Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Mastozoología neotropical
versão impressa ISSN 0327-9383versão On-line ISSN 1666-0536
Mastozool. neotrop. vol.23 no.1 Mendoza jun. 2016
ARTÍCULO
Precipitation drives reproductive activity in male Microcavia australis in the monte desert
Natalia Andino1, Marisa Nordenstahl1,2, Miguel Fornés3,4, José Priotto3,5, and Stella Giannoni1,2
1 Interacciones Biológicas del Desierto (INTERBIODES). Facultad de Ciencias Exactas, Físicas y Naturales. Universidad Nacional de San Juan (UNSJ), Av. Ignacio de la Roza 590 (Oeste), J5402DCS Rivadavia, San Juan, Argentina [Correspondence: Natalia Andino <nandino@unsj-cuim.edu.ar>].
2 CIGEOBIO (Centro de Investigaciones de la Geósfera y la Biosfera), UNSJ-CONICET. Av. Ignacio de la Roza 590 (Oeste), J5402DCS Rivadavia, San Juan, Argentina.
3 Consejo Nacional de Investigaciones Científicas y Técnicas, Av. Rivadavia 1917, C1033AAJ, Buenos Aires, Argentina.
4 Laboratorio de Investigaciones Andrológicas de Mendoza, Instituto de Histología y Embriología de Mendoza, Facultad de Ciencias Médicas, Universidad Nacional de Cuyo, Av. Libertador 80, Centro Universitario, 5500 Mendoza, Argentina.
5 Departamento de Ciencias Naturales, Facultad de Ciencias Exactas, Fisicoquímicas y Naturales, Universidad Nacional de Río Cuarto, Ruta Nacional 36, Km 601, Córdoba, Argentina.
Recibido 19 agosto 2015.
Aceptado 3 febrero 2016.
Editor asociado: C Galliari
ABSTRACT.
Desert areas represent heterogeneous environments where animals must reproduce under extreme conditions, and where a combination of environmental factors may contribute to trigger or inhibit reproduction. Microcavia australis is a caviomorph rodent that occurs in arid and semiarid habitats of Argentina. We examined how reproductive activity in male M. australis from a Monte Desert population is responsive to environmental conditions, including precipitation. Our results showed that reproductive activity of these animals is predicted exclusively by precipitation. This research adds new evidence to the ecology of this species, which could explain its wide distribution.
RESUMEN.
Las precipitaciones conducen la actividad reproductiva en machos de Microcavia australis en el desierto del Monte.
Las áreas desérticas representan ambientes heterogéneos donde los animales deben reproducirse bajo condiciones extremas y donde una combinación de factores ambientales puede contribuir para iniciar o inhibir la reproducción. Microcavia australis es un roedor caviomorfo que vive en hábitats áridos y semiáridos de Argentina. Examinamos cómo la actividad reproductiva en machos de M. australis en una población del Desierto del Monte responde a condiciones ambientales, incluyendo la precipitación. Nuestros resultados mostraron que la actividad reproductiva de estos animales es predicha exclusivamente por la precipitación. Este estudio incorpora nuevas evidencias a la ecología de esta especie que podrían explicar su amplia distribución.
Key words: Environmental factors; Precipitation; Reproduction; Rodents.
Palabras clave: Factores ambientales; Precipitación; Reproducción; Roedores.
INTRODUCTION
In desert rodents, a combination of environmental factors, as opposed to single factors, act as signals to trigger or inhibit reproductive (Bronson and Heideman, 1994) events that sometimes match the times with greater water and food availability and favorable climate conditions (Bronson, 1989; Degen, 1997). Accordingly, some desert rodent species may use ambient temperature as a signal for reproduction and may postpone it under fluctuating temperature conditions (Prendergast et al., 2001). In other cases, such as Microtus californicus and Peromyscus californicus, day length has also been shown to be a cue for the onset of reproduction (Nelson et al., 1983; Nelson et al., 1995). Alternatively, high precipitation may also be a condition for successful reproduction in these extreme environments, mainly through its influence on plant productivity and, hence, on food availability (Wingfield and Kenagy, 1991; Mateos-Quesada and Carranza, 2000).
