Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Mastozoología neotropical
versión impresa ISSN 0327-9383versión On-line ISSN 1666-0536
Mastozool. neotrop. vol.23 no.1 Mendoza jun. 2016
NOTA
New records of Lampronycteris brachyotis in Brazil
Marcus V. Brandão1, Patrício A. da Rocha2, Poliana Mendes3, Paulo V. S. Bernardo3, Irineu N. Cunha4, Paul F. Colas-Rosas5, Mônica A. Pedroso6, Carla C. de Aquino7, and Caroline C. Aires6
1 Programa de Pós-Graduação em Diversidade Biológica e Conservação, Universidade Federal de São Carlos, Campus Sorocaba, Sorocaba, SP, Brazil. Rod. João Leme dos Santos (SP-264), km 110, 18052-78. Bairro Itinga, Sorocaba/ São Paulo, Brasil. [Corresponding author: Marcus V. Brandão <puerabio@gmail.com>]
2 Programa de Pós-Graduação em Zoologia, Universidade Federal da Paraíba, Campus I, Departamento de Sistemática e Ecologia, 58059-900, João Pessoa, Paraíba, Brazil.
3 Programa de Pós-Graduação em Ecologia e Evolução, Instituto de Ciências Biológicas, Universidade Federal de Goiás, Campus Samambaia, 74690-000, Goiânia, Goiás, Brazil.
4 Universidade Braz Cubas, campus da Sede, Avenida Francisco Rodrigues Filho, 1233 - Mogilar, Mogi das Cruzes, 08773-380, Brazil.
5 Biophilium Consultoria Ambiental, 12940-210, Atibaia, São Paulo, Brazil.
6 Universidade de Mogi das Cruzes, Campus da Sede, CEP 087809-11. Vila Partenio, Mogi das Cruzes, São Paulo, Brazil.
7 Museu de Zoologia da Universidade de São Paulo, Mastozoologia, Caixa Postal 42694. CEP 04299-970. São Paulo, SP, Brazil.
Recibido 21 diciembre 2015.
Aceptado 7 marzo 2016.
Editor asociado: R Gregorin.
ABSTRACT.
Lampronycteris is a monotypic genus distributed throughout the Neotropical region. Brazil presents the largest number of reports of L. brachyotis, most of which occur within Amazonia, with only limited records in Cerrado and Atlantic Forest. The present report provides an updated distribution of L. brachyotis in these three biomes, including the first records for the states of Rondônia and Goiás and additional records in the states of Mato Grosso, Pará and São Paulo. We highlight that mesic areas in open formations such as Cerrado might support relictual populations of L. brachyotis, highlighting the importance of these areas for bat diversity.
RESUMEN.
Nuevos registros de Lampronycteris brachyotis en Brasil.
Lampronycteris es un género monotípico de amplia distribución en la región neotropical. Brasil tiene el mayor número de registros de L. brachyotis concentrados en la Amazonía y solo unos pocos registros en el Cerrado y Bosque Atlántico. Este artículo ofrece una actualización de la distribución de L. brachyotis en estos tres biomas, con el primer registro en Rondônia y Goiás, y registros adicionales en los estados de Mato Grosso, Pará y São Paulo. Con los registros presentados aquí, destacamos que los ambientes mésicos presentes en bosque estacional seco, como el Cerrado, pueden mantener poblaciones relictuales de L. brachyotis, destacando la importancia de estas áreas para la diversidad de murciélagos.
Key words: Amazon; Atlantic Forest; Cerrado; Chiroptera; Distribution; Orange-throated bat.
Palabras clave: Amazonía; Bosque Atlantico; Cerrado; Chiroptera; Distribución; Murciélago orejón de garganta amarilla.
The orange-throated bat Lampronycteris brachyotis (Dobson, 1879), originally treated as subgenus within Micronycteris Gray, 1866 (Sanborn, 1949), is now considered the single representative of the genus Lampronycteris Sanborn, 1949 (Simmons, 2005). This decision was based on morphological data (Simmons and Voss, 1998) and later consolidated in studies using morphological, karyological, and molecular data (Wetterer et al., 2000). Therefore, nowadays, it is considered a wide distributed monotypic genus in Neotropical region (Williams and Genoways, [2008]).
