Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Mastozoología neotropical
versão impressa ISSN 0327-9383versão On-line ISSN 1666-0536
Mastozool. neotrop. vol.22 no.2 Mendoza dez. 2015
ARTÍCULO
Ectoparasites associated with bats in northeastern Tolima, Colombia
Andrea del Pilar Tarquino-Carbonell1, Karina A. Gutiérrez-Díaz1, Emma Y. Galindo-Espinosa1, Gladys Reinoso-Flórez1, Sergio Solari2, and Ricardo Guerrero3
1 Grupo de Investigación en Zoología, Universidad del Tolima, Barrio Santa Helena, 730006 Ibagué, Tolima, Colombia [Correspondencia: Andrea del Pilar Tarquino-Carbonell <atarquino@ut.edu.co>]
2 Grupo Mastozoología, Instituto de Biología, Universidad de Antioquia, 050010 Medellín, Antioquia, Colombia.
3 Instituto de Zoología y Ecología Tropical, Universidad Central de Venezuela, 1050 Caracas, Venezuela.
ABSTRACT.
This study reports data on the prevalence and mean intensity of ectoparasites associated with bats in northeastern Tolima, Colombia. We captured 140 bats representing 21 species and 5 families. Most individuals represented the Phyllostomidae (84%), and Carollia perspicillata was the most frequently captured species (35%). Parasites were found in 14 of these species (66%), 8 of which (29.28%) were parasitized by Streblidae, 6 (14.28%) by Spinturnicidae, 5 (9.28%) by Macronyssidae, and 5 (7.85%) by Argasidae. Ectoparasites represented 5 families and 24 species; the most abundant was Trichobius joblingi (16.85%), followed by Radffordiella desmodi (11.04%). This research represents the first report on ectoparasites of bats in northern Tolima (Colombia).
RESUMEN.
Ectoparásitos asociados con murciélagos en el noreste de Tolima, Colombia.
El presente estudio registra los ectoparásitos asociados a murciélagos en el noreste del Tolima, Colombia, con datos sobre su prevalencia e intensidad promedio. Se capturaron 140 murciélagos distribuidos en 5 familias y 21 especies, siendo la familia Phyllostomidae la más abundante (84%), y la especie de mayor frecuencia Carollia perspicillata (35%). De las especies de murciélagos colectadas, 14 (66%) se hallaron parasitadas; 8 de estas (29.28%) fueron parasitadas por la familia Streblidae, 6 (14.28%) por Spinturnicidae, 5 (9.28%) por Macronyssidae y 5 especies (7.85%) parasitadas por Argasidae. Cinco familias y 24 especies de ectoparásitos fueron encontradas, de las cuales Trichobius joblingi fue la más abundante (16.85%) seguida por Radffordiella desmodi (11.04%). Esta investigación representa el primer reporte sobre las especies de ectoparásitos de murciélagos del norte del Tolima (Colombia).
Key words: Argasidae; Chiroptera; Host-Parasite relationships; Mesostigmata; Streblidae.
Palabras clave: Argasidae; Chiroptera; Mesostigmata; Relaciones parásito-hospedero; Streblidae.
Recibido 24 abril 2015.
Aceptado 17 junio 2015.
Editor asociado: M Lareschi
INTRODUCTION
Because they cover a wide trophic spectrum and often exhibit high habitat specificity, bats (Chiroptera) represent a good model for analyzing the consequences of habitat fragmentation and environmental changes (Pérez-Torres and Ahumada, 2004; Jones et al., 2009). Bats also represent a key group for understanding the evolution and ecology of parasitism, because their ectoparasites are often specialized, with many taxa closely associated with particular species of ectoparasites (Dick and Patterson, 2006).