The southern mountain cavy (M. australis) is one of the smallest caviomorph rodents (250 g; Tognelli et al., 2001). This herbivorous, diurnal species is widely distributed in Argentina, from Jujuy south to Santa Cruz, across a variety of semiarid and arid lands (Redford and Eisenberg, 1992). Southern mountain cavies live in groups (Rood, 1967; 1972; Taraborelli and Moreno, 2009; Andino et al., 2011) of different size and composition, with a maximum of eight males and five females plus the offspring (Andino et al., 2011). Previous studies of behavior, physiology, and renal and digestive morphology have revealed the plasticity of M. australis to cope with the challenges of the arid lands it inhabits (Sassi et al., 2007; Taraborelli and Moreno, 2009; Andino et al., 2011; Tejo et al., 2014). A single study of reproduction, performed by Vélez et al. (2010) in a semiarid region of Argentina at the Monte-Cardonal ecotone, showed that precipitation, minimum temperature and day length are positively correlated with testicular weight of the male southern mountain cavy and seem to be important to its reproductive activity. However, it is still necessary to further investigate reproduction in environments with other features such as our study area (a hyper arid ecosystem with harsh climatic conditions). This information will help understand to what extent the plasticity reported in other aspects of the species morphology and physiology (Sassi et al., 2007; Taraborelli and Moreno 2009; Andino et al., 2011; Tejo et al., 2014) is also present in reproduction.
Even though all desert areas are characterized by low humidity, wide temperature ranges, and unpredictable food availability, they represent heterogeneous environments. Animals occurring in these settings often face strong changes in environmental conditions, in time and space, and must sometimes reproduce under extreme conditions (e.g., temperatures extremely high in summer and below freezing in winter, low humidity during day followed by cold nights, strong winds and unstable sandy soils that hinder the growth of vegetation, Randall, 2007). We hypothesized that, to face this heterogeneity of biotic and abiotic conditions, male southern mountain cavies adjust reproductive activity by responding to short-term environmental cues rather than seasonality. To test this hypothesis, we examined the variation in testicular and epididymal weight track variation in environmental conditions. Particularly, we predict that males will increase testicular and epidiymal weight with increases of temperature, precipitation, and plant cover, but not with day length.
METHODS
Study area
The study was conducted in El Leoncito National Park (31°47' S, 69°'17' W, 2484 m a.s.l., San Juan province, Argentina), a protected area belonging to the Argentine National System of Protected Zones. The climate is arid (cold and dry), and mean annual temperature is 15.6 °C, with marked diurnal and seasonal variation (winter range: -4 to 20 ºC, summer range: 8 to 32 ºC; Márquez and Dalmasso, 2003; Fig. 1). Mean annual precipitation is below 100 mm, mostly in the form of snow and hail and concentrated during the colder months (April through August). Precipitation during the warmer months (November-December) is below 10 mm (Le Houérou, 1999). The study site within the park was Ciénaga del Medio, an area of the Monte Desert and influenced by the Puna desert. The site has low total plant cover, reaching a maximum of 21.9% (Andino et al., 2011). The shrub stratum is dominated by the creosote bush Larrea nitida, which is abundant (reaching 90% cover) throughout the year, and a sparse and low-lying herbaceous layer that represents 10% of plant cover (Andino et al., 2011).
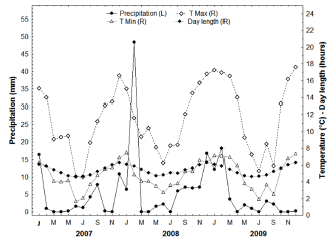
Fig. 1. Climatic conditions and day length in El Leoncito National Park during the study period.
Data
During 1191 trap-days we captured 19 adult male southern mountain cavies from a 1.8-ha area, using Havahart-type traps. Nine capture periods were carried out from 2007 to 2009, including all four seasons (autumn: March 2007, 2008, May 2009; winter: June 2007, July 2009; spring: September 2007, 2008; summer: December 2007, 2008). In each sampling month, we placed traps in active runways, usually under the canopy of creosote bush and near burrow entrances, for 10 consecutive days approximately. We registered the body weight of each captured male. In each sampling period, we gathered climate data and measured plant cover. Mean daily minimum (Tmin) and maximum air temperature (Tmax), total monthly precipitation (P) and humidity (H) were obtained from records of the weather station located in the study area. Day length (DL: i.e, photoperiod) data were obtained from the Astronomical Applications Department of the U.S. Naval Observatory website. We also calculate thermal amplitude (TA) as difference between Tmax and Tmin. The record of plant cover (PC) data was performed throughout the study site along five 20-m-long transects (sample), each with three quadrats (6 x 6 m each subsample) at 1-m intervals along each transect.
