Lopes et al., 2013).
The present work deals with chromosomal variability of the Ctenomys Corrientes group that exhibits one of the widest ranges of diploid numbers within the genus. Demes of tuco-tucos from this group are patchily distributed in a vast area under the wide influence of the Iberá marsh and its natural channels and lagoons, in the Argentinean province of Corrientes, between the Paraná and Uruguay rivers. Sandy soils, predominant in this area, are favorable habitats for tuco-tucos, though their proximity to the wetlands makes these habitats temporary and spatially fragmented. The dynamics of habitat availability (via expansions and contractions) imposes putative contacts between neighboring populations and/or splitting and divergence of demes.
The pioneering works of Contreras y Scolaro (1986), Ortells et al. (1990) and Ortells y Barrantes (1994) initiated chromosomal studies in the Ctenomys Corrientes group. Ortells et al. (1990) showed that high karyotypical variability, with chromosomal numbers ranging from 2n = 42 to 2n = 70, characterized this group. Chromosomal numbers and morphology have been described for some karyomorphs in the mentioned contributions, as well as in subsequent ones (García et al., 2000; Argüelles et al., 2001). However, for several karyomorphs only chromosomal (2n) and fundamental (FN) numbers have been published without any data on chromosomal morphology (Giménez et al., 2002; Lanzone et al., 2007). The purpose of the present work is to expand the chromosomal information of the Ctenomys Corrientes group, particularly of the Iberá subgroup that comprises the tuco-tucos that inhabit the margins of the Iberá marsh (Caraballo et al., 2012). We found 3 new karyomorphs, described another 5 from which only 2n and FN values were available, and confirmed chromosome number and morphology of another 6 previously published karyomorphs.
MATERIALS AND METHODS
Specimens
This study included 47 individuals from 15 localities of the province of Corrientes (Argentina). Animals were live-captured with modified Oneida Victor Nr. 0 snap traps. Guidelines of the American Society of Mammalogists (Gannon et al., 2007) were followed. Well-preserved skulls were deposited in the Colección de Mastozoología of the Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”. Trapping localities with their geographical location (Fig. 1) as well as field / catalog numbers (when available) were recorded: Estancia la Tacuarita (27° 58’ 42.7” S; 56° 33’ 40.6’’ W), specimens 203, 204 and 205; Saladas Centro (28º 14’ 20.3’’ S; 58º 37’ 40.4’’ W), specimens 128 and 129; Saladas Sur (28º 17’ 37.5’’ S; 58º 41’ 19.2’’ W), specimen 134; San Alonso (28° 17’ 76” S; 57° 24’ 45’’ W), specimens 186, 187, 188 and 190; Estancia San Luis (28º 6’ 43.7’’ S; 58º 51’ 48.1’’ W), specimens 236/ 26484, 237/26485, 238/26486 and 239/26487; Goya (29° 11’ 17.2” S ; 59° 12’ 36.7’’ W), specimens 181 to 184; San Roque (28° 41’ S; 58° 42’ W), specimens 135 to 137; Chavarría (28° 58’ S; 58° 35’ W), specimens 149 to 153; Curuzú Laurel (27º 55’ 24.4’’ S; 57º 29’ 23.5’’ W), specimens 220 to 222; Loreto (27º 44’ 43.7’’ S ; 57º 14’ 35.2’’ W), specimens 156, 223/26479 and 224; Contreras Cué (28° 5’ 28.6” S ; 56° 33’ 53.7’’ W), specimens 199, 207, 208, 210 and 211; Mbarigüí (27° 33’ S; 57° 31’ W), specimen 174; San Miguel (28° 0’ 58.6” S; 57° 36’ 19.2’’ W), specimens 214/26478, 216 and 217; Paraje Caimán (28° 3’ 3.1” S ; 57° 40’ 38.4’’ W), specimens 225, 226/26480, 227/26481 to 228/26482, and Paraje Sarandicito (30° 14’ 43.1” S; 59° 33’ 46.1’’ W), specimens 212/26476 and 213/26477.