There have been relatively few studies of bat ectoparasites in Colombia. Early studies of obligate ectoparasitic Diptera in Colombia and Panama highlighted the presence of the families Streblidae (16 species) and Nycteriibidae (2 species) (Bequaert, 1940). Machado-Allison and Antequera (1969) reported the distribution and hosts of spinturnicid mites, including the presence of the genus Spinturnix. Subsequently, Marinkelle and Grose (1981) published a list of ectoparasites in Colombian bats of the orders Diptera, Hemiptera and Siphonaptera, as well as 5 families of mites, reporting a total of 88 species. Recently, Dick et al. (in press) developed a catalogue of Streblidae of Colombia, reporting 73 species recorded in Colombia. Despite these efforts, information on Colombian bat ectoparasites is still scarce (Tarquino-Carbonell, 2014). We studied associations between ectoparasites and their chiropteran hosts in a fragment of tropical dry forest in the northern department of Tolima, presenting data on prevalence and intensity of infestation, and determining interdependence between ectoparasites and hosts.
MATERIALS AND METHODS
Study area
The study was carried out in Chorrillo, a site of approximately 50 ha of fragmented forest, in the Ambalema municipality in northeastern Tolima, Colombia (4° 26’ N, 74°4 8’ W) approximately 273 m above sea level. The average annual temperature is 25.7 °C, and relative humidity is 80%. Rainfall averages 3116 mm annually (Hijmans et al., 2005). The vegetation cover and climatic conditions correspond to tropical dry forests (Holdridge, 1967).
Field Methods
The material was collected between August and November 2012, 3 nights per month, every night from 18:00 to 00:00 h. Five mist nets of 12 x 3 m were used at the understory and checked every 30 min. Bats were extracted from the mist net using a red flashlight and separated into individual bags to prevent contamination between hosts. The handling and collection of the organisms were made following the guidelines of the American Society of Mammalogists (Gannon et al., 2007; Sikes et al., 2011). Morphometric data were taken using a digital caliper with 0.1 mm precision. Ectoparasites were collected with fine forceps and placed in Eppendorf tubes containing 70% ethanol, each one labeled with the capture number from each bat.
Laboratory methods
Bats were prepared as standard museum specimens and identified using keys and descriptions from Gardner (2008). Mites were mounted in Hoyer’s and Lactophenol medium (Faraji and Bakker, 2008), and examined using a Nikon Labophot microscope YF21-E with phase contrast and Nikon DS-Fi1 camera, and a Leica MS-5 stereomicroscope. Insects were examined in ethanol. We identified Macronyssidae mites using Radovsky (1967), Spinturnicidae with Herrin and Tipton (1975). We used Kohls et al. (1965) to identify the species of Ornithodoros. Diptera were identified with the keys of Wenzel (1976) and pictorial keys from Graciolli and Carvalho (2001). The specimens collected as samples are preserved in the Zoological Collection of the University of Tolima (CZUT).
Data analysis
To evaluate the association of ectoparasites among different hosts, we used the following indices (Bush, 1997):
Prevalence: the number of hosts infected with 1 or more individuals of a particular parasite species (or taxonomic group) divided by the number of hosts examined for that parasite species.
Mean intensity: the average intensity of a particular species of parasite among the infected members of a particular host species
To determine if there are associations between the parasite and the bat taxa, a contingency table was realized to each of the families of parasites found and a Fisher’s test was applied like in another Studies (Almeida et al., 2011). Variables such as number of parasitic hosts and presence of two or more parasite families at the same time are taken into account in this contingency table, testing for significant differences between each parasite family with respect to the other families
RESULTS
We recorded 140 bats belonging to 21 species and 5 families. Phyllostomidae was the most abundant family with the highest number of species registered (Table 1). The most frequently captured bat species were Carollia perspicillata (35%), Artibeus planirostris (14%) and Desmodus rotundus (11%). Species in the subfamily Phyllostominae, as well as in the families Emballonuridae and Vespertilionidae, were less common in general.
Table 1 Bat species reported in this study.

Associations between ectoparasites and bats
Eight bat species (29.28%) were parasitized by streblid flies, 6 species (14.28%) by spinturnicids, 5 species (9.28%) by the macronyssids and 5 species (7.85%) by argasid ticks (Table 2). A total of 69 hosts representing 14 bat species were found to be parasitized. Some species of ectoparasites collected were found in coexistence on the same host; in particular ~64% of these associations were found on D. rotundus. The most common association between ectoparasites families was between Streblidae and Spinturnicidae.
Table 2 Parasitic associations found in this study.
