Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Mastozoología neotropical
versión impresa ISSN 0327-9383versión On-line ISSN 1666-0536
Mastozool. neotrop. vol.23 no.2 Mendoza dic. 2016
ARTÍCULO
Small mammals and microhabitats in Araucaria forests of Neuquén, Argentina
John D. Shepherd and Rebecca S. Ditgen
Biology Department, Mercer University, Macon, GA 31207, USA [Correspondence: <Shepherd_JD@mercer.edu>]
Recibido 6 noviembre 2015.
Aceptado 12 abril 2016.
Editor asociado: ML Guichón
ABSTRACT.
We used mark-recapture techniques to sample small mammals in two Araucaria araucana-Nothofagus forest plots at dry and mesic ends of a moisture gradient in Neuquén, Argentina. In summer and fall trapping sessions from 2004 to 2007, we had 678 captures (323 individuals) of 6 species in a total of 5300 trap nights. Abrothrix hirta (64% of captures) was captured in every trapping session, but Oligoryzomys longicaudatus (26%) was trapped only in the fall. Both species had lower body weights when Araucaria seed crop was very small. O. longicaudatus appears to migrate into Araucaria forest from other habitats to exploit the autumn seed fall. We measured 9 canopy and 19 understory variables around 100 trap sites and used factor analysis to identify 4 canopy and 6 understory factors in each forest. We used regression to model capture-vegetation relationships. Features of the understory had greater influence than did the canopy. Capture-vegetation models were more complex in the moist forest than in the dry forest. In the moist forest, more A. hirta and O. longicaudatus were caught in patches of bamboo (Chusquea culeou), and away from grass and open areas, but these species differed in capture rates in other kinds of understory vegetation. There was less overlap between species’ microhabitats in the dry forest. More individuals of both species were caught away from patches of fallen logs, but A. hirta and O. longicaudatus, responded differently to other features of the understory. Spatial, temporal and behavioral differences in the way A. hirta and O. longicaudatus use these forests and its Araucaria seed falls promote coexistence and community complexity.
RESUMEN.
Pequeños mamíferos y microhábitats en bosques de Araucaria de Neuquén, Argentina.
Utilizamos métodos de marcado-captura-recaptura para muestrear pequeños mamíferos en un bosque húmedo y un bosque seco de Araucaria araucana-Nothofagus en Neuquén, Argentina. Durante 4 años, en verano y otoño, se realizaron 678 capturas (323 individuos) de 6 especies en un total de 5300 trampa noches. Abrothrix hirta (64% de capturas) se capturó en cada trampeo, pero Oligoryzomys longicaudatus (26%) se capturó solo en otoño. Ambas especies de ratones presentaron menor peso cuando la producción de semillas de Araucaria fue muy baja. O. longicaudatus parece migrar de otros hábitats hacia los bosques de Araucaria para explotar la caída de semillas en otoño. Medimos 9 variables del dosel y 19 variables del sotobosque alrededor de 100 sitios de trampa y utilizamos análisis factorial para identificar 4 factores propios del dosel y 6 factores propios del sotobosque en cada bosque. Utilizamos modelos de regresión para encontrar relaciones entre las capturas y los factores de vegetación. Las tasas de captura estuvieron más influidas por características del sotobosque que por las del dosel. Los modelos de captura-vegetación resultaron más complejos en el bosque húmedo que en el bosque seco. En el bosque húmedo, más individuos de A. hirta y O. longicaudatus fueron capturados en parches de caña, y lejos de pasto y zonas abiertas, pero estas especies difieren en las tasas de captura en otros tipos del sotobosque. Hubo menos solapamiento entre microhábitats en el bosque seco. Ambas especies fueron capturadas más frecuentemente lejos de parches de troncos caídos, pero A. hirta y O. longicaudatus respondieron de manera diferente a otras características del sotobosque. Diferencias espaciales, temporales y de comportamiento en la manera de uso de estos bosques por A. hirta y O. longicaudatus promueven la coexistencia y la complejidad de la comunidad.
Key words: Abrothrix hirta; Araucaria araucana; Oligoryzomys longicaudatus.
Palabras clave: Abrothrix hirta; Araucaria araucana; Oligoryzomys longicaudatus.
INTRODUCTION
The small mammal fauna of temperate Andean forests has both high diversity and endemism (Pearson and Pearson, 1982; Pearson, 1983). Even though medium-sized species are lacking, richness equals that of other temperate and even tropical forests. This fauna has been the subject of numerous studies in broadleaf forests of southern beech (Nothofagus spp.) and in Valdivian rainforests. Previous work has focused on biodiversity and biogeography (Pearson and Pearson, 1982; Pearson, 1983); distribution along elevational and ecological gradients (Patterson et al., 1989; Kelt, 1996); demography (Murúa et al., 1986, 1987; González et al., 1989; Meserve et al., 1991, 1999; Polop et al., 2010); diet (Murúa et al., 1980; Murúa and González,1981; Meserve et al., 1988; Polop et al., 2014a, 2015); autoecology (Pearson, 1984, 1995; Kelt, 1994); habitat associations (Murúa and González, 1982; Patterson et al., 1990; Kelt et al., 1994, 1999; Guthmann et al., 1997; Lozada and Guthmann, 1998; Lozada et al., 2000; Piudo et al., 2005); and epidemiology (Piudo et al., 2011; Andreo et al., 2012, 2014).