In the laboratory, we sacrificed the captured males following the Guidelines on Euthanasia of the American Veterinary Association (AVMA 2013), and the procedure used was approved by the Commission of Bioethics (number 490) of the National University of San Juan, Argentina. Testes and epididymes were removed, weighed with a precision scale (AND model HR-200, d = 0.001 mg) and their mean weight was calculated following Vélez et al. (2010).
Statistical analysis
To assess inter-annual and seasonal variations in body weight, testicular weight and epididymal weight, we generated independent sets of General Linear Models (GLM; Logan, 2010). We used as explanatory variables the year and season of year when each male was captured. To test for the effect of environmental factors on reproductive activity of male southern mountain cavies we used Generalized Linear Mixed Models (GLMM; Zuur et al., 2009) with Gaussian distribution of errors to model each response variable (testicular weight change and epididymal weight change) independently. The following: Tmax, Tmin, DL, P, H, and PC were included as potential explanatory environmental variables. However, H and Tmax, which were correlated with P and Tmin, respectively (r > 0.7) and were therefore removed following (Neter et al., 1990). The year of study and season were included as in the models as random variables. Akaike's information criterion corrected for small sample size (AICc), was calculated to evaluate the models that best fitted the data (Burnham and Anderson, 2002 ). The AICc weight of a model (wi) was calculated based on all candidate models according to Burnham and Anderson (2002), representing the likelihood that a particular model is the best model, given the data and the candidate models considered. Support for each of the predictor variables was estimated by summing wi across all models that contained them. Predictor variables with good support presented high parameter-likelihood values (wi near 1). The relative importance (RI) of each predictor variable was also evaluated for each candidate model (Burnham and Anderson, 2002). All statistical analyses were carried out with R 3.2.3 (R Development Core Team, 2015) software.
RESULTS
Body weight varied among seasons (Fig. 2) but not among years (F3,14 = 0.24; p = 0.62). After including body weight as a covariate, there was significant seasonal variation in testicular weight that was higher in summer and lowest in autumn and winter (Fig. 2). The lowest testicular weight was found in autumn (1.36 ± 0.24 g); it increased to 1.59 ± 0.33 g in winter, and 2.55 ± 0.21 g in spring, with a peak in summer (3.02 ± 0.26 g). The lowest epididymal weight was recorded in autumn (0.55 ± 0.09 g) and increased in winter (0.80 ± 0.12 g; Fig. 2). In spring, epididymal weight was of 0.65 ± 0.08 and in summer it reached a value of 0.67 ± 0.10 g (Fig. 2).
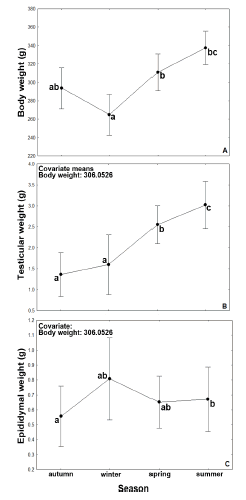
Fig. 2. Seasonal variation of body weight (A), testicular weight (B) and epididymal weight (C) of male southern mountain cavies between 2007 and 2009. Body weight was used as a covariate in the analysis of testicular weight and epididymal weight. Letters (a, b, c) indicate significant differences (Tukey test; P<0.05). Means ( ± SE) are shown.
Analysis of the environmental explanatory variables revealed that precipitation was the single most important variable impacting measures of reproductive activity (Table 1). For both response variables (testicular weight and epididymal weight) the best model, based on AICc criteria, had precipitation as the only explanatory variable (Table 1), which accounted for 89% of variation in testicular weight and 61% of variation in epididymal weight, with a relative importance (RI) of 0.99 and 0.69, respectively (Table 1).
Table 1 Relationships between testicular and epididymal weight and environmental factors (precipitation: P; mean minimum temperature: Tmin; plant cover: PC; day length: DL, and thermal amplitude: TA) were examined using Generalized Linear Mixed Models (GLMM). We used as random variable the year of study and season. For each reproductive variable the table shows: best model, minimum AICc (Akaike's Information Criterion corrected for small sample size), maximum Akaike weight (wi), % of the total deviance explained by the fixed factor (marginal R2) and by the fixed factor and random factor (conditional R2). The relative importance of the climatic variables is also indicated.