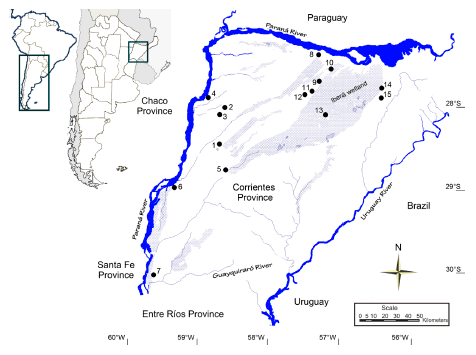
Fig. 1. Map showing sampling localities. Numbers correspond to the following localities: 1-San Roque, 2-Saladas Centro, 3-Saladas Sur, 4-Estancia San Luis, 5-Chavarría, 6-Goya, 7-Paraje Sarandicito, 8-Mbarigüí, 9-Curuzú Laurel, 10-Loreto, 11-San Miguel, 12-Paraje Caimán, 13-San Alonso, 14-Estancia La Tacuarita, 15-Contreras Cué.
Chromosome preparations
Metaphase preparations were obtained from bone marrow following Ford and Hamerton (1956) and stained with Giemsa. Chromosomes were classified according to Levan et al. (1964) and FNs were computed considering autosomes and sexual chromosomes. For each specimen a minimum of 20 metaphases was analyzed. For each specimen a karyogram was considered reliable when at least five different metaphases yielded the same assembly. Chromosomes were classified into three groups: sexual chromosomes (see Results), and biarmed and telocentric autosomes. Chromosomes were joined in pairs according with their morphology and size, in decreasing order.
RESULTS
In Table 1 we present a summary of the karyomorphs of the Ctenomys Corrientes group published in the literature. In the present paper, we described a total of 15 karyomorphs. The sexual chromosome system for the Corrientes group is XX/XY; both sexual chromosomes were biarmed, as was characterized by Ortells et al. (1990). The identification of sexual chromosomes was based on the differences between males and females from each population, as well as contrasting our karyomorphs with previously reported ones (Ortells et al., 1990). In each karyomorph the sexual pair was included in a third group apart from the classification of biarmed and telocentric chromosome pairs (Figs. 2-6).
Table 1 Karyomorphs of the Ctenomys Corrientes group. 1: Ortells et al. (1990), 2: Argüelles et al. (2001), 3: Giménez et al. (2002) and 4: Lanzone et al. (2007).

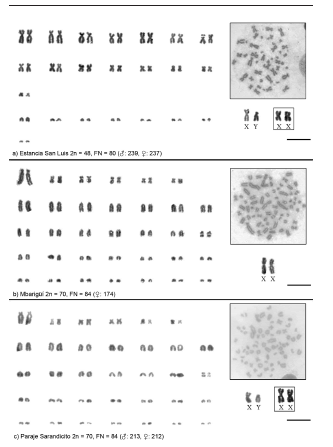
Fig. 2. Giemsa-stained karyomorphs of the Ctenomys Corrientes group. Localities, 2n and FN and specimen field number are indicated in each panel. Scale = 10 μm.

Fig. 3. Giemsa-stained karyomorphs of the Ctenomys Corrientes group. Localities, 2n and FN and specimen field number are indicated in each panel. Scale = 10 μm.
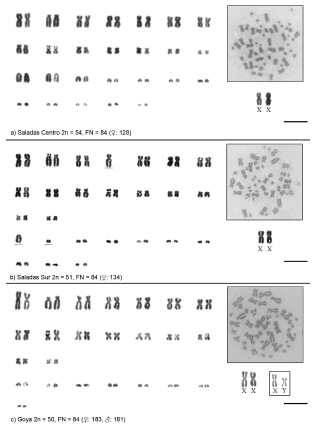
Fig. 4. Giemsa-stained karyomorphs of the Ctenomys Corrientes group. Localities, 2n and FN and specimen field number are indicated in each panel. Scale = 10 μm.

Fig. 5. Giemsa-stained karyomorphs of the Ctenomys Corrientes group. Localities, 2n and FN and specimen field number are indicated in each panel. Scale = 10 μm.

Fig. 6. Giemsa-stained karyomorphs of the Ctenomys Corrientes group. Localities, 2n and FN and specimen field number are indicated in each panel. Scale = 10 μm.