Much less attention has been paid to small mammals in forests of the ancient conifer, Araucaria araucana (Araucariaceae). This dominant forest tree is an Andean endemic classified as Vulnerable in 2005 and now Endangered because of poor regeneration and continued decline under pressure from fire, logging, seed harvest, and overgrazing (Hechenleitner et al., 2005; Premoli et al., 2015). Small mammals are predators of Araucaria seeds (Sanguinetti and Kitzberger, 2009, 2010) and play a key role in seed (piñón) dispersal (Shepherd and Ditgen, 2013). They are also a sensitive indicator of human impacts because their populations respond to changes in the structure of the forest understory (Shepherd and Ditgen, 2005). However, little is known about community dynamics and how the vegetation affects these mammals. This study examines the dynamics of the small mammal assemblage and the microhabitat associations of the two most common species in two Araucaria-Nothofagus forests.
METHODS
Study site
This study was conducted in mixed forests of A. araucana, Nothofagus pumilio, and N. antartica (Fagaceae) at an elevation of 1200 m a.s.l. in southwestern Neuquén Province in Parque Nacional Lanín (Argentina) at its border with Parque Nacional Villarica (Chile). This forest is protected from most human disturbance because of its location on an international border between customs and immigration posts. As a result, there are no livestock and there is no firewood collection, but some human harvesting of seeds is permitted (Shepherd and Ditgen, 2005). Populations of feral exotic species (especially wild boar, Sus scrofa) impact the forest and compete for seeds with small mammals (Sanguinetti and Kitzberger, 2010).
The study area was less than 7 km northeast of the summit of Volcan Lanín. At this site, A. araucana had peak mast years of seed production in 2000 (>30 mean cones per tree), 2007 (35 cones/tree) and 2013 (about 50 cones/tree) (Sanguinetti and Kitzberger, 2008; Sanguinettii 2014). This study was conducted during years of very low (2005, 2 cones/ tree), intermediate (2004, 2006; 11-18 cones/tree), and masting (2007, 35 cones/tree) seed production (Sanguinettii, 2014).
We trapped in two areas that differed in forest structure and physical environment. Although they were only about 0.5 km apart, they were separated by a road and shrubby non-forest. One area (“MOIST FOREST”: 39.58140º S, 71.45983º W) was pro tected on the north and west by an older volcanic ridge. Topsoil was dark brown and a small stream flowed near its edge. The canopy was a mixture of A. araucana and N. pumilio, and the understory contained large clumps of bamboo (Chusquea culeou). An earlier study found six species of small mammals in the understory of this forest (Shepherd and Ditgen, 2005). In a second area (“DRY FOREST”: 39.58427º S, 71.45979º W) closer to the volcano’s steep slopes, there were swaths of loose, unvegetated, sand-to-pebble-sized volcanic sediments. This area contained more grassy areas (Festuca pallescens) and dense thickets of N. antarctica.
Trapping
In each forest we established 10 x 10 grids of 100 traps 10 m apart. Each grid consisted of 25 large (model XLF15) and 75 small (model LFATDG) Sherman live traps, with every other row containing alternating large and small traps. Large traps were included because of the presence of Norway rats (Rattus norvegicus), but an earlier study (Shepherd and Ditgen, 2005) found no difference in trap success for the two different trap sizes. The Moist Forest was trapped in the summer (February) and during the autumn Araucaria seed fall (late March-early May) from 2004 until 2007, except in the summer of 2005 when circumstances prevented trapping (seven trapping sessions). The Dry Forest was trapped from the fall of 2005 to the fall of 2007 (five trapping sessions).
Traps were baited with a mixture of rolled oats and ground peanuts and checked in the early morning. Trapped animals were identified to species, weighed and measured, marked with a numbered stainless steel ear tag (model 1005-1, National Band and Tag Company, Newport, KY, USA), and released at their capture location. Trapping continued for five nights, except when disrupted by snowfall or vandalism. We report the number of different individuals caught per 100 trap nights as a measure of relative abundance.
For each recaptured animal, we calculated the distance between capture sites. As a measure of movement, we recorded the maximum distance between traps at which an animal was captured. We used a multi-way, main-effects ANOVA to test for the influence of forest type, year, season, sex, and number of captures on weights and maximum distance moved. This conservative analysis ignores interactions between factors, which would be better studied with a more balanced sampling design.
We calculated the correlation between the number of individuals captured per 100 trap nights and published seed crop estimates (Sanguinetti, 2014). Since small mammal populations are widely reported to increase in response to more abundant food (Ostfeld and Keesing, 2000), we would expect positive correlation of autumn seed crop and spring mammal abundance so we calculated one-tailed probabilities from a t-test.
Vegetation sampling
The vegetation of each forest was sampled with 144 circular quadrats (5 m radius) in a 12 x 12 grid centered on the area trapped. Each trapping location was the center of a quadrat. Within the quadrat, the circumference of all trees (≥ 10 cm diameter 1.5 m from ground) was measured and recorded by species. We also counted saplings (< 10 cm diameter 1.5 m from ground) by species and measured circumferences of dead trees without recording species. We recorded the diameter and length of fallen logs greater than 10 cm diameter and calculated the cylindrical volume of each log.
Ground cover was sampled with four 5-meter line segments oriented 45º from the lines of travel between quadrats. Ten sample points were located on each line segment, every 50 cm from the quadrat center. At each sample point, we recorded the presence of woody and herbaceous species, as well as logs and coarse woody debris. Leaf litter was only recorded at points without vegetation cover, coarse woody debris or logs. Preliminary observations suggested that some species might have particular ecological significance because they provided dense cover (C. culeou) or occupied more open areas (Festuca pallescens). Other species (Adenocaulon chilense, Lathyrus magellanicus, Vicia nigricans) were common in disturbed areas like those created by foraging wild boar. These and tree seedlings were recorded by species while all others were recorded as “herb” or “shrub.”