DISCUSSION
We found evidence of seasonal variation in the reproductive activity of adult male southern mountain cavies, a finding that should be regarded as preliminary, given the reduced number of animals examined. Our results suggest that precipitation would be the main (if not the sole) ecological factor driving variation in the size of reproductive organs of male southern mountain cavies. Our findings are at odds with previous studies of the same species at a site with mean annual precipitation of 324.5 mm, mean annual temperature of 10.5 ºC and mean annual plant cover of 50% (Vélez et al., 2010); that is to say, a slightly arid site with different environmental conditions compared to our hyper arid study area. These authors postulated that precipitation, temperature, and day length seem to be important for the testis cycle of southern mountain cavy males, although their results were only marginally significant, probably due to the low number of replicas (14 individuals). Even so, our results and those by Vélez et al. (2010) show that populations occurring in different habitats exhibit differences in their reproductive activity, though new investigations with larger sample sizes are necessary to detect effects not found in either study.
Positive correlations between precipitation and breeding have been reported in African (Perrin and Swanepoel, 1987), Australian (Breed, 1990), Asian (Rogovin, 1985) and North American desert rodents (Randall, 1993). Precipitation might have direct (i.e. water availability) and indirect effects (via plant productivity and food availability) on reproductive activity by allowing the species to increase its body weight sufficiently to reproduce. In our study site, precipitation occurs in two episodes (winter and summer) and, similar to other desert dwelling species (Bronson, 1989), reproduction occurred during the rainy months and was suppressed in autumn months when rains were absent, as found by Bukovetzky et al. (2012) in Acomys cahirinus.
Water plays a major role in reproduction of rodents (Schwimmer and Haim, 2009; Prakash and Ghosh, 1975) and might affect hormone regulation, increasing gonadotropin (GnRH) secretion, regulating testosterone secretion by Leydig cells, and promoting sperm production (Bronson, 2009). Desert rodents generally do not drink free water but satisfy their water needs from food in the form of preformed and metabolic water. In the study area, plant cover is approximately stable year-round, likely due to the presence of shrubs of Larrea nitida, a perennial species that reaches 90% of vegetation cover and is a dominant component in this species' diet (nearly 85%), whereas herbs are scarcely represented (10%), restricted to summer, and barely present (5%) in the diet (Sassi et al., 2010). Although plant cover is maintained approximately stable throughout the year, the nutritional quality of the diet varies between stations with increased fiber content in the dry season (Sassi et al., 2007). Although our results show that the reproductive activity of this cavy was not influenced by plant cover, Larrea plants comprise hygroscopic shrubs (Taylor, 1968) that are consumed by some mammal species to obtain performed water (Baxter and Hansson, 2001). The inverted conical shape of Larrea plants, which have all primary external stems with angles > 45°, allows the plant to maximize water collection during precipitation, increasing water availability for animals. Some desert rodent species (Otomys, Rhabdomys, Mastomys, Microtus) consume bark of trees and shrubs and obtain performed water through debarking (Baxter and Hansson, 2001). Campos et al. (2006) found that southern mountain cavy debarked on L. divaricata in winter to obtain water. We found cavies associated with L. nitida shrubs with debarked branches in winter (N Andino and S Giannoni unpublished data). In the cold period, cavies probably obtain performed water and energy through debarking activity, begin ning reproductive activity in this period. On the other hand, in warm months, food supply increases when shrubs grow. Larrea shrubs provide high quality food due to the greater protein content of the shoots and performed water (Sinclair et al., 2006). Shoots are known to be important in increasing reproductive activity in spring-summer; however, we were unable to detect changes in shoots biomass among seasons. The method used to estimate plant cover may have not been sensitive enough to detect subtle changes, such as an increase in the shoots of shrubs, but food supply for herbivores is known to be higher in summer at high latitudes, as in the Monte Desert region of South America (Sinclair et al., 2006). During spring and summer the individuals increase the corporal mass and adipose tissue, this last stores water, which can then be obtained from the oxidation of fat increasing the reproduction in the warm months (Ghosh, 1975).