The current known distribution of L. brachyotis ranges throughout southern Mexico, Guatemala, Panama, Colombia, Venezuela, Bolivia, Trinidad and Tobago, Guyana, Suriname, Ecuador, Peru, and Brazil (Weinbeer and Kalko, 2004; Acosta and Aguanta, 2005; Simmons, 2005; Williams and Genoways, [2008]; Tirira et al., 2010). Most of the reports for the species are in Brazil, with records through the Amazon in the states of Acre, Amazonas, Amapá, Mato Grosso, and Pará (Sampaio et al., 2003; Bernard et al., 2011a; Miranda et al., 2015). There are scarce reports for the Atlantic Forest, most of them from the state of São Paulo (Taddei and Pedro, 1996; Gimenez and Ferrarezzi, 2004; Geraldes, 2005), and only one record in each of the following states: Bahia (Faria et al., 2006), Espírito Santo (Peracchi and Albuquerque, 1993), and Paraná (Scultori et al., 2009). Finally, recent reports have mentioned this species to be present in the Cerrado formations of the state of Mato Grosso (Louzada et al., 2015; Oliveira and Faria, 2015) (Fig. 1; Table 1).

Fig. 1. Map showing the known localities for L. brachyotis. Stars represent the new records here presented and circles are records taken from literature. Localities numbered as in Table 1.
Table 1 Localities at which the occurrence of the L. brachyotis has been confirmed. The code numbers refer to the points showed in Fig. 3.
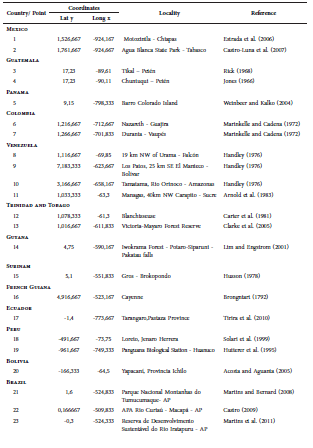
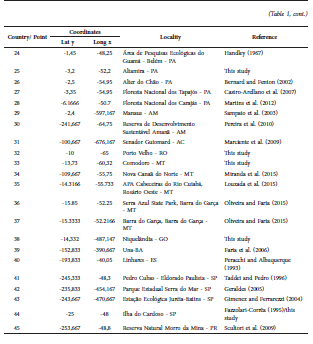
Herein, we provide additional records of L. brachyotis for São Paulo, Pará and Mato Grosso, and the first record for the states of Rondônia and Goiás. All these records fill gaps in the distribution of the species in Brazil and improve the knowledge about its habitat usage.
We recorded five specimens of L. brachyotis (Fig. 1; Table 1), two from the Cerrado, two from the Amazon, and one from the Atlantic Forest (detailed information in Table 2). All individuals were netted at ground level and handled in accordance with Sikes et al. (2011), fixed in 10% formaldehyde and preserved in 70% ethanol, and the skulls were removed and cleaned (Fig. 2). External and cranial measurements are in Table 3. The specimens are housed at the zoological collections of the Museu de Zoologia da Universidade de São Paulo (MZUSP) and of the Universidade Federal de Goiás (MZUFG).
Table 2 Information about the five specimens of Lampronycteris brachyotis reported in the present study.
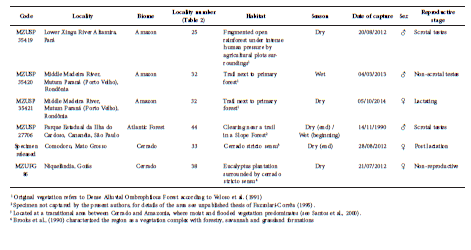
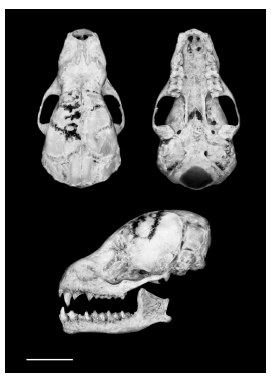
Fig. 2. Dorsal, ventral and lateral views of the skull and lateral view of the mandible of Lampronycteris brachyotis (MZUSP 27706) from Ilha do Cardoso, São Paulo, Brazil. Scale bar = 6 mm.
Table 3 Sex, external and cranial measurements of the Lampronycteris brachyotis specimens collected during the present study.