Microhabitat analysis
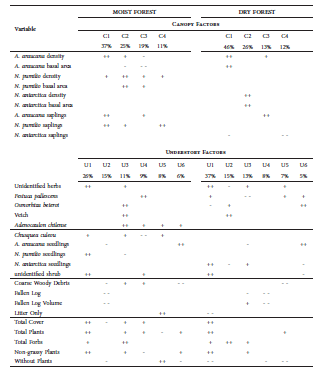
For each grid, tree and sapling counts were log transformed; understory percentage cover estimates were normalized with an arcsine square root transformation. Estimates were expressed as z-scores for each variable. We separated vegetation data into 9 canopy and 19 understory variables (Table 4). Factor analysis (Statistica 7.0, Statsoft Corporation) combined correlated variables into orthogonal components of canopy and understory variation. Because small mammals could be expected to respond to both major and minor vegetation components, we extracted 4 canopy and 6 understory factors, which accounted for the majority of cumulative variation in the data. We used variables significantly correlated with each vegetation factor/component to describe it.
Table 4 Results of factor analysis for canopy and understory vegetation in the Moist and Dry Araucaria araucana Forests. Each Canopy (C1-C4) and Understory factor (U1-U6) is listed with the percentage variance explained. Blanks indicate absence of variable or no significant correlation. Symbols indicate significant correlation of variable with factor (p < 0.05, df = 99): +, - indicate 0.195 < r < 0.600; ++, - - indicate r > 0.600
We regressed traps’ capture counts on traps’ surrounding vegetation to identify the effect of habitat relationships. For the two most common species, we used Poisson regression for the two trapping grids. We treated all canopy and understory vegetation components as potential predictors of small mammal captures, using the full model (with 10 vegetation parameters) as our null hypothesis. Because of its possible confounding effects, we examined overdispersion using the complete Poisson model (Cameron and Trivedi, 1990, 2005) within the R package AER (Kleiber and Zeileis, 2015), which measures overdispersion with the α statistic and provides a formal test. We also considered negative binomial (Linden and Mantyniemi, 2011) and zero-inflated models (Agarwal et al., 2002; Martin et al., 2005) as alternative model classes that might better fit overdispersed data. The added logistic component of the zero-inflated models accounts for the “extra” zeroes in the data and is reported separately in the results.
We used R (3.1.2) to find the best model in each of four model classes (Poisson, P; negative binomial, NB; zero-inflated Poisson, ZIP; zero-inflated negative binomial, ZINB), by eliminating vegetation parameters from the full model until we arrived at a subset of vegetation components with the lowest corrected Akaike Information Criterion (AICc) (Burnham et al., 2011; Cooch and White, 2014). We similarly selected the best overall model by comparing the best models in each model class.
RESULTS
While both forests had about the same density of A. araucana, the standing crop of trees (basal area) in the Moist Forest was 38% more than that of the Dry Forest (Table 1). With more than twice the saplings and 3 times the seedling cover, Araucaria reproduction was also much higher in the Moist Forest. Lenga (N. pumilio) was 10% of the trees in the Moist Forest; small, often shrubby, ñire (N. antarctica) made up almost half of the trees in the Dry Forest. The more closed Moist Forest understory had more dense patches of bamboo (C. culeou), Araucaria saplings and Araucaria seedlings. Nearly half of the more open Dry Forest understory was covered by coirón (F. pallescens), which made up less than 20% of the Moist Forest understory. The cover and total volume of fallen logs were higher in the Moist Forest.
Table 1 Composition and structure canopy and understory vegetation of two Araucaria araucana-Nothofagus forests in Parque Nacional Lanín, Neuquén, Argentina. Canopy tree and sapling densities were measured as stems/ ha, and basal area (stem cross section 1.5 m from ground) as dm2/ha. Understory fallen log volume was estimated in dm3; all other understory variables were measured as percent cover.

Small Mammals
We captured 323 individuals of six species in a total of 5300 trap nights in both forests (Table 2, Fig. 1). The most common species, Abrothrix hirta, was the only one captured in all trapping sessions. Oligoryzomys longicaudatus, the second most common species, was never caught in the summer (2500 trap nights), but was caught during every fall trapping session (2800 trap nights). Chelemys macronyx was caught about equally in the two forests, but Loxodontomys micropus and Abrothrix olivaceous were caught much more often in the Moist Forest and the Dry Forest respectively. Rattus norvegicus was caught in very low numbers. Total captures and trap success were higher in the Moist Forest than in the Dry Forest. Seasonal increases in captures (from summer to fall) were due largely to the appearance of O. longicaudatus; seasonal and annual variation in captures was much higher for O. longicaudatus than for A. hirta. The relatively large numbers of A.hirta and O. longicaudatus captured allowed statistical analyses for these species that were not possible for the four species caught less often.
Table 2 Small mammal captures in live trapping of the Moist and Dry Araucaria araucana Forests from summer 2004 to fall 2007. Table lists all captures, including recaptures. Percentage recaptures is the proportion of individuals of species that were recaptured at least once. Overall trap success for each forest is measured as captures per 100 trap nights.


Fig. 1. Individual small mammals captured per 100 trap nights in the Moist and Dry Araucaria araucana Forest grids in the summer (S) and fall (F) during four years in Lanín National Park, Neuquén, Argentina. Dashed line shows fall seed production (cones/tree) from Sanguinetti (2014). A.h.: Abrothrix hirta, O.l.: Oligoryzomys longicaudatus, OTHER: Loxodontomys micropus, Abrothrix olivaceus, Chelemys macronyx, and Rattus norvegicus.