Estancia San Luis
The four individuals, two females (236 and 237) and two males (238 and 239) sampled at estancia San Luis were homozygous 2n = 48 and FN = 80 (Fig. 2a). The autosomal complement comprises 15 biarmed and 8 telocentric pairs. On the 7th biarmed pair, a secondary constriction was observed. The X chromosome is a large metacentric, while the Y chromosome is a medium-sized submetacentric. The karyomorph found in Estancia San Luis is indistinguishable from that found in Costa Mansión, Ctenomys roigi’s type locality (Ortells et al., 1990), less than 10 km from Estancia San Luis.
Mbarigüí
The chromosome complement of a female (174) from Mbarigüí, the type locality of Ctenomys dorbignyi was 2n = 70 FN = 84, confirming the characteristic karyotype of this species, which possesses the highest diploid number of the genus (Fig. 2b). The autosomal complement consists of 6 biarmed and 28 telocentric pairs. The 14th monobrachial pair presents a secondary constriction. This karyomorph shows the same morphology and chromosome number as the one described by Ortells et al. (1990), corresponding to the same locality. We identified the X chromosome as a large metacentric, also in accordance with Ortells et al. (1990).
Paraje Sarandicito
A male (213) and a female (212) captured at the locality of Paraje Sarandicito had a 2n = 70 FN = 84 karyomorph (Fig. 2c) distinguishable from the one found in Mbarigüí (Fig. 2b) for having a submetacentric second pair instead of metacentric, in agreement with Argüelles et al. (2001). The Y chromosome is a medium-sized submetacentric, in agreement with previous descriptions (Ortells et al., 1990; Argüelles et al., 2001).
Chavarría
Specimens sampled in Chavarría depicted the same karyomorph. Three males (149, 150 and 153) and two females (151 and 152) shared a karyomorph 2n = 56 and FN = 84 (Fig. 3a). The autosomal complement of the 2n = 56 FN = 84 comprises 13 biarmed and 14 telocentric pairs, while the sexual pair consists of a medium-sized metacentric X chromosome and a small submetacentric Y chromosome. A secondary constriction was found in the 8th biarmed pair. For the same locality, Giménez et al. (2002) and Lanzone et al. (2007) mentioned a karyomorph 2n = 58 FN = 84. However, these publications did not include any photographic material to corroborate chromosome number.
San Roque
Two females (135 and 136) and one male (137) from San Roque were analyzed. The 3 specimens had a karyomorph 2n = 62 and FN = 84, consisting of 11 biarmed and 30 telocentric autosomal pairs (Fig. 3b). A secondary constriction was observed in the 5th biarmed pair. The X chromosome is a medium-sized submetacentric, while the Y chromosome is a medium-sized metacentric. This karyomorph matches the one described for the same population by Ortells et al. (1990).
Saladas Centro
The two females (128 and 129) sampled in the locality of Saladas Centro had 2n = 54 FN = 84. A total of 14 biarmed and 12 telocentric pairs compose the autosomal complement (Fig. 4a). The 9th biarmed pair bears a secondary constriction, and the X chromosome is a large metacentric, in correspondence with an identical karyomorph found in this population by Ortells et al. (1990). These authors found two additional karyomorphs 2n = 55 and 2n = 56, both with FN = 84, which correspond to heterozygous and homozygous morphs originated by a centric fusion/fission event comprising the 7th biarmed pair.
Saladas Sur
Saladas Sur is a previously undescribed locality placed 7.3 km far from Saladas Centro in southwest direction. The karyomorph of a female (134) was 2n = 51 FN = 84 constituting a new 2n record for the Corrientes group (Fig. 4b). The autosomal complement consists of 15 biarmed pairs plus one biarmed chromosome in heterozygosis, and 8 telocentric pairs plus two telocentric chromosomes in heterozygosis. One of the chromosomes of the biarmed pairs—probably the 4th—was “orphan” and could be involved in a fusion/fission event with two telocentric chromosomes. This Robertsonian rearrangement should be confirmed by banding techniques. The X chromosome is a large metacentric, identified in comparison with the karyomorph found in the neighboring population of Saladas Centro. The 11th biarmed pair bears a secondary constriction.
Goya
The chromosome complement of the 2 females (183 and 184) and 2 males (181 and 182) sampled in Goya, the type locality of C. perrensi, was 2n = 50 FN = 84, in accordance with the karyotype previously published (Ortells et al., 1990). This karyomorph comprises 8 telocentric and 16 biarmed autosomal pairs (Fig. 4c). A secondary constriction was observed in the 9th biarmed pair. The X chromosome is a large metacentric while the Y chromosome is a medium-sized submetacentric.