Trillmich (2000) suggests that a combination of low temperature and short photoperiod might influence reproduction in cavies in laboratory conditions. The relationship between reproduction in small rodents and day length is frequently related to latitude (Bronson and Perrigo, 1987). Several investigations predict a decreased responsiveness to photoperiod from high to low latitude regions, so that in areas below 30° latitude some rodents may stop using day length as a cue of beginning of reproduction (Bronson, 1985; Bronson and Perrigo, 1987; El-Barky et al., 1999). However, other authors suggest that response to photoperiod is relatively plastic because many rodent species from low latitude are non-photoperiodic, whereas populations of the same species living at other latitudes are responsive to changes in the photoperiod (Lynch, 1981; Dark et al., 1983; Nelson et al., 1983; Nelson et al., 1995). The latter could explain our results, because our study population occurs at a relative low latitude and unresponsive to photoperiod compared with photoperiodic dependence findings by Vélez et al. (2010) at higher latitudes. Furthermore, we did not find an effect of minimum temperature in our study, which is also in disagreement with the results of Vélez et al. (2010). In our site, orientation and architecture of galleries generate a stable and temperate microclimate (Taraborelli et al., 2009), and communal nesting in these animals (Ebensperger et al., 2006) may contribute to decrease the energy costs of thermoregulation. Again, limited sample sizes compromise the ability to detect subtle effects.
Alternatively, it is known that social factors influence the reproductive function of males (Demas and Nelson, 1998; Gouat et al., 2003). In males with relatively long reproductive activity, the fine tuning of reproduction responds to the estrus cycle of females, which shed ova and mate once they have sufficient food resources and other conditions for ensuring successful reproduction (Kenagy and Bartholomew, 1985). In our study site, Taraborelli and Moreno (2009) performed behavioral observations of southern mountain cavy and registered sexual behaviors from August to March, with the first litters observed in September and the last ones in March. The gestation period of southern cavy females is approximately 56 days (Tognelli et al., 2001), and mating begins in August, as observed by Taraborelli and Moreno (2009). However, histological studies showed sperm in the tail of the epididymis in males captured in July (Vélez et al., 2010; Andino unpublished data), confirming that males start reproductive activity earlier than females.
ACKNOWLEDGEMENTS
We are indebted to Parques Nacionales de Argentina, particularly to park rangers of Parque Nacional El Leoncito. We sincerely thank the team of IHEM for help with laboratory work. We thank to Luis Ebensperger and the anonymous reviewer for their valuable suggestions. Jorgelina Brasca and Nelly Horak assisted us with English editing. Funding was provided by CICITCA-UNSJ (RES. 018-14/CS; SG).
LITERATURE CITED
1. AMERICAN VETERINARY MEDICAL ASSOCIATION (AVMA). 2013. Guidelines for the euthanasia of animals: 2013 Edition. [ Links ]
2. ANDINO N, L REUS, F CAPPA, V CAMPOS, and S GIANNONI. 2011. Social environment and agonistic interactions: Strategies in a small social mammal. Ethology 117:992-1002. [ Links ]
3. BAXTER R and L HANSSON. 2001. Bark consumption by small rodents in the northern and southern hemispheres. Mammal Review 31:47-59. [ Links ]
4. BREED WG. 1990. Comparative studies on the timing of reproduction and foetal number in six species of Australian rodents (Muridae: Hydromyidae). Journal of Zoology (London) 221:1-10. [ Links ]
5. BRONSON FH. 1985. Mammalian reproduction: An ecological perspective. Biological Reproduction 32:1-26. [ Links ]
6. BRONSON FH. 1989. Mammalian Reproductive Biology. The University of Chicago Press, Chicago and London. [ Links ]