We had hypothesized that captures would be positively correlated with current or previous Araucaria seed production, but this was not supported by our results (Fig. 1). Fall captures of A. hirta were not significantly correlated with the current (r = -0.67, n = 7, p = 0.051) or previous (r = 0.63, n = 7, p = 0.064) seed crops; summer samples were too few to make correlation useful. Likewise, O. longicaudatus fall captures were not correlated to the current (r = 0.29, n = 7, p = 0.263) or previous (r = 0.29, n = 7, p = 0.270) Araucaria seed crops.
The year of capture affected individual weights of A. hirta and O. longicaudatus, primarily because weights were lower in the intermast year 2005 than in other years (Table 3). Weights were not significantly different in the two forests. Males were heavier in O. longicaudatus, but not in A. hirta. There was a small seasonal weight increase in A. hirta.
Table 3 Results of main effects ANOVA on the weights of Abrothrix hirta and Oligoryzomys longicaudatus in the Moist and Dry Araucaria araucana Forests. Weights are given for variables in which there was a significant difference. For both species, Bonferroni post hoc comparison showed that 2005 was different from other years (p < 0.01).

For A. hirta, the maximum distance moved by an individual was 100 m during a trapping period, as much as 92 m in a single night, and 15% of recaptured individuals were only caught at one trap site. Oligoryzomys longicaudatus moved as much as 70 m during a trapping period, as much as 56 m in a single night, with only 9% of recaptures at only one trap site. With fewer than five recaptures each, L. micropus, A. olivaceous, and Chelemys macronyx had maximum measured move distances of 72 m, 54 m, and 14 m respectively.
For A. hirta, there was no significant effect of forest type (F1,111 = 0.36, p = 0.55), year (F3,111 = 0.35, p=0.79), season (F1,111 = 1.44, p = 0.23), or sex (F1,111 = 1.31, p=0.25) on the maximum distance an individual moved dur ing a trapping session. There was a significant effect for the number of nights an individual was captured (F3,111 = 9.19, p< 0.001), and a Bonferroni post hoc comparison showed this resulted from the difference between individuals captured twice and those captured more than twice (Fig. 2). Because O. longicaudatus was only trapped in the fall and there were fewer recaptures, only the effects of year and number of captures were tested . For this species, there was no significant effect of year (F3,35 = 1.60, p = 0.21) or number of captures (F2,35 = 0.90, p = 0.39) on maximum move distance (Fig. 2).

Fig. 2. Maximum distance (mean ± standard error) between capture sites for recaptured individuals in the Moist and Dry Araucaria araucana Forests. (A. hirta: n = 128; O. longicaudatus: n = 43).
Microhabitat
The extracted components of the factor analysis accounted for over 90% of variation in the canopy vegetation and 75-85% of variation in the understory of the two forests (Table 4). In both forests the first two canopy factors (C1, C2) were correlated with abundance of trees of the dominant A. araucana and then of the subdominant Nothofagus species. The fourth factor (C4) in both cases accounted for variation in the density of Nothofagus saplings. Measures of overall plant cover were the most important component (U1) of both understories. Fallen logs and areas of Araucaria seedlings contributed understory components in both forests. In the Moist Forest, a single factor (U4) separated areas of bamboo (C. culeou) from open areas of grass (F. pallescens).
Capture-vegetation relationships were best fit by a variety of model classes (Table 5): two Poisson models, two negative binomial models, and two zero-inflated Poisson models. Capture data itself varied greatly in the degree of overdispersion. Moist Forest models contained more (5 to 7) parameters than those for the Dry Forest (3 parameters). Models comprised 0-2 canopy components and 2-6 understory components.
Table 5 Models of captures regressed on vegetation components for two species in the Moist and Dry Araucaria araucana Forests. The best reduced and full models are shown for each species/season. The number of model parameters is shown along with the vegetation Canopy (Cx) and Understory (Ux) components (Table 3) included in the model. Below the models in each case, the results are reported for a test of overdispersion using the complete Poisson model; see text for explanation. (P: Poisson; NB: Negative Binomial; ZIP: Zero- Inflated Poisson; ZINB: Zero-Inflated Negative Binomial).

In the Moist Forest, both rodent species (Table 4, Fig. 3a) were caught more in bamboo away from grass (U4), away from open leaf litter (U5), and away from forb patches (U3). Only O. longicaudatus was caught more away from large Araucaria (C3) and away from logs (U2). Summer and fall A. hirta captures were similar: increased by Nothofagus saplings (C4), logs (U2), bamboo (U4), and Araucaria seedlings (U6); and decreased in grassy areas (U4) and open leaf litter (U5). For A. hirta in the fall (Fig. 3b), overall plant cover (U1) and forbs (U3) increased capture probability.

Fig. 3. Coefficient estimates for best models (Table 4) of captures and vegetation components in the Moist Forest. a. count model coefficients and b. zero-inflated logit model coefficients. Error bars show the 90% confidence interval.
In the Dry Forest, there was less overlap between the two species (Fig. 4). More O. longicaudatus were caught with higher plant cover (U1) and ñire seedlings (U6); fewer were caught among logs (U4), Araucaria seedlings and grass (U6). For A. hirta in the summer high densities of ñire saplings (C4) and high plant cover (U1) decreased both the number caught (Fig. 4a) and probability of capture (Fig. 4b). Coarse woody debris and low grass cover (U5) increased the number of captures. In the fall more A. hirta were caught where there was less Araucaria (C1), fewer logs (U4) and more coarse woody debris (U5).