Paraje Caimán
In the population sampled at Paraje Caimán, from a total of 4 specimens, 2 females (225 and 228) were heterozygous with 2n = 45 (Fig. 5b) while a female (226) and a male (227) had 2n = 46 (Fig. 5a), all of which had FN = 78. In the morph with 2n = 46 the autosomal complement consists of 15 biarmed and 7 telocentric pairs. The sexual pair is heteromorphic; the X chromosome is a large submetacentric, whereas the Y chromosome is a small submetacentric. The 6th biarmed pair, a medium-sized submetacentric, bears a secondary constriction. In the 2n = 45 morph, the 3rd biarmed and the first two major telocentric chromosomes resulted “orphans”, being probably involved in a fusion/fission event that originated the heterozygous condition. The 2n = 44, FN = 78 morph was not found in this population, likely due to insufficient sampling. However, the occurrence of this karyomorph is highly probable in this population, and it would be indistinguishable from the one found in San Alonso (Fig. 5c). In a previous article Giménez et al. (2002) stated that in Paraje Caimán, in addition to the 2n = 46, a 2n = 47 morph was found. But, like in the case of Chavarría, these karyomorphs cannot be compared because there is no photographic material available.
San Alonso
San Alonso is a sandy patch located in the central area of the Provincial Reserve Esteros del Iberá. It is an island surrounded by shallow marshes that could eventually reconnect with the mainland. Two males (186 and 188) and two females (187 and 190) sampled in San Alonso had 2n = 44 and FN = 78, previously undescribed karyomorph for the Corrientes group. The autosomal complement consisted of 16 biarmed and 5 telocentric pairs, with a secondary constriction observed in the 7th biarmed pair (Fig. 5c). The sexual pair was heteromorphic, including a large submetacentric X chromosome and a small submetacentric Y chromosome.
San Miguel
The sample from San Miguel consisted of two males (214 and 217) and one female (216), with a 2n = 44 and FN = 76 karyomorph (Fig. 6a). In this case, the autosomal complement presented 15 biarmed and 6 telocentric pairs. Within the telocentric group, the two major pairs resemble the ones found in Paraje Caimán. The sexual pair was heteromorphic, including a large submetacentric X chromosome and a small submetacentric Y chromosome. This karyomorph was already published by Giménez et al. (2002) as well as by Lanzone et al. (2007) for this locality but again lacking photographic support.
Contreras Cué
A total of 5 specimens were sampled at Contreras Cué. Four of the individuals, two females (207 and 208) and two males (210 and 211) had 2n = 42 and FN = 76 (Fig. 6b) while a female (199) was heterozygous with 2n = 41 and the same fundamental number (Fig. 6c). The autosomal complement of the 2n = 42 karyomorph consists of 16 biarmed and 4 telocentric pairs. The sexual pair is submetacentric: the X is a large chromosome, while the Y is small. The female with 2n = 41 had one extra small meta-centric chromosome, and lacked two telocentric chromosomes of the autosomal complement. When compared with the karyomorph 2n = 42, these differences are most probably due to a centric fusion.
Curuzú Laurel
Two females (220 and 221) and a male (222) captured at the locality of Curuzú Laurel were homozygous with 2n = 42 and FN = 76, in agreement with the karyomorph published by Ortells et al. (1990) corresponding to the same locality. This karyomorph is indistinguishable from the 2n = 42 described for Contreras Cué (Fig. 6b).
Loreto
A total of three tuco-tucos were captured in Loreto, two males (156 and 224) and a female (223) that were homozygous with a karyomorph that was identical to the 2n = 42 found in Contreras Cué (Fig. 6b). The 2n and FN found in this locality coincide with the ones previously published by Giménez and collaborators (2002).
Estancia La Tacuarita
Cytogenetic analyses were carried out for three individuals sampled at Estancia La Tacuarita, two females (203 and 204) and a male (205), The karyomorph was homozygous and indistinguishable from the other 2n = 42 described in this paper (Fig. 6b), and also reported by Giménez et al. (2002) although without photographic support.