7. BRONSON FH. 2009. Climate change and seasonal reproduction in mammals. Philosophical Transactions of the Royal Society B 364:3331-3340. [ Links ]
8. BRONSON FH and PD HEIDEMAN. 1994. Seasonal regulation of reproduction in mammals. Pp. 541- 583, in: The Physiology of Reproduction 2nd Edition (E Knobil and JD Neill, eds.). Raven Press, New York. [ Links ]
9. BRONSON FH and G PERRIGO. 1987. Seasonal regulation of reproduction in muroid rodents. American Zoologist 27:929-940. [ Links ]
10. BUKOVETZKY E, H SCHWIMMER, F FARES, and A HAIM. 2012. Photoperiodicity and increasing salinity as environmental cues for reproduction in desert adapted rodents. Hormones and Behaviour 61:84-90. [ Links ]
11. BURNHAM KP and DR ANDERSON. 2002. Model selection and multimodel inference: A practical information-theoretic approach. Springer-Verlag, New York. [ Links ]
12. CAMPOS C, C BORGHI, S GIANNONI, A MANGEAUD, and M TOGNELLI. 2006. Bark consumption of creosote bush (Larrea cuneifolia): Effect of branch survival and reproduction. Ecología Austral 16:1-6. [ Links ]
13. DARK J, I ZUCKER, and GN WADE. 1983. Photoperiodic regulation of body mass, food intake and reproduction in meadow voles. American Journal of Physiology 245:334-338. [ Links ]
14. DEGEN AA. 1997. Ecophysiology of small mammals. Springer-Verlag, New York. [ Links ]
15. DEMAS GE and RJ NELSON. 1998. Social, but not photoperiodic, influences on reproductive function in male Peromyscus aztecus. Biological Reproduction 58:385-389. [ Links ]
16. EBENSPERGER, L., P. TARABORELLI, S. GIANNONI, M. HURTADO, C. LEÓN and F. BOZINOVIC. 2006. Nest and space use in a highland population of the southern mountain cavy (Microcavia australis). Journal of Mammalogy 87(5):834-840. [ Links ]
17. EL-BARKY H, W ZAHRAN, and T BARTNESS. 1999. Control of reproductive and energetic status by environmental cues in a desert rodent, Shaw's Jird. Physiology & Behavior 66:657-666. [ Links ]
18. GHOSH PK. 1975. Thermo-regulation and water economy in Indian Desert Rodents. Pp 397-412, in: Rodents in desert environments (I Prakash and PK Ghosh, eds.). The Hague: Dr. W. Junk b.v. [ Links ]
19. GOUAT P, C FÉRON, and S DEMOURON. 2003. Seasonal reproduction and delayed sexual maturity in mound-building mice Mus spicilegus. Reproduction Fertililty and Development 15:1-9. [ Links ]
20. KENAGY GJ and GA BARTHOLOMEW. 1985. Seasonal reproductive patterns in five coexisting California desert rodent species. Ecological Monographs 55:371- 397. [ Links ]
21. LE HOUÉROU HN. 1999. Estudios e investigaciones ecológicas de las zonas áridas y semiáridas de Argentina. IADIZA-CRICYT, Mendoza. [ Links ]
22. LYNCH GR, HEATH HW, and CM JOHNSTON. 1981. Effect of geographical origin on the photoperiodic control of reproduction in the white-footed mouse, Peromyscus leuopus. Biological Reproduction 25:475- 480. [ Links ]
23. LOGAN M. 2010. Biostatistical Design and Analysis Using R: A Practical Guide. Wiley, West Sussex. [ Links ]
24. MÁRQUEZ J and A DALMASSO. 2003. Las comunidades vegetales de los ambientes húmedos del Parque Nacional El Leoncito, San Juan, Argentina. Multequina 12:55-67. [ Links ]
25. MATEOS-QUESADA P and J. CARRANZA. 2000. Reproductive patterns of roe deer in central Spain. Etología 8:17-20. [ Links ]
26. NELSON RJ, J DARK, and I ZUCKER. 1983. Influence of photoperiod and water availability on reproduction of male California voles (Microtus californicus). Journal Reproduction and Fertility 69:473-477. [ Links ]
27. NELSON RJ, D GUBERNICK, and J BLOM. 1995. Influence of photoperiod, green food, and water availability on reproduction in male California mice (Peromyscus californicus). Physiology & Behavior 57:1175-1180. [ Links ]
28. NETER J, W WASSERMAN, and M KUTER. 1990. Applied linear statistical models: Regression, analysis of variance, and experimental designs. 2nd edition. R.D. Irwin (Homewood, III) Publisher. [ Links ]
29. PRAKASH I and PK GOSH. 1975. Rodents in desert environments. The Hague: Dr. W. Junk. [ Links ]