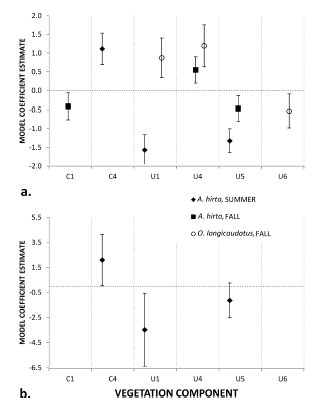
Fig. 4. Coefficient estimates for best models (Table 4) of captures and vegetation components in the Dry Forest. a. count model coefficients and b. zero-inflated logit model coefficients. Error bars show the 90% confidence interval.
Characteristics of capture sites of species with few captures in the Moist Forest indicated that C. macronyx were found in or adjacent to patches of bamboo and L. micropus were caught in a variety of understory vegetation. In the Dry forest, A. olivaceus, C. macronyx, L. micropus were all caught within and adjacent to patches of shrubby N. antarctica. Abrothrix olivaceus was also caught in more open grassy areas.
DISCUSSION
Small Mammals
The Araucaria forest species reported in this study are also found in Nothofagus forests (Pearson and Pearson, 1982) where they were characterized as forest species (A. olivaceus, C. macronyx), an invasive exotic (R. norvegicus) and three wide-ranging species (A. hirta, O. longicaudatus, L. micropus). Dromiciops gliroides was caught in the Moist Forest in 2002 and 2003 (Shepherd and Ditgen, 2005; Ditgen, unpublished), but was not seen in the 4 years of this study.
In Valdivian forest, low over-winter survival extinguished local O. longicaudatus populations or left them near-zero in the spring (Murúa et al., 1986; Meserve et al., 1991), but reproduction by immigrants allowed rapid recovery. At the forest-steppe ecotone, its populations had a high coefficient of variation and short residence time (Guthmann et al., 1997). This life history makes its populations potentially irruptive (Kelt, 1994; Meserve et al., 1991; Sage et al., 2007). In this study, fall captures varied 8- to 10-fold from year to year.
Oligoryzomys longicaudatus has been described as a dietary opportunist (Polop et al., 2014a, 2015), a seed-eating species (Meserve et al., 1988; González et al., 1989) and an Araucaria seed predator (Shepherd and Ditgen, 2013), so we might expect it to respond to changes in seed crops. Elsewhere population peaks were a year after bumper seed crops (González et al., 1989; Gallardo and Mercado, 1999). Lower body weight during a year of few Araucaria seeds suggests an immediate effect of seed abundance on body condition, but capture rates were not correlated with current or previous seed crops. In this same area, the synchronous masting of A. araucaria and C. coleou did not produce the expected rodent population explosion (Guichón et al., 2014). This lack of response to changing resources awaits further study.
Abrothrix hirta is the most common species in these forests and in other forests of the southern Andes (Pearson, 1983, 1995; Meserve et al., 1991). Elsewhere, A. hirta has high survival rates (Meserve et al., 1991) and relatively stable intra-annual populations (Kelt, 1996; Guthmann et al., 1997; Meserve et al., 1999). Our study showed a maximum of 3-fold seasonal and annual variation. While its capture rates were not correlated with Araucaria seed production, the influence of the seed availability was seen in the lower average weights during an intermast year, and slightly higher fall weights.
Differing population dynamics for A. hirta and O. longicaudatus may reflect contrasting diets and behavioral niches. In Nothofagus forests, seed-eating O. longicaudatus persists through patch population dynamics, exploiting locally ephemeral resources in heterogeneous habitat with its high mobility and local migration (Murúa et al., 1986; Meserve et al., 1991). Our results suggest summer seed supplies in Araucaria forests may be inadequate to support this species. Oligoryzomys longicaudatus may move into Araucaria forest from nearby habitats (e.g.: meadows, N. pumilio-N. nervosa forest, N. antarcticathickets) in the fall to take advantage of a locally abundant, but temporally isolated, seed source. This hypothesis awaits confirmation. In contrast, A. hirta is an omnivore in temperate Chilean rainforest with seeds a small part of its diet (Meserve et al., 1988) and is an arthropod predator at the forest-steppe ecotone (Polop et al., 2015). It is the dominant Araucaria seed consumer in these forests and extends seed availability by scatterhoarding (Shepherd and Ditgen, 2013). This omnivore’s shifting-diet lifestyle does not require inter-habitat mobility and allows more stable local populations.
The average maximum move distances (40+ m for A. hirta and 20+ m for O. longicaudatus) are consistent with what is known of their handling of Araucaria seeds. Abrothrix hirta carries seeds to many small caches, some of which are 40 m from seed sources (Shepherd and Ditgen, 2013); increasing short term movement within the trapping period may reflect more wide-ranging movement among burrows, seeds, and caches within its home range. Oligoryzomys longicaudatus consumes seeds in situ and moved seeds only an average of 7.9 m (Shepherd and Ditgen, 2013). This non-resident fall visitor moves seeds short distances and consumes them before moving to find more seeds. Its low recapture rate may result from longer movements between habitat patches. Movement distances we measured are consistent with home ranges measured for O. longicaudatus (0.073 to 0.253 ha, Murúa et al., 1986) and for 20-40 g rodents (0.12 to 0.81 ha, Harestad and Bunnell, 1979).