Lampronycteris brachyotis is a peculiar species of the subfamily Micronycterinae (sensu Baker et al., 2003), easily distinguished by its small (less than 16 mm) and peculiar shaped ears (short and pointed; see Fig. 3); calcar about the same length as the foot; upper incisors chisel shaped and in line with canines; second phalanx of the middle finger conspicuously longer than the first; the dorsal pelage is orange while the ventral is yellowish-orange; the lower rim of the horseshoe of the noseleaf is defined by a ridge; and the lower lip presents two smooth tubercles separated by a V-shaped groove (see Fig. 3) (Dobson, 1879; Williams and Genoways, [2008]).
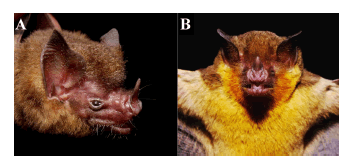
Fig. 3. Live individuals of Lampronycteris brachyotis. A - From Porto Velho, Rondônia (MZUSP 35420). B - From Comodoro, Mato Grosso. Note the peculiar shape of the ears (short and pointed), the yellowish-orange pelage, the lower rim of the horseshoe of the noseleaf defined by a ridge, and presence of two smooth tubercles separated by a V-shaped groove at the lower lip.
Our specimens include one non-reproductive female, collected in July (from Goiás), one lactating female in October (from Rondônia), and one post-lactating female in August (from Mato Grosso) (Table 2). The literature reports lactating females in July (from Mexico; Estrada et al., 2006), and pregnant and lactating females in July (from Guatemala; Rick, 1968).
In spite of the fact that the wide distribution of L. brachyotis includes both the Atlantic Forest and the Amazon in Brazil, until recently there was a large gap of records between those areas (see Williams and Genoways, [2008]). That gap corresponds to dry/open vegetation areas, such as Caatinga and Cerrado. Recently, Rocha et al. (2015), using distribution models of forest dwelling bats, predicted a pattern of low suitability for L. brachyotis in dry seasonal forest areas, suggesting that its presence along the Cerrado and Caatinga would be associated to mesic phytophysiognomies, sparsely found within these biomes. Corroborating this statement, both recent records from Cerrado (Louzada et al., 2015; Oliveira and Faria, 2015) are from gallery forest. Furthermore, two of the new records provided here (Comodoro and Niquelandia), although coming from areas of Cerrado sensu stricto, are comprised within regions characterized by a complex of phytophysiognomies, including mesic areas.
It is possible that these mesic savanna areas may be acting as stepping-stones (i.e. discontinuous patches that connect otherwise isolated patches) between more suitable biomes to L. brachyotis, such as the Amazon and Atlantic Forest, and can explain the occurrence and persistence of this rare species in the Cerrado (see Irwin and Taylor, 2000; Loehle, 2007). Alternatively, they may represent isolated local populations. Additional sampling and mark-recapture data are needed to document the distribution and understand the demography of this species in the area (see Bernard et al., 2011b). With the recent literature records and additional ones reported here, we highlight that mesic areas in semiarid vegetation such as Cerrado and Caatinga, might support peripheric and relictual populations or assist in dispersion.
Acknowledgements.
We are grateful to Arcadis Logos S/A for field campaigns, to Dr. Mario de Vivo and Juliana Gualda de Barros from MZUSP, to Paulo B. Passos Filho and Thais C. Lira for the help during fieldworks, to Anglo American pcl Company for financial support for the field work carried out in Niquelândia municipality, as well as the biodiversity project in partnership between Universidade Federal de Goiás and Anglo American. Additionally we thank Instituto Chico Mendes for the licenses provided for field works. PAR (processes 501701/2013-3 and 150407/2015-7) thanks CNPq for research stipends.