DISCUSSION
In the present work we characterized 3 new karyomorphs: 2n = 44 FN = 78 from San Alonso (Fig. 5c) plus two heterozygous 2n = 51 FN = 84 from Saladas Sur (Fig. 4b) and 2n = 45 FN = 78 from Paraje Caimán (Fig. 5c). We also showed another 5 karyomorphs from which only 2n and FN numbers have been published but without photographs depicting the morphology and size of the chromosomes: those from Chavarría (Fig. 3a), Paraje Caimán (Fig. 5a), San Miguel (Fig. 6a), Contreras Cué (Fig. 6b and c), Loreto (see Results) and estancia La Tacuarita (see Results). We also reanalyzed the previously described karyomorphs, confirming those from Mbarigüí, the type locality of C. dorbignyi (Fig. 2b), Paraje Sarandicito (Fig. 2c), Goya, the type locality of C. perrensi (Fig. 4c), San Roque (Fig. 3b), Saladas Centro (Fig. 4a) and Curuzú Laurel (see Results). The finding of a new population of C. roigi (Estancia San Luis) is important because this is a critically endangered species (Bidau et al., 2008).
New chromosomal data from the Corrientes group confirm that FNs 76, 78, 80 and 84 characterize the karyomorphs of the group. Fundamental numbers 76 and 78 were found in the Iberá subgroup; FN = 80 was only found in the karyomorph from Estancia San Luis (which is indistinguishable from that of Costa Mansión, the type locality of C. roigi) and FN = 84 was found in the remainders.
The karyomorphs of the Iberá subgroup that share FN = 76 are very similar (see Results) with 2n = 41, 42 and 44. The same occurs with those that share FN = 78 that depict 2n = 44, 45 and 46. Differences in 2n without changes in FN might be mainly explained by Robertsoninan events, although banding techniques are required to confirm it and to identify the chromosomes involved. Since within each FN, 2n range is limited, the rearrangements involved might be easy to track by G-banding techniques.
The karyomorphs that share FN = 84, on the contrary, include a broader range of chromosomal numbers, from 2n = 56 to 2n = 70. In these cases, although Robertsonian changes are also probably the most frequent rearrangement, as those described by Lanzone et al. (2002) and Lanzone et al. (2007), the relationships among the karyomorphs might be more difficult to establish. The ample range of chromosomal numbers suggests a more complex scenario that may include parallelisms, reversions and/ or convergences.
New chromosomal data shown in this paper broaden the chromosomal information of the Ctenomys Corrientes group, essential to understand the role of chromosomal variability in speciation and hybridization processes. However, phylogeny (Caraballo et al., 2012), population genetics (Gómez-Fernández et al. 2012), morphology and ecology should also be taken into account to establish species boundaries in this interesting group of tuco-tucos.
ACKNOWLEDGEMENTS
This work was supported by grants from the Agencia Nacional de Investigaciones Científicas y Técnicas (PICT 3836/1) and Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 5776 and PIP 2012-2014) from Argentina. Authors thank Drs. Lidia Poggio and Liliana María Mola from the Laboratorio de Citogenética y Evolución, Departamento de Ecología Genética y Evolución. Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, and also Rodrigo Álvarez, Pablo Belluscio, María Jimena Gómez Fernández, Marcelo Kittlein, Fernando Mapelli, Patricia Mirol, Matías Mora, Vanina Raimondi, Verónica Trucco Cano, and Laura Wolfenson for assistance at the field work. We also like to thank Diana Avedikian, Verónica Romero, Cecilia Kopuchian and Sergio Rodríguez Gil, two anonymous reviewers whose comments have improved the manuscript, and the editor for his suggestions.