Microhabitat Associations
The degree of overdispersion affected selection of capture-vegetation regression models. The least overdispersed capture data were best fit with simple Poisson regressions, while more strongly overdispersed data required negative binomial or zero-inflated Poisson models. These results caution against a priori selection of a single analytical model.
Components of the understory vegetation were more important than features of the canopy in explaining capture success. The importance of statistically minor components of the vegetation (those that explain a relatively small portion of overall variability) in all the models indicates that these species exploit subtle differences in microhabitat. The Moist Forest had a more closed, spatially heterogeneous, understory with intermingled patches of bamboo, grass, and Araucaria seedlings. The Dry Forest had dense clumps of shrubby N. antarctica, but larger open areas of grass, leaf litter and woody debris. More complex models (i.e., with more parameters) in the Moist Forest may simply reflect its greater structural and spatial complexity.
Abrothrix hirta and O. longicaudatus are both wide-ranging species, found in a variety of forest and steppe-edge habitats (Pearson, 1983, 1995; Kelt, 1994, 1996). Abrothrix hirta was more abundant in shorter forests at higher elevations in a Chilean Valdivian-N. dombeyi forest, while O. longicaudatus appeared equally at all elevations (Patterson et al., 1989). Along the same gradient, these species overlapped greatly in terms of the habitat characteristics of capture sites (Patterson et al., 1990). Oligoryzomys longicaudatus was most abundant in Nothofagus forests in wet years, but in brushlands in a dry year (Andreo et al., 2012). Oligoryzomys longicaudatus and A. hirta were abundant in the small mammal assemblages of shrubland and Nothofagus forest, but much less common in open pasture (Polop et al., 2014b).
While they broadly overlap in habitat use at a coarse scale, these species appear to select microhabitats non-randomly at a finer scale (Patterson et al., 1990; Kelt et al., 1999). Discriminant analysis separated A. olivaceous and O. longicaudatus with structural habitat variables that affected visibility from above and from the side (Murúa and González, 1982). For a pre-cordilleran Valdivian forest, Kelt et al. (1994) found that A. hirta and O. longicaudatus were two of the most similar species, separated only by the preference of A. hirta for higher overall cover and areas of more shrubs. At a forest-steppe ecotone they had similar associations with shrub cover, but the preference of O. longicaudatus was significantly stronger than that of A. hirta (Lozada et al., 2000). Some vegetation characteristics (cover of understory grasses, shrubs, and vascular plants) were important at a fine scale in our study, but were not significant in separating the species along an elevation gradient (Patterson et al., 1989).
We found overlap in microhabitat use by these two species in their apparent preference for the dense cover of bamboo and avoidance of grassy, open areas. Nonetheless, A. hirta preferred the dense cover of bamboo, but not dense patches of shrubs; O. longicaudatus preferred both. Abrothrix hirta used fallen logs much more than O. longicaudatus. Abrothrix hirta used Araucaria seedlings, N. pumilio saplings, and coarse woody debris; O. longicaudatus was found among N. pumilio trees, but avoided patches of forbs. Their use of these forests was broadly similar, but subtly different.
Since A. hirta is the most important seed harvester in these forests (Shepherd and Ditgen, 2013), we thought it might use its environment differently when rich patches of Araucaria seeds appear on the forest floor. There is little support for this idea in our results. The density and size of Araucaria trees had a negative effect on summer captures in the Moist Forest and on fall captures in the Dry Forest. There were few other seasonal differences. Seed predation by wild boar in areas with less dense cover (Sanguinetti and Kitzberger, 2010) may simply reinforce a preference for dense cover.
We have shown that details of the structure and composition of the forest understory affect the distribution of the two most common small mammals. Anecdotal evidence suggests the same is true for the less common species. Because domestic livestock and feral exotic species can severely alter this environment (Shepherd and Ditgen, 2005; Sanguinetti and Kitzberger, 2010), conservation of these species and their role in in the Araucaria forest ecosystem (Shepherd and Ditgen, 2013) will be enhanced by maintenance of intact forest understories.
ACKNOWLEDGEMENTS
We thank the Delegación Regional of Administración de Parques Nacionales for permission to work in Parque Nacional Lanín. The Biology Department of Mercer University provided equipment and logistic support. Some financial support was provided by the Fulbright Scholarship Program. Javier Sanguinetti and anonymous reviewers helped improve earlier versions of the manuscript.