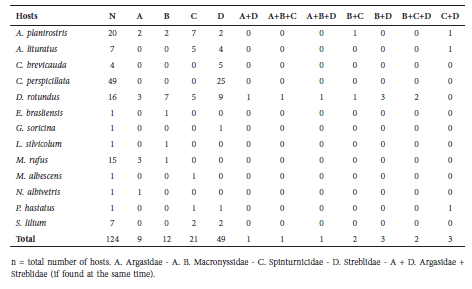
Streblidae. Eleven bat fly species were recorded for this family. The most abundant bat fly is Trichobius parasiticus, present in D. rotundus, and Trichobius joblingi, found on C. perspicillata. The bat flies reported in this study (Table 3) represent 18% of all species registered in Colombia (Guerrero, 1997).
Table 3 Bat fly associations found in this study.
The most abundant bat fly was T. joblingi, found on both species of Carollia. Four species of Trichobius were found on five species of bats.
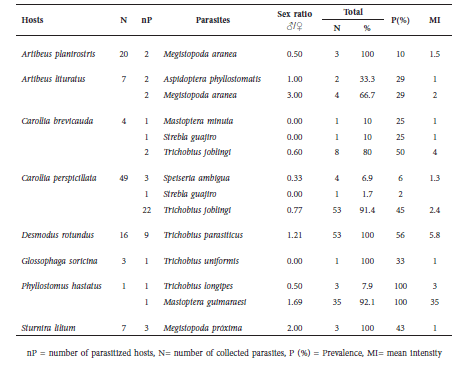
Spinturnicidae. Six spinturnicid species were found in association with six host species (Table 4). The most abundant species was Periglischrus iheringi, followed by Periglischrus ojasti. We found adult males, females and, in smaller numbers, male protonymphs of P. iheringi on two host species (Table 4).
Table 4 Spinturnicid mite associations found in the study.
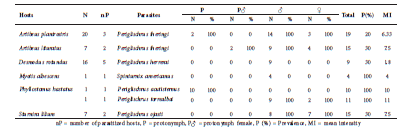
Macronyssidae. Four macronyssid species were present on 12 individuals of five bat species (Table 5). The most frequently encountered species was Raddfordiella desmodi, only found in D. rotundus, followed by Macronyssoides conciliatus in A. planirostris.
Table 5 Macronyssid mite associations found in the study

Argasidae. This family was represented by only two species (Table 6); however, they were found in large numbers and on five different host species, including diverse subfamilies with different trophic guilds (i. e. fisher bat and vampire bat).
Table 6 Argasid tick associations found in the study

We also found Trombiculidae larvae on a Saccopteryx bilineata male, although they could not be identified to species due to deterioration of the proboscis.
The frequency data of Streblidae was significantly associated (Fisher’s p = 0.003) with the frequency values of each of the other three families of parasites found in this study. No other significant associations were found between families.
DISCUSSION
Phyllostomidae was the family with greatest species richness and abundance (Table 1). This is consistent with results of studies of bat richness in other Colombian tropical dry forests (Ballesteros et al., 2007; Sánchez et al., 2007; Mantilla-Meluk, 2009). These results might be attributed to the variety of trophic guilds that this family presents and their wide geographic distribution in the country (Ballesteros et al., 2007; Sánchez et al., 2007; Mantilla-Meluk, 2009). In terms of species richness and local areas of importance for bat conservation, Chorrillo has almost a third of the bat species reported for the Department of Tolima (Galindo-Espinosa et al., 2010; Gutierrez et al., 2010; Solari et al., 2013).
Species of Carollia are characterized by their adaptation to changes in habitat conditions, a pattern applicable to various Neotropical areas (Kalko, 1998; Simmons and Voss, 1998; Soriano, 2000; Willig et al., 2007). Artibeus planirostris can move easily between open and fragmented areas, eating a wide variety of fruits, allowing seed dispersion within forests (Cadena et al. 1988). The evidence indicates that D. rotundus is especially abundant in areas with a high density of domestic animals (Sánchez et al., 2010). This factor could explain their high abundance in the study site during the sampling period.