30. PERRIN MR and P SWANEPOEL. 1987. Breeding biology of the bushveld gerbil Tatera leucogaster in relation to diet, rainfall and life history. South African Journal of Zoology 22:218-227. [ Links ]
31. PRENDERGAST BJ, LJ KRIEGSFELD, and RJ NELSON. 2001. Photoperiodic polymorphism in rodents: Neuroendocrine mechanisms, costs, and functions. Quarterly Review of Biology 76:293-325. [ Links ]
32. RANDALL JA. 1993. Behavioural adaptations of desert rodents (Heteromyidae). Animal Behaviour 45:263- 287. [ Links ]
33. RANDALL JA. 2007. Environmental constraints and the evolution of sociality in semi-fossorial desert rodents. Pp 368-379, in: Rodent societies an ecological & evolutionary perspective (J Wolff and P Sherman, eds.). The University of Chicago Press, Ltd. London. [ Links ]
34. R CORE TEAM. 2015. R: A language and environment for statistical computing. R foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. [ Links ]
35. REDFORD KH and JF EISENBERG. 1992. Mammals of the neotropics: the southern cone. University of Chicago Press. Chicago. [ Links ]
36. ROGOVIN KA. 1985. A comparative analysis of behavior in supergeneric groups of jerboas (Rodentia, Dipodidae). Zoologicheskii Zhurnal 64:1702-1711. [ Links ]
37. ROOD J. 1967. Observaciones sobre la ecología y el comportamiento de los Caviinae de la Argentina (Mammalia, Rodentia). Zoología Platense. Investigaciones Zoológicas y Paleontológicas 1:1-6. [ Links ]
38. ROOD J. 1972. Ecological and behavioural comparisons of three genera of Argentine cavies. Animal Behaviour Monographs 5:1-83. [ Links ]
39. SASSI P, C BORGHI, and F BOZINOVIC. 2007. Spatial and seasonal plasticity in digestive morphology of cavies (Microcavia australis) inhabiting habitats with different plant qualities. Journal of Mammalogy 88(1):165-172 [ Links ]
40. SASSI P, E CAVIEDES-VIDAL, R ANTON, and F BOZINOVIC. 2010. Plasticity in food assimilation, retention time and coprophagy allow herbivorous cavies (Microcavia australis) to cope with low food quality in the Monte desert. Comparative Biochemistry and Physiology Part A 155:378-382. [ Links ]
41. SCHWIMMER H and A HAIM. 2009. Physiological adaptations of small mammals to desert ecosystems. Integrative Zoology 4:357-366. [ Links ]
42. SINCLAIR A, J FRYXELL, and G CAUGHLEY. 2006. Wildlife ecology, conservation and management. Second Edition. Blackwell Publishing. [ Links ]
43. TARABORELLI P and P MORENO. 2009. Comparing composition of social groups, mating system and social behaviour in two populations of Microcavia australis. Mammalian Biology 74:15-24. [ Links ]
44. TARABORELLI P, N BORRUEL, A SANDOBAL, and S GIANNONI. 2009. Influence of biotic and abiotic factors on the structure of burrows of the cavy Microcavia australis. Mastozoología Neotropical 16(2):411-421. [ Links ]
45. TAYLOR CR. 1968. Hygroscopic food: A source of water for desert antelopes? Nature 219:181-182. [ Links ]
46. TEJO P, G DIAZ, N ANDINO, and C BORGHI. 2014. Renal intraspecific variation along and aridity gradient detected by new renal indices in a desert herbivorous rodent. Journal of Experimental Zoology Part A 321:348-356. [ Links ]
47. TOGNELLI MF, CM CAMPOS, and RA OJEDA. 2001. Microcavia australis. Mammalian Species 648:1-4. [ Links ]
48. TRILLMICH F. 2000. Effect of low temperature and photoperiod on reproduction in the female wild Guinea pig (Cavia aperea). Journal of Mammalogy 81: 586-594. [ Links ]
49. VÉLEZ S, P SASSI, C BORGHI, M MONCLUS, and M FORNÉS. 2010. Effect of climatic variables on seasonal morphological changes in the testis and epididymis in the wild rodent Microcavia australis from the Andes mountains, Argentina. Journal Experimental Zoology Part A 313(8):474-483. [ Links ]
50. WINGFIELD JC and GJ KENAGY. 1991. Natural regulation of reproductive cycles. Pp 230-235, in: Vertebrate endocrinology: Fundamentals and biomedical implications (MP Schurbman and RE Jones, eds.). Academic Press, New York. [ Links ]
51. ZUUR AF, EN IENO, NJ WALKER, AA SAVELIEV, and GM SMITH. 2009. Mixed effect models and extensions in ecology with R. Springer, New York, USA. [ Links ]