LITERATURE CITED
1. AGARWAL DK, AE GELFAND, and S CITRON-POUSY. 2002. Zero-inflated models with application to ecological count data. Environmental and Ecological Statistics 9:341-355. [ Links ]
2. ANDREO V, M NETELER, D ROCCHINI, C PROVENSAL, S LEVIS, X PORCASI, A RIZZOLI, M LANFRI, M SCAVUZZO, N PINI, D ENRIA, and J POLOP. 2014. Estimating hantavirus risk in southern Argentina: A GIS-based approach combining human cases and host distribution. Viruses 6:201-222. [ Links ]
3. ANDREO V, C PROVENSAL, S LEVIS, N PINI, D ENRIA, and J POLOP. 2012. Summer-autumn distribution and abundance of the hantavirus host, Oligoryzomys longicaudatus, in northwestern Chubut, Argentina. Journal of Mammalogy 93(6):1559-1568. [ Links ]
4. BURNHAM KP, DR ANDERSON, and KP HUYVAERT. 2011. AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behavioral Ecology and Sociobiology 65:23-35. [ Links ]
5. CAMERON AC and PK TRIVEDI. 1990. Regression-based Tests for Overdispersion in the Poisson Model. Journal of Econometrics 46:347-364. [ Links ]
6. CAMERON AC and PK TRIVEDI. 2005. Microeconometrics: Methods and Applications. Cambridge University Press. [ Links ]
7. COOCH E and G WHITE. 2014. Program Mark – A gentle introduction, 13th Edition. http://www.phidot.org/software/mark/docs/
8. GALLARDO MH and CL MERCADO. 1999. Mast seeding of bamboo shrubs and mouse outbreaks in southern Chile. Mastozoología Neotropical 6(2):103-111. [ Links ]
9. GONZÁLEZ L, R MURÚA, and C JOFRÉ. 1989. The effect of seed availability on population density of Oryzomys in southern Chile. Journal of Mammalogy 70(2):401-403. [ Links ]
10. GUICHÓN ML, FA MILESI, M MONTEVERDE, L PIUDO, and J SANGUINETTI. 2014. Efectos de la floración masiva de caña colihue (Chusquea culeou) y la superproducción de semillas de araucaria (Araucaria araucana) a diferentes niveles de la trama trófica. Informe Final. [ Links ]
11. GUTHMANN N, M LOZADA, JA MONJEAU, and KM HEINEMANN. 1997. Population Dynamics of five sigmodontine rodents of northwestern Patagonia. Acta Theriologica 42(2):143-152 [ Links ]
12. HARESTAD AS and FL BUNNELL. 1979. Home range and body weight – A reevaluation. Ecology 60(2):389-402.
13. HECHENLEITNER P, MF GARDNER, PI THOMAS, C ECHEVERRÍA, B ESCOBAR, P BROWNLESS, and C MARTÍNEZ. 2005. Plantas amenazadas del centro-sur de Chile. Distribución, conservación y propagación. Primera Edición. Universidad Austral de Chile and Royal Botanical Garden of Edinburgh. 188 pp. [ Links ]
14. KELT DA. 1994. The natural history of small mammals from Aisén Region, southern Chile. Revista Chilena de Historia Natural 67:183-207. [ Links ]
15. KELT DA. 1996. Ecology of small mammals across a strong environmental gradient in southern South America. Journal of Mammalogy 77(1):205-219. [ Links ]
16. KELT DA, PL MESERVE, and BK LANG. 1994. Quantitative habitat associations of small mammals in a temperate rainforest in southern Chile: The importance of ecological scale. Journal of Mammalogy 75:890-904. [ Links ]
17. KELT DA, PL MESERVE, BD PATTERSON, and BK LANG. 1999. Scale dependence and scale independence in habitat associations of small mammals in southern temperate rainforest. Oikos 85(2):320-334. [ Links ]
18. KLEIBER C and A ZEILEIS. 2015. AER: Applied Econometrics with R. R package version 1.1, http://CRAN.R-project.org/package=AER. [ Links ]
19. LINDÉN A and S MÄNTYNIEMI. 2011. Using the negative binomial distribution to model overdispersion in ecological count data. Ecology 92(7):1414-1421. [ Links ]
20. LOZADA M and N GUTHMANN 1998. Microhabitat selection under experimental conditions of three sigmodontine rodents. Ecoscience 5(1):51-55. [ Links ]
21. LOZADA M, N GUTHMANN, and N BACCALA. 2000. Microhabitat selection of five sigmodontine rodents in a forest-steppe transition zone in northwestern Patagonia. Studies on Neotropical Fauna and Environment 35(2):85-90. [ Links ]
22. MARTIN TG, BA WINTLE, JR RHODE S , PM KUHNERT,SA FIELD, SJ LOW-CHOY, AJ TYRE, and HP POSSINGHAM. 2005. Zero tolerance ecology: Improving ecological inference by modelling the source of zero observations. Ecology Letters 8:1235-1246. [ Links ]
23. MESERVE PL, BK LANG, R MURÚA, A MUÑOZ-PEDREROS, and LA GONZÁLEZ. 1991. Characteristics of a terrestrial small mammal assemblage in a temperate rainforest in Chile. Revista Chilena de Historia Natural 64:157-169. [ Links ]
24. MESERVE PL, BK LANG, and BD PATTERSON. 1988. Trophic relationships of small mammals in a Chilean temperate rainforest. Journal of Mammalogy 69:721- 730. [ Links ]
25. MESERVE PL, DR MARTÍNEZ, JR RAU, R MURÚA, BK LANG, and A MUÑOZ-PEDREROS. 1999. Comparative demography and diversity of small mammals in precordilleran temperate rainforests of southern Chile. Journal of Mammalogy 80(3):880-890. [ Links ]
26. MURÚA R and LA GONZÁLEZ. 1981. Estudios de preferencias y hábitos alimentarios en dos especies de roedores cricétido. Medios Ambiente 5:115-124. [ Links ]
27. MURÚA R and LA GONZÁLEZ. 1982. Microhabitat selection in two Chilean cricetid rodents. Oecologia 52:12-15 [ Links ]