The low number of species from the families Emballonuridae and Vespertilionidae could be related to limitations of sampling methods and the flight behavior of the species, because these families are characterized by high flight between and above the forest canopy (Bergallo et al., 2003; Simmons and Voss, 1998). Moreover, relative to phyllostomids, their echolocation allows them to better detect and avoid mist nets (Ortegón-Martínez and Pérez-Torres, 2007). Specifically, members of Phyllostominae usually hunt their prey at higher open spaces or forest edges (Montenegro and Romero-Ruiz, 1999), thus explaining their low catch.
Associations between ectoparasites and bats
Streblid flies are typical and widespread in the Neotropics (Carrrejo and González-Obando, 1992). This family lives mainly on bats of the family Phyllostomidae, which are also widely distributed and abundant throughout the Neotropics (Guerrero, 1996). On the other hand, the most species-rich genus was Trichobius. Species in this genus are generally quite mobile with greater ability to fly relative to other bat flies. For example, T. joblingi, a winged fly that lives in roost sites often shared by two or more host species, actively moves outside the body of the host (Guerrero, 1996; Dick and Patterson, 2006). In fact, this genus is usually quite abundant in studies of this type (Guerrero, 1996; Dick and Patterson, 2006; Almeida et al., 2011).
Aspidoptera phyllostomatis has been reported on Artibeus jamaicensis (Guerrero, 1997), but there is no record of its occurrence on Artibeus planirostris. However, changes in Artibeus taxonomy for northern South America (Larsen et al., 2007, 2010) and recent reviews show that A. jamaicensis is restricted to the Colombian Caribbean region (Solari et al., 2013). For the Magdalena valley of Department of Tolima, these bats must be recognized as A. planirostris (Solari et al., 2013). Therefore, in this study we note that the species of Artibeus recorded in northern Tolima is A. planirostris, which was parasitized by Aspidoptera phyllostomatis.
The incidence of Streblidae was very high for some bat species such as C. perspicillata and D. rotundus (Table 3), although the prevalence in this study is lower than that previously reported in Colombia (Marinkelle and Grose, 1981) for the same species. Low host specificity has been proposed for these bat flies, as many species of bats that share the same roost sites are exposed to similar parasites (Theodor, 1957). On the other hand, Streblidae are very common among phyllostomid species, which could explain this finding compared to groups of mites already reported in other studies (Guerrero, 1996). Bat flies are often dependent on the presence of the other groups; i.e., bat flies will be associated with particular species depending on which bat species are present in particular roosts.
The high interdependence found for streblids in the contingency table reflects by abundance of Streblidae on C. perspicillata and D. rotundus, revealing their strongest association in relation to other groups of ectoparasites. Thus, bat flies on C. perspicillata were significantly more likely to be the only parasites on their host to be found in association with other parasites. The significant results for the other 3 families of ectoparasites and their equal proportions suggest that the remaining families were associated uniformly for this study. Taxa exhibiting the lowest ectoparasite prevalence and mean intensity were those species that shelter in open areas. Also, caves allow greater horizontal transfer of ectoparasites relative to open habitats (Guerrero, 1993; Guerrero, 1996; Dick and Paterson, 2006)
It has been proposed that the presence or absence of one parasitic fly species may facilitate the presence or absence of another fly species, by representing the persistence of two species of parasites in a host over time (Dick and Gettinger, 2005). In some cases, these associations occur even when the parasites are in different genera (Dick and Gettinger, 2005). The degree of specificity in bat flies as obligate ectoparasites has been debated and some studies suggest a high specificity (Dick and Gettinger, 2005), although higher host densities could provide a substrate rich for bat flies and might be affecting associations of these flies (Guerrero, 1993; Dick y Paterson, 2006). However, certain factors that influence the specificity such as physical isolation, climate, competition, predation and physiological and morphological adaptation are recognized (Marshall, 1976, 1982).
The dynamics of mites as obligate ectoparasites of bats, unlike other groups such as Streblidae, have not been studied in recent decades and little is known about the regional distribution of species in the case of Colombia (Bequaert, 1940; Machado-Allison and Antequera, 1969; Marinkelle and Grose, 1981). These studies only include new species descriptions, reflecting the paucity of recent studies on taxonomy of ectoparasites and parasite-host relationship.