LITERATURE CITED
1. ARGÜELLES CF, P SUÁREZ, MD GIMÉNEZ, and CJ BIDAU. 2001. Intraspecific chromosome variation between different populations of Ctenomys dorbignyi (Rodentia, Ctenomyidae) from Argentina. Acta Theriologica 46:363-373. [ Links ]
2. BIDAU C, E LESSA, and R OJEDA. 2008. Ctenomys roigi. The IUCN Red List of Threatened Species. Version 2014.3. <www.iucnredlist.org>. Downloaded on 10 March 2015. [ Links ]
3. CARABALLO DA, GA ABRUZZESE, and MS ROSSI. 2012. Diversity of tuco-tucos (Ctenomys, Rodentia) in the Northeastern wetlands from Argentina: Mitochondrial phylogeny and chromosomal evolution. Genetica 140:125-136. [ Links ]
4. CONTRERAS JR and JA SCOLARO. 1986. Distribución y relaciones taxonómicas entre los cuatro núcleos geográficos disyuntos de Ctenomys dorbignyi en la provincia de Corrientes, Argentina (Rodentia, Ctenomyidae). Historia Natural 6:21-30. [ Links ]
5. COOK JA, S ANDERSON, and TL YATES. 1990. Notes on Bolivian Mammals 6: The genus Ctenomys (Rodentia, Ctenomyidae) in the highlands. American Museum Novitiates 2980:1-27. [ Links ]
6. FREITAS TRO. 2007. Ctenomys lami: The highest chromosome variability in Ctenomys (Rodentia, Ctenomyidae) due to a centric fusion/fission and pericentric inversion system. Acta Theriologica 52:171-180. [ Links ]
7. FORD CE and JL HAMERTON. 1956. A colchicine, hypotonic citrate, squash sequence for mammalian chromosomes. Stain Technology 31:247-251. [ Links ]
8. GANNON WL and RS SIKES. 2007. The Animal Care and Use Comitee of the American Society of Mammalogists. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. Journal of Mammalogy 88:809-823. [ Links ]
9. GARCÍA L, M PONSA, J EGOZCUE, and M GARCÍA. 2000. Comparative chromosomal analysis and phylogeny in four Ctenomys species (Rodentia, Octodontidae). Biological Journal of the Linnean Society 69:103-120. [ Links ]
10. GIMÉNEZ MD, PM MIROL, CJ BIDAU, and JB SEARLE. 2002. Molecular analysis of populations of Ctenomys (Caviomorpha, Rodentia) with high karyotypic variability. Cytogenetic Genome Research 96:130-136. [ Links ]
11. LANZONE C, CJ BIDAU, MD GIMÉNEZ, and JL SANTOS. 2002. Synaptic behaviour and morphological modifications of the X and Y chromosomes during pachytene in three species of Ctenomys (Rodentia, Caviomorpha, Ctenomyidae). Genome 45:1110-1115. [ Links ]
12. LANZONE C, MD GIMÉNEZ, JL SANTOS, and CJ BIDAU. 2007. Meiotic effects of Robertsonian translocations in tuco-tucos of the Ctenomys perrensi superspecies (Rodentia: Ctenomyidae). Caryologia 60:233-244. [ Links ]
13. LEVAN A, K FREDGA, and AA SANDBERG. 1964. Nomenclature for centromeric position on chromosomes. Hereditas 52:201-220. [ Links ]
14. LOPES CM, SSF XIMENES, A GAVA, and TRO FREITAS. 2013. The role of chromosomal rearrangements and geographical barriers in the divergence of lineages in a South American subterranean rodent (Rodentia: Ctenomyidae: Ctenomys minutus). Heredity 111:293–305.
15. ORTELLS MO and GE BARRANTES. 1994. A study of genetic distances and variability in several species of the genus Ctenomys (Rodentia: Octodontidae) with special references to a probable causal role of chromosomes in speciation. Biological Journal of the Linnean Society 53:189-208. [ Links ]
16. ORTELLS MO, JR CONTRERAS, and OA REIG. 1990. New Ctenomys karyotypes (Rodentia, Octodontidae) from north-eastern Argentina and from Paraguay confirm the extreme chromosomal multiformity of the genus. Genetica 82:189-201. [ Links ]
17. REIG OA and P KIBLISKY. 1969. Chromosome multiformity in the genus Ctenomys (Rodentia, Octodontidae). A progress report. Chromosoma 28:211-244. [ Links ]
18. TOMASCO IH and EP LESSA. 2007. Phylogeography of the tuco-tuco Ctenomys pearsoni: mtDNa variation and its implication for chromosomal differentiation. Pp. 859-882, in: The quintessential naturalist: Honoring the life and legacy of Oliver P. Pearson (DA Kelt, EP Lessa, J Salazar-Bravo, and JL Patton eds.). University of California Publications in Zoology, Berkeley. [ Links ]
19. WOODS C and C KILPATRICK. 2005. Infraorder Hystricognathi Brandt, 1855. Pp. 1538-1600, in: Mammal species of the world: A taxonomic and geographic reference (DE Wilson and DM Reeder eds.). John Hopkins University Press, Baltimore. [ Links ]