28. MURÚA R, L GONZÁLEZ, and C JOFRÉ. 1980. Experimental food preferences of two southern Chilean rodents. Journal of Mammalogy 61:138-140. [ Links ]
29. MURÚA R, L GONZÁLEZ, and PL MESERVE. 1986. Population ecology of Oryzomys philippii (Rodentia: Cricetidae) in southern Chile. Journal of Animal Ecology 55(1):281-293. [ Links ]
30. MURÚA R, PL MESERVE, L GONZÁLEZ, and C JOFRÉ. 1987. The small mammal community of a Chilean temperate rain forest: Lack of evidence of competition between dominant species. Journal of Mammalogy 68(4):729-738. [ Links ]
31. OSTFELD RS and F KEESING. 2000. Pulsed resources and community dynamics of consumers in terrestrial ecosystems. Trends in Ecology and Evolution 15(6):232-237. [ Links ]
32. PATTERSON BD, PL MESERVE, and BK LANG. 1989. Distribution and abundance of small mammals along an elevational transect in temperate rainforests of Chile. Journal of Mammalogy 70(1):67-78. [ Links ]
33. PATTERSON BD, PL MESERVE, and BK LANG. 1990. Quantitative habitat associations of small mammals along an elevational transect in temperate rainforests of Chile. Journal of Mammalogy 71(4):620-633. [ Links ]
34. PEARSON OP 1983. Characteristics of a mammalian fauna from forests in Patagonia, southern Argentina. Journal of Mammalogy 64:476-492. [ Links ]
35. PEARSON OP. 1984. Taxonomy and natural history of some fossorial rodents of Patagonia, southern Argentina. Journal of Zoology 202(2):225-237. [ Links ]
36. PEARSON OP. 1995. Annotated keys for identifying small mammals living near Nahuel Huapi National Park and Lanín National Park, southern Argentina. Mastozoología Neotropical 2:99-148. [ Links ]
37. PEARSON OP and AK PEARSON. 1982. Ecology and biogeography of the southern rainforests of Argentina. Pp. 129-142, in: Mammalian biology in South America (MA Mares and HH Genoways, eds.). Volume 6. Special Publication Series, Pymatuning Laboratory of Ecology, University of Pittsburgh. [ Links ]
38. PIUDO L, MJ MONTEVERDE, S GONZALES CAPRIA, P PADULA, and CARMANCHAHI P. 2005. Distribution and abundance of sigmodontine rodents in relation to hantavirus in Neuquén, Argentina. Journal of Vector Ecology 30(1):119-25. [ Links ]
39. PIUDO L, MJ MONTEVERDE, RS WALKER, and RJ DOUGLASS. 2011. Rodent community structure and Andes virus infection in sylvan and peridomestic habitats in norwestern Patagonia, Argentina. Vector Borne Zoonotic Disease 11(3):315-24. [ Links ]
40. POLOP FJ, MC PROVENSAL, N PINI, SC LEVIS, JW PRIOTTO, D ENRÍA, G CALDERÓN, F COSTA, and J POLOP. 2010. Temporal and spatial host abundance and prevalence of Andes Hantavirus in Southern Argentina. Ecohealth 7:176-184. [ Links ]
41. POLOP FJ, L SEPÚLVEDA, A PELLIZA SBRILLER, J POLOP, and MC PROVENSAL. 2014a. Food habits of Oligoryzomys longicaudatus (Rodentia) in a steppe– forest transitional area of Argentinean Patagonia. Ecología Austral 24:304-310.
42. POLOP FJ, L SEPÚLVEDA, A PELLIZA SBRILLER, J POLOP, and MC PROVENSAL. 2014b. Spatial and temporal variation of terrestrial rodent assemblages in Cholila, Chubut Province, Argentina. Studies on Neotropical Fauna and Environment 49(2):151-157. [ Links ]
43. POLOP FJ, L SEPÚLVEDA, A PELLIZA SBRILLER, J POLOP, and MC PROVENSAL. 2015. Estructura de la dieta de roedores sigmodontinos en arbustales del ecotono bosque-estepa del suroeste de Argentina. Ecología Austral 24:304-310. [ Links ]
44. PREMOLI A, P QUIROGA, and M GARDNER. 2015. Araucaria araucana. The IUCN Red List of Threatened Species. Version 2015.2. <www.iucnredlist.org>. Downloaded on 25 August 2015. [ Links ]
45. SAGE RD, OP PEARSON, J SANGUINETTI, and A PEARSON. 2007. Ratada 2001: A rodent outbreak following the flowering of bamboo (Chusquea culeou) in southwestern Argentina. Pp 177-224, in: The Quintessential Naturalist: Honoring the Life and Legacy of Oliver P. Pearson (DA Kelt, EP Lessa, J Salazar-Bravo, and JL Patton, eds.). University of California Publications in Zoology 134:1-981. [ Links ]
46. SANGUINETTI J. 2014. Producción de semillas de Araucaria araucana (Molina K. Koch) durante 15 años en diferentes poblaciones del Parque Nacional Lanín (Neuquén-Argentina). Ecología Austral 24:265-275. [ Links ]
47. SANGUINETTI J and T KITZBERGER. 2008. Patterns and mechanisms of masting in the large-seeded southern hemisphere conifer Araucaria araucana. Austral Ecology 33:78-87. [ Links ]
48. SANGUINETTI J and T KITZBERGER. 2009. Efectos de la producción de semillas y la heterogeneidad vegetal sobre la supervivencia de semillas y el patrón espacio-temporal de establecimiento de plántulas en Araucaria araucana. Revista Chilena de Historia Natural 82:319-335. [ Links ]
49. SANGUINETTI J and T KITZBERGER. 2010. Factors controlling seed predation by rodents and non-native Sus scrofa in Araucaria araucana forests: Potential effects on seedling establishment. Biological Invasions 12:689-706. [ Links ]
50. SHEPHERD JD and RS DITGEN. 2005. Human use and small mammal communities of Araucaria forests in Neuquén, Argentina. Mastozoología Neotropical 12(2):217-226. [ Links ]
51. SHEPHERD JD and RS DITGEN. 2013. Rodent handling of Araucaria araucana seeds. Austral Ecology 38:23- 32. [ Links ]