The Spinturnicidae family, found only on the wings and tail membranes of the host, exhibit a life cycle morphologically adapted and modified relative to other Mesostigmata (Rudnick, 1960). In general, this family can be easily found in any given bats group of the New World due to their morphological, ecological and behavioral conditions (Dowling, 2006). Periglischrus iheringi is commonly found in species of Artibeus and Uroderma, and other species as S. lilium (Herrin and Tipton, 1975). This species has been reported in Colombia in several leaf-nosed bat species including some species of Glossophaga (Marinkelle and Grose, 1981) without references to A. planirostris, which might indicate contamination or misidentification of the host. However, the association between these two species has already been extensively described from previous works in Brazil (Almeida et al., 2008; Silva et al., 2013).
Periglischrus ojasti has been reported in Colombia parasitizing S. lilium and S. ludovici, while P. acutisternus has primarily been reported in association with species of Phyllostomus, as we found in this study (Marinkelle and Grose, 1981). In the Neotropics, Spinturnix americanus has been described parasitizing Brazilian Myotis spp. (Silva et al., 2013). Ours is the first report of this association for the region of Tolima.
The proportion of Macronyssidae was low relative to other groups, such as streblids. Their range of movement is much lower than other groups, gives them greater specificity in relation to other groups of ectoparasites (Radovsky, 1967). We found no males of this family on any hosts, likely because Macronyssidae males do not consume blood (Radosky, 1967). The low number of macronyssid mites collected in this study makes it difficult to compare their prevalence with other studies, which reported higher numbers of mites. For example, Radffordiella desmodi species has been found in association with Desmodus in Costa Rica (Rojas et al., 2008). Steatonyssus occidentalis has been found in some genus of Vespertilionidae (Radovsky, 1967). Macronyssoides conciliatus can be found in Molossus genus (Radovsky, 1967), and the recently described P. bakeri (Morales- Malacara and Guerrero, 2007) can be found in some Phyllostomidae. The low number of specimens collected in this study may indicate contamination, so it is necessary to obtain more samples to gain an accurate understanding of their prevalence.
Argasid ticks can be found in well-established colonies of bats, where high transfer of ectoparasites occurs (Guerrero, 1996). Ornithodoros hasei has been found in association with species of the genus Artibeus, Phyllostomus, Noctilio, Rhogeeesa and Molossus for Colombia (Marinkelle and Grose, 1981). Ornithodoros (Pavloskyella) natalinus has only been reported previously on bats of the genus Natalus (Capinera, 2008). We present the first report of Ornithodoros (Pavloskyella) natalinus on individuals of D. rotundus for this region.
Results from our study suggest this locality can provide important information about bat-ectoparasites relationships in dry forests, an ecosystem that has previously been only rarely studied. In particular, we found that bat species most frequently parasitized (C. perspicillata, D. rotundus and A. planirostris) are those which inhabit transformed spaces and secondary vegetation, exhibiting habitat flex ibility. This habitat preference, in addition to ectoparasite ecology, roosting site, bat biology and host behavior, influence the association and competition between different ectoparasites families and the parasite load of the hosts. Our data relating to prevalence of major groups and species of ectoparasites present in bat populations of northern Tolima could help inform and provide direction for further research on these topics.
ACKNOWLEDGEMENTS
To Viviana García, Azucena Ramirez, Fabian Santos, Kevin Gonzalez, Daniel Duran, Enryque Torres, Steven Liévano, Luisa Beltran, Daniela Ortiz, and Angela Navarro for their field supportment. To the Instituto de Zoología y Ecología Tropical from the Universidad Central de Venezuela and the people who made it possible to stay in this country to review the material. To Luz Stella Sierra, Jenny Sierra, Jennifer Sierra, Paola Mojica for their help in Caracas. To the people from Chorrillo for receiving us very generously in the location. We are very grateful to Carl Dick and an anonymous reviewer for their suggestions. Special thanks to Erin Kane for their comments. The lead author is especially grateful to Fredy Quintero for his support in this work. This research was funded by the Comité Central de Investigaciones and Grupo de Investigación en Zoología of University of Tolima.