LITERATURE CITED
1. ACOSTA SL and AF AGUANTA. 2005. Nota sobre un nuevo registro de murciélago (Lampronycteris brachyotis) para Bolivia. Kempffiana 1:65-69. [ Links ]
2. ARNOLD ML, RJ BAKER, and RL HONEYCUTT. 1983. Genic differentiation and phylogenetic relationships within two New World bat genera. Biochemical Systematics and Ecology 11:295-303. [ Links ]
3. BAKER RJ, SR HOOFER, CA PORTER, and RA VAN DEN BUSSCHE. 2003. Diversification among New World leaf-nosed bats: An evolutionary hypothesis and classification inferred from digenomic congruence of DNA sequence. Occasional Papers, Museum Texas Tech University 230:1-32. [ Links ]
4. BERNARD E and MB FENTON. 2002. Species diversity of bats (Chiroptera: Mammalia) in forest fragments, primary forests and savannas in Central Amazonia, Brazil. Canadian Journal of Zoology 80:1124-1140. [ Links ]
5. BERNARD E, VC TAVARES, and E SAMPAIO. 2011a. Updated compilation of bat species (Chiroptera) for the Brazilian Amazonia. Biota Neotropica 11:1-12. [ Links ]
6. BERNARD E, LMS AGUIAR, and RB MACHADO. 2011b. Discovering the Brazilian bat fauna: A task for two centuries? Mammal Review 41(1):23-39. [ Links ]
7. BRONGNIART A. 1792. Catalogue de mammifères envoyés de Cayenne par M. le Blond. Actes Société d'Histoire Naturelle. Paris 1:115. [ Links ]
8. BROOKS RR, RD REEVES, AJM BAKER, JA RIZZO, and H DIAZ FERREIRA. 1990. The Brazilian Serpentine Plant Expedition (BRASPEX), 1988. National Geographic Research. 6:205-219. [ Links ]
9. CARTER CH, HH GENOWAYS, RS LORENGNARD, and RJ BAKER. 1981. Observations on bats from Trinidad, with a checklist of species occurring on the island. Mammalogy Papers: University of Nebraska State Museum. Paper 179:1-28. [ Links ]
10. CASTRO IJ. 2009. Assembleia de morcegos (Mammalia: Chiroptera) da Área de Proteção Ambiental do Rio Curiaú, Amapá. Master Thesis, Ecologia, Programa de Pós-Graduação em Biodiversidade Tropical, Universidade Federal do Amapá [ Links ].
11. CASTRO-ARELLANO I, SJ PRESLEY, LN SALDANHA, MR WILLIG, and JM WUNDERLE JR. 2007. Effects of reduced impact logging on bat biodiversity in terra firme forest of lowland Amazonia. Biological Conservation 138:269-285. [ Links ]
12. CASTRO-LUNA AA, VJ SOSA and G CASTILLO-CAMPOS. 2007. Bat diversity and abundance associated with the degree of secondary succession in a tropical forest mosaic in south-eastern Mexico. Animal Conservation 2:219-228. [ Links ]
13. CLARKE FM, DV PIO, and PA RACEY. 2005. A Comparison of logging systems and bat diversity in the Neotropics. Conservation Biology 19:1194-1204. [ Links ]
14. DOBSON GE. 1879. Notes on recent additions to the collection of Chiroptera in the Muséum d'Histoire Naturelle at Paris, with descriptions of new and rare species. Proceedings of the Zoological Society of London 1878:873-880. [ Links ]
15. ESTRADA CG, A DAMON, CS HERNÁNDEZ, LS PINTO and GI NÚNEZ. 2006. Bat diversity in montane rainforest and shaded coffee under different management regimes in southeastern Chiapas, Mexico. Biological Conservation 132:351-361. [ Links ]
16. FARIA D, B SOARES-SANTOS, and E SAMPAIO. 2006. Bats from the Atlantic rainforest of southern Bahia, Brazil. Biota Neotropica 6:2-13. [ Links ]
17. FAZZOLARI-CORRÊA S. 1995. Aspectos sistemáticos, ecológicos e reprodutivos de morcegos na Mata Atlântica. Doctoral Dissertantion, IB, Universidade de São Paulo, São Paulo. [ Links ]
18. GERALDES MP. 2005. Diversidade e estratificação altitudinal de conjuntos taxonômicos de morcegos na Mata Atlântica da Serra do Mar, São Paulo. Doctoral Dissertation Thesis, Universidade de São Paulo, São Paulo. [ Links ]