LITERATURE CITED
1. ALMEIDA JC, SS SILVA, NM SERRA-FREIRE, and MP VALIM. 2011. Ectoparasites (Insecta and Acari) associated with bats in southeastern Brazil. Journal of Medical Entomology 48:753-757. [ Links ]
2. BALLESTEROS J, J RACERO, and M NÚÑEZ. 2007. Diversidad de murciélagos en cuatro localidades de la zona costanera del departamento de Córdoba- Colombia. Revista de la Facultad de Medicina Veterinaria y Zootecnia de la Universidad de Córdoba (MVZ) 12:1013-1019. [ Links ]
3. BEQUAERT JC. 1940. Moscas parásitas pupiparas de Colombia y Panamá. Revista de la Academia Colombiana de Ciencias Exactas y Físicas Naturales 3(12):414.418 [ Links ]
4. BERGALLO H, C ESBÉRARD, M RIBEIRO, V LINS, R MANGOLIN, and G MELO. 2003. Bat species richness in Atlantic Forest: What is the Minimum Sampling Effort? Biotropica 35:278-288. [ Links ]
5. CAPINERA JL. 2008. Encyclopedia of Entomology. Springer Science and Business Media. 4346 pp. [ Links ]
6. CADENA A, J ÁLVAREZ, F SÁNCHEZ, C ARIZA, and A ALBESIANO. 1988. Dieta de los murciélagos frugívoros en la zona árida del río Chicamocha (Santander, Colombia). Boletin de la Sociedad de Concepción 69:47-53. [ Links ]
7. CARRREJO NS and R GONZÁLEZ-OBANDO. 1992. Introducción al conocimiento de los Diptera. Cali, Valle: Colección de Edición Previa. Universidad del Valle. Serie Investigaciones. [ Links ]
8. DICK CW and D GETTINGER. 2005. A faunal survey of streblid bat flies (Diptera: Streblidae) associated with bats in Paraguay. Journal of Parasitology 91:1015-1024. [ Links ]
9. DICK CW and B PATTERSON. 2006. Bat flies as obligate ectoparasites of bats. Pp. 54-66, in: Micromammals and macroparasites: How are they and how interact? (S Morand, ed.). Editorial Springer. Museum Smitsonian, New York. [ Links ]
10. DICK CW, G GRACIOLLI, and R GUERRERO. (In press). 30. Family Streblidae. In: Catalogue of Streblidae of Colombia. Special volume of Zootaxa. 25 pp. [ Links ]
11. DOWLING AP. 2006. Mesostigmatid mites as parasites of small mammals. Pp. 103-117 in: Micromammals and macroparasites: From evolutionary ecology to management (S Morand, B Krasnov, and R Poulin, eds.). Springer-Verlag, Tokyo. [ Links ]
12. FARAJI F and FA BAKKER. 2008. Modified method for clearing, staining and mounting plant-inhabiting mites. European Journal Entomology 105:793-795. [ Links ]
13. GALINDO-ESPINOSA EY, KA GUTIÉRREZ, and G REINOSO. 2010. Lista de quirópteros del departamento del Tolima, Colombia. Biota Colombiana 11:107-116. [ Links ]
14. GANNON WL, RS SIKES, and THE ANIMAL CARE AND USE COMMITTEE OF THE AMERICAN SOCIETY OF MAMMALOGISTS. 2007. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. Journal of Mammalogy 88:809-823. [ Links ]
15. GARDNER AL. 2008. Mammals of South America: Xenarthrans, Shrew, and Bats. The University of Chicago Press, Chicago, USA. 669 pp. [ Links ]
16. GRACIOLLI G and CJ DE CARVALHO. 2001. Moscas ectoparásitas (Diptera, Hippoboscoidea) de morcegos (Mammalia, Chiroptera) do Estado do Paraná. II. Streblidae. Chave pictórica para géneros e espécies. Revista brasileira da Zoologia 1:907-960. [ Links ]
17. GUERRERO R. 1993. Catálogo de los Streblidae (Diptera: Pupipara) parásitos de murciélagos (Mammalia: Chiroptera) del nuevo mundo. I. Clave para los géneros y Nycterophiliinae. Acta Biológica Venezuelica 14:61-75. [ Links ]
18. GUERRERO R. 1996. Estudio preliminar de los murciélagos de Pakitza, Parque Nacional Manú (Perú). Pp. 643-657, in: Biodiversidad del Sureste del Perú (DE Wilson and A Sandoval, eds.). Smithsonian Institution, Lima. [ Links ]
19. GUERRERO R. 1997. Catálogo de los Streblidae (Diptera: Pupipara) parásitos de murciélagos del nuevo mundo. VII. Lista de Especies, hospedadores y países. Acta Biológica Venezuelica 17:9-24. [ Links ]
20. GUTIERREZ KA, E GALINDO, and G REINOSO. 2010. Nuevos registros de quirópteros para el departamento del Tolima, Colombia. Revista Tumbaga 5:27-37. [ Links ]
21. HERRIN CS and VJ TIPTON. 1975. Spinturnicid Mites of Venezuela (Acarina: Spinturnicidae) (Vol. 2). Provo, Biological Series, Utah, USA. [ Links ]
22. HIJMANS RJ, SE CAMERON, JL PARRA, PG JONES, and A JARVIS. 2005. Very high resolution interpolated climate curfaces for global land areas. International Journal of Climatology 25:1965-1978 [ Links ]
23. HOLDRIDGE LR. 1967. Life Zone Ecology. Tropical Science Center. San José, Costa Rica. [ Links ]
24. JONES G, DS JACOBS, TH KUNZ, MR WILLIG, and PA RACEY. 2009. Carpe noctem: the importance of bats as bioindicators. Endangered Species 8:93-115. [ Links ]
25. KALKO E. 1998. Organization and diversity of tropical bats communities through space and time. Zoology 101:281-297 [ Links ]
26. KOHLS G, D SONENSHINE, and C CLIFFORD. 1965. The Systematics of Subfamily Ornithodorinae (Acarina: Argasidae) II. Identification of the larvae of the Western Hemisphere and description of three new species. Annals of the Entomological Society of America 58:331-364. [ Links ]
27. LARSEN PA, SR HOOFER, MC BOZEMAN, SC PEDERSEN, HH GENOWAYS, CJ PHILLIPS, DE PUMO and RJ BAKER. 2007. Phylogenetics and phylogeography of the Artibeus jamaicensis complex based on cytochrome-b DNA sequences. Journal of Mammalogy 88:712-727 [ Links ]
28. LARSEN PA, MR MARCHÁN-RIVADENEIRA, and RJ BAKER. 2010. Natural hybridization generates mammalian lineage with species characteristics. Proceedings of the National Academy of Sciences 107:11447-11452 [ Links ]
29. SILVA CL and G GRACIOLLI. 2013. Prevalence, mean intensity of infestation and host specificity of Spinturnicidae mites (Acari: Mesostigmata) on bats (Mammalia: Chiroptera) in the Pantanal, Brazil. Acta Parasitologica 58:174-9. [ Links ]
30. MACHADO-ALLISON C and R ANTEQUERA. 1969. Notas sobre Mesostigmata neotropicales V. Algunos datos sobre la distribución y huéspedes de los Spinturnicidae de Colombia (Acarina, Mesostigmata, Spinturnicidae). Caldasia 10:371-376. [ Links ]
31. MANTILLA-MELUK H. 2009. Phyllostomid Bats of Colombia: Annotated Checklist, Distribution, and Biogeography. Museum of Texas Tech University. Special Publications. Lubbock [ Links ]
32. MARINKELLE C and ES GROSE. 1981. A list of ectoparasites of Colombian bats. Revista de Biología Tropical 29:11-20. [ Links ]
33. MARSHALL AG. 1976. Host-specificity amongst arthropods ectoparasitic upon mammals and birds in the New Hebrides. Ecological Entomology 1(3):189-199. [ Links ]
34. MARSHALL AG. 1982. Ecology of Insects Ectoparasites on Bats. Pp. 369-401, in: Ecology of Bats (T Kunz and TH Kunz, eds.). Plenum Press, New York. [ Links ]
35. MONTENEGRO O and H ROMERO-RUIZ. 1999. Murciélagos del sector sur de la Serranía del Chiribiquete, Caquetá, Colombia. Revista de la Academia Colombiana de Ciencias 23:641-649. [ Links ]