19. GIMENEZ EA and H FERRAREZZI. 2004. Diversidade de morcegos no sudeste da Mata Atlântica. Pp. 314-330, in: Estação Ecológica Juréia-Itatins: ambiente físico, flora e fauna (OAV Marques and W Duleba, eds.) Ribeirão Preto, SP. [ Links ]
20. HANDLEY Jr CO. 1967. Bats of the canopy of an Amazonian forest. Atas do Simpósio sobre a Biota Amazônica 5:211-215. [ Links ]
21. HANDLEY Jr CO. 1976. Mammals of the Smithsonian Venezuelan Project. Brigham Young University Science Bulletin. Biological Series 20:1-89. [ Links ]
22. HUSSON AM. 1978. Order Chiroptera. Pp. 80-86, in: The Mammals of Suriname (EJ Brill, ed.). Zoologische Monographieen van het Rijksmuseum van Natuurlijke Historie, Leiden, NED. [ Links ]
23. HUTTERER R, N VERHAAGH, J DILLER, and R PODLOUCKYL. 1995. An inventory of mammals observed at Panguana Biological Station, Amazon Peru. Ecotropica 11:3-20. [ Links ]
24. IRWIN AJ and PD TAYLOR. 2000. Evolution of dispersal in a stepping-stone population with overlapping generations. Theoretical Population Biology 58:321- 328. [ Links ]
25. JONES JK. 1966. Bats from Guatemala. University Kansas, Publications Museum Natural History 16:439-472. [ Links ]
26. LIM BK and MD ENGSTROM. 2001. Species diversity of bats (Mammalia: Chiroptera) in Iwokrama Forest, Guyana, and the Guianan subregion: Implications for conservation. Biodiversity and Conservation 10:613-657. [ Links ]
27. LOEHLE C. 2007. Effect of ephemeral stepping stones on metapopulations on fragmented landscapes. Ecological Complexity 4:42-47. [ Links ]
28. LOUZADA NSV, ACMM LIMA, LM PESSôA, JLP CORDEIRO, and LFB OLIVEIRA. 2015. New records of phyllostomid bats for the state of Mato Grosso and for the Cerrado of Midwestern Brazil (Mammalia: Chiroptera). Check List 11(3):1644. [ Links ]
29. MARCIENTE R and AM CALOURO. 2009. Mammalia, Chiroptera, Phyllostomidae, Lampronycteris brachyotis (Dobson, 1879): First record in Acre, Brazil. Check List. Journal of Species Lists and Distribution 5:886-889. [ Links ]
30. MARINKELLE CJ and A CADENA. 1972. Notes on bats new to the fauna of Colombia. Mammalia 36:49-58. [ Links ]
31. MARTINS ACM and E BERNARD. 2008. Inventários biológicos rápidos da fauna de morcegos de cinco localidades do Parque Nacional Montanhas do Tumucumaque, Amapá. Rapid Assessment Program Bulletin of Biological Assessment 48:59-65. [ Links ]
32. MARTINS ACM, E BERNARD, R GREGORIN, and WAS DA SILVA. 2011. Filling data gaps on the diversity and distribution of Amazonian bats (Chiroptera): The case of Amapá, easternmost Brazil. Sociedade Brasileira de Zoologia 28:177-185. [ Links ]
33. MARTINS FD, AF CASTILHO, J CAMPOS, FM HATANO, and SG ROLIM. 2012. Fauna da Floresta Nacional de Carajás, estudos sobre vertebrados terrestres. São Paulo, Nitro Imagens. 236 pp. [ Links ]
34. MIRANDA JMD, L ZAGO, F CARVALHO, MBG RUBIO, and IP BERNARDI. 2015. Morcegos (Mammalia: Chiroptera) da região do Médio Rio Teles Pires, Sul da Amazônia, Brasil. Acta Amazonica 45:89-100. [ Links ]
35. OLIVEIRA SF and KC FARIA. 2015. Extension of the known geographic distribution of Lampronycteris brachyotis (Dobson, 1879) (Mammalia, Chiroptera, Phyllostomidae): First records from the Cerrado of the Brazilian Midwest, in the state of Mato Grosso. Check List 11(3):1635. [ Links ]
36. PERACCHI AL and ST DE ALBUQUERQUE. 1993. Quirópteros do Município de Linhares, Estado do Espirito Santo, Brasil (Mammalia, Chiroptera). Revista Brasileira de Biologia 53:575-581. [ Links ]
37. PEREIRA MJR, JT MARQUES, and JM PALMEIRIM. 2010. Vertical stratification of bat assemblages in flooded and unflooded Amazonian forests. Current Zoology 56:469-478. [ Links ]