36. MORALES-MALACARA JB and R GUERRERO. 2007. A New Species of Parichoronyssus (Acari: Dermanyssoidea: Macronyssidae) from Bats of the Genus Phyllostomus (Chiroptera: Phyllostomidae) in Peru and Venezuela, with Keys to the Species of Parichoronyssus. Journal of Medical Entomology 44:8-13. [ Links ]
37. ORTEGÓN-MARTÍNEZ D and J PÉREZ-TORRES. 2007. Estructura y composición del ensamblaje de murciélagos (Chiroptera) asociado a un cafetal con sombrío en la Mesa de los Santos (Santander) Colombia. Actualidades biológicas 29:215-228. [ Links ]
38. PÉREZ-TORRES and J AHUMADA. 2004. Murciélagos en bosques altoandinos, fragmentados y continuos, en el sector occidental de la Sabana de Bogotá (Colombia). Universitas Scientiarum Revista de la Facultad de Ciencias Pontificia Universidad Javeriana 9(1):33-46. [ Links ]
39. RADOVSKY FJ. 1967. The Macronyssidae and Laelapidae (Vol. 46). University of California Press, Berkeley and Los Angeles, California. [ Links ]
40. ROJAS A, A JIMÉNEZ, M VARGAS, M ZUMBADO, and M HERRERO. 2008. Ectoparasites of the common vampire bat (Desmodus rotundus) in Costa Rica: Parasitism rates and biogeographic trends Mastozoología Neotropical 15:181-187. [ Links ]
41. RUDNICK A. 1960. A revision of the mites of the familiy Spinturnicidae (Acarina). University California Publications Entomology 17:157-284. [ Links ]
42. SÁNCHEZ F, J ÁLVAREZ, C ARIZA, and A CADENA. 2007. Bat assemblage structure in two dry forests of Colombia: Composition, species richness, and relative abundance. Mammals Biology 72:82-92. [ Links ]
43. SÁNCHEZ-CORDERO V, F BOTELLO, G MAGAÑA-COTA, and J IGLESIAS. 2010. Vampire bats, Desmodus rotundus, feeding on white-tailed deer, Odocoileus virginianus. Short Note. Mammalia 74:55-56. [ Links ]
44. SIKES RS, WL GANNON, and THE ANIMAL CARE AND USE COMMITTEE OF THE AMERICAN SOCIETY OF MAMMALOGISTS 2011. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. Journal of Mammalogy 92:235-253. [ Links ]
45. SIMMONS NB and RS VOSS. 1998. The Mammals of Paracou, French Guiana: A Neotropical lowland rainforest fauna. Part 1. Bats. Bulletin of the American Museum of Natural History, New York, 237:1-219. [ Links ]
46. SOLARI S, Y MUÑOZ-SABA, JV RODRÍGUEZ-MAHECHA, TR DEFLER, HE RAMÍREZ-CHAVES, and F TRUJILLO. 2013. Riqueza, endemismo y conservación de los mamíferos de Colombia. Mastozoología Neotropical 20:301-365. [ Links ]
47. SORIANO PJ. 2000. Functional structure of bat communities in tropical rainforest and Andean cloud forest. Ecotropicos 13(1):1-20. [ Links ]
48. TARQUINO-CARBONELL AP. 2014. Ectoparásitos asociados a la quiropterofauna en la vereda Chorrillo, Tolima, Colombia. Tesis de Pregrado. Facultad de Ciencias Básicas. Universidad del Tolima. Ibagué, Tolima, Colombia. [ Links ]
49. THEODOR O. 1957. Parasitic adaptation and host-parasite specificity in the pupiparous Diptera. Pp. 50-63, in: First symposium on host specificity among parasites of vertebrates (E Mayr, ed.). Neuchâtel, Switzerland: Université de Neuchâtel. [ Links ]
50. WENZEL RL. 1976. The streblid batflies of Venezuela (Diptera: Streblidae). Brigham Young University Science Bulletin (Biological Series) 20:177 pp. [ Links ]
51. WILLIG MR, SJ PRESLEY, CP BLOCH, CL HICE, SP and ANOVIAK y MM DÍAZ. 2007. Phyllostomid bats of lowland Amazonia: effects of habitat alteration on abundance. Biotropica 39:737-746 [ Links ]