38. RICK AM. 1968. Notes on bats from Tikal, Guatemala. Journal of Mammalogy 49:516-520. [ Links ]
39. ROCHA PA, SF FERRARI, A FEIJÓ, and SF GOUVEIA. 2015. Zoogeography of South American forest-dwelling bats: Disjunct distributions or sampling deficiencies? PLoS ONE 10(7):e0133276. [ Links ]
40. SAMPAIO EM, EKV KALKO, E BERNARD, B RODRÍGUEZ-HERRERA and CO HANDLEY Jr. 2003. A Biodiversity assessment of bats (Chiroptera) in a tropical lowland rainforest of central Amazonia, including methodological and conservation considerations. Studies on Neotropical Fauna and Environment 38:1731. [ Links ]
41. SANBORN CC. 1949. Bats of the genus Micronycteris and its subgenera. Fieldiana Zoology 31:215-33. [ Links ]
42. SANTOS JR, M KEIL, LS ARAUJO, MS PARDI LACRUZ, JCS KRAMER, and O KANDLER. 2000. Biomass estimation of forest and savanna transition vegetation zone by JERS-1 and SIR-C backscatter data. International Congress for Photogrammetry and Remote Sensing, 19. Amsterdam, Netherlands. [ Links ]
43. SCULTORI C, D DIAS, and AL PERACCHI. 2009. Mammalia, Chiroptera, Phyllostomidae, Lampronycteris brachyotis (Dobson, 1879): First record in the state of Paraná, southern Brazil. Check List 5:872-875. [ Links ]
44. SIKES RS, WL GANNON, and THE ANIMAL CARE AND USE COMMITTEE OF THE AMERICAN SOCIETY OF MAMMALOGISTS. 2011. Guidelines of the American Society of Mammalogists for the Use of Wild Mammals in Research. Journal of Mammalogy 92:235-253. [ Links ]
45. SIMMONS NB. 2005. Order Chiroptera. Pp. 312-529, in: Mammal Species of the World: A Taxonomic and Geographic Reference (DE Wilson and DM Reeder, eds.). Johns Hopkins University Press, Baltimore, OH. [ Links ]
46. SIMMONS NB and RS VOSS. 1998. The mammals of Paracou, French Guiana: A Neotropical lowland rainforest fauna, Part 1. Bats. Bulletin of the American Museum of Natural History 237:1-219. [ Links ]
47. SOLARI S, V PACHECO, and E VIVAR. 1999. Nuevos registros distribucionales de murciélagos peruanos. Revista Peruana de Biologia 6:152-159. [ Links ]
48. TADDEI VA and WA PEDRO. 1996. Micronycteris brachyotis (Chiroptera, Phyllostomidae) from the state of São Paulo, Brazil. Revista Brasileira de Biologia 56:217-222. [ Links ]
49. TIRIRA DG, CE BOADA, and SF BURNEO. 2010. Mammalia, Chiroptera, Phyllostomidae, Lampronycteris brachyotis (Dobson, 1879): First confirmed record for Ecuador. Check List 6:237-238. [ Links ]
50. VELOSO HP, ALR RANGEL-FILHO, and JCA LIMA. 1991. Classificação da vegetação brasileira, adaptada a um sistema universal. MEFP/IBGE/DRNEA, Rio de Janeiro. [ Links ]
51. VIZOTTO LD and VA TADDEI. 1973. Chave para determinação de quirópteros brasileiros. Revista da Faculdade de Filosofia, Ciências e Letras de São José do Rio Preto, Boletim de Ciências 1:1-72. [ Links ]
52. WEINBEER M and EKV KALKO. 2004. Morphological characteristics predict alternate foraging strategy and microhabitat selection in the orange-bellied bat, Lampronycteris brachyotis. Journal of Mammology 85:1116-1123. [ Links ]
53. WETTERER AL, MV ROCKMAN, and NB SIMMONS. 2000. Phylogeny of phyllostomid bats (Mammalia, Chiroptera): Data from diverse morphological systems, sex chromosomes, and restriction sites. Bulletin of the American Museum of Natural History 248:1-200. [ Links ]
54. WILLIAMS SL and HH GENOWAYS. 2007 [2008]. Subfamily Phyllostominae Gray, 1825. Pp. 255-300, in: Mammals of South America (AL Gardner, ed.). Volume 1: Marsupials, Xenarthrans, Shrews, and Bats. Chicago and London: The University of Chicago Press, Chicago, IL. [ Links ]














