Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Mastozoología neotropical
Print version ISSN 0327-9383On-line version ISSN 1666-0536
Mastozool. neotrop. vol.24 no.2 Mendoza Dec. 2017
ARTÍCULO
Notes on the taxonomy of Calomys laucha (Rodentia, Cricetidae), with the designation of a neotype
Pablo Teta1, Raúl E. González-Ittig2, Enrique M. González3, Ulyses F. J. Pardiñas4, and Jorge Salazar-Bravo5
1 División Mastozoología, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Buenos Aires, Argentina. [Correspondence: Pablo Teta <antheca@yahoo.com.ar>]
2 Instituto de Diversidad y Ecología Animal (IDEA), CONICET-UNC and Facultad de Ciencias Exactas, Físicas y Naturales, Universidad Nacional de Córdoba, Córdoba, Argentina.
3 Museo Nacional de Historia Natural, Montevideo, Uruguay.
4 Instituto de Diversidad y Evolución Austral (IDEAus, CONICET), Puerto Madryn, Chubut, Argentina.
5 Department of Biological Sciences, Texas Tech University, Lubbock, Texas 79409, USA.
Recibido 29 noviembre 2016.
Aceptado 27 abril 2017.
Editor asociado: E Palma
ABSTRACT.
Calomys laucha (Fischer, 1814) is a small phyllotine rodent (<20 g), broadly distributed from northern Paraguay and southeastern Bolivia to southern Brazil, Uruguay and central Argentina. We provide an update on the genetic composition of this species. Our results support previous findings and indicate that C. laucha is composed of two main clades, one encompassing populations of southern Brazil, Uruguay and the Argentinean Mesopotamia, and another including those from the remainder of the distribution in Argentina, Bolivia and Paraguay. The boundary between these groups appears to converge along the Paraná-Paraguay rivers, which we presume act as a geographic barrier. As in other species of mammals described by Félix de Azara, the type locality of C. laucha is uncertain. Previous authors restricted it to eastern Paraguay based partially on historical misinterpretations. This restriction is inappropriate because currently only Calomys tener is recorded there. To stabilize the taxonomy of C. laucha we designate a neotype for the species, fixing with this act its terra typica to a locality in northern Buenos Aires province, in central-eastern Argentina.
RESUMEN.
Notas sobre la taxonomía de Calomys laucha (Rodentia, Cricetidae), con la designación de un neotipo.
Calomys laucha (Fischer, 1814) es un pequeño roedor filotino (<20 g), ampliamente distribuido desde el norte de Paraguay y el sureste de Bolivia hasta el sur de Brasil, Uruguay y el centro de Argentina. En esta contribución, ofrecemos una actualización sobre la composición genética de esta especie. Nuestros resultados apoyan hallazgos previos que indican que la especie está compuesta por dos clados principales, uno que abarca las poblaciones del sur de Brasil, Uruguay y la Mesopotamia argentina y otro que incluye las del resto de Argentina, Bolivia y Paraguay. Los límites entre ambos grupos parecen estar relacionados con los ríos Paraná- Paraguay, que actuarían como barrera geográfica. Al igual que en otras especies de mamíferos descritas por Félix de Azara, la localidad tipo de C. laucha es incierta. Autores previos la restringieron al este de Paraguay, basándose parcialmente en interpretaciones históricas erróneas. Esta restricción es inapropiada, porque según nuestro conocimiento actual, solo C. tener estaría presente allí. Para estabilizar la taxonomía de C. laucha hemos designado un neotipo para este taxón, fijando con este acto su terra typica a una localidad en el norte de la provincia de Buenos Aires, centro-este de Argentina.
Key words: Calomys tener; Félix de Azara; Phyllotini; Sigmodontinae.
Palabras clave: Calomys tener; Félix de Azara; Sigmodontinae; Phyllotini.
INTRODUCTION
Calomys laucha (Fischer, 1814) is a small phyllotine rodent (<20 g), widely distributed from northern Paraguay and southeastern Bolivia to southern Brazil, Uruguay and central Argentina (Salazar-Bravo 2015). The species name was coined by Fischer (1814: 71) as Mus Laucha et Lauchita, based on the “rat septième ou rat laucha” described by the Spanish naturalist Félix de Azara in 1801. The original description was also taken by Olfers (1818: 209), who used it as a reference for his M[us]. laucha. Like other several forms described by Azara, the absence of a type specimen and the uncertainty about the exact location of the collection site have been decisive in the subsequent complex taxonomic history of this species (Tate 1932).
Hershkovitz (1962) revised C. laucha, including as synonyms several nominal forms that today are recognized as valid species (e.g., C. hummenlincki, C. musculinus, C. tener) and restricted its type locality to Asunción, eastern Paraguay. Massoia et al. (1968) redefined the concept of C. laucha, referring its terra typica to the vicinities of Buenos Aires, Argentina, and removing from under its synonymy the currently accepted C. musculinus (Thomas, 1913). Other nominal taxa, such as C. tener (Winge, 1887) and C. hummelincki (Husson, 1960) have not been studied and compared to the detail that Massoia et al. (1968) employed in comparing C. laucha and C. musculinus. However, phylogenetic analyses of mitochondrial genes, coupled with chromosomal counts, spermatic morphology and skull morphometrics have been useful in assigning specific status to these species (e.g., Olds 1988; Pérez- Zapata et al. 1997; Bonvicino & Almeida 2000; Salazar-Bravo et al. 2001). Most of subsequent authors maintained Paraguay as the type locality of C. laucha (e.g., Musser & Carleton 2005; Salazar-Bravo 2015), following the restriction made by Hershkovitz (1962).
In a recent contribution, González-Ittig et al. (2014), based on the analyses of cytochrome b (Cyt-b) sequences, recognized two main clades in the current distribution of C. laucha, one encompassing populations in southern Brazil and Uruguay and another including those from the remainder of the distribution in Argentina, Bolivia and Paraguay. However, these authors did not include specimens from the Argentinean Mesopotamia, leaving some doubts about the limits between both clades. Finally, González-Ittig et al. (2014) recorded only C. tener in eastern Paraguay, challenging the traditional view of this area as occupied by C. laucha.
With the above described scenario, the present work has two objectives: 1) to provide, based on cytochrome b (Cyt-b) sequences, an update on the genetic composition of eastern and western clades of C. laucha, increasing the sampling made by González-Ittig et al. (2014); 2) to stabilize the taxonomy of this widespread sigmodontine, by designating a neotype and assigning a new type locality for the species.
MATERIALS AND METHODS
We expanded and complemented the Cyt-b gene matrix of González-Ittig et al. (2014) by incorporating sequences of individuals from new localities in Uruguay and Argentina: Uruguay: Rincón del Colorado (Canelones), Punta Gorda (Colonia), Estancia Loma del Queguay (Paysandú), Estancia Los Paraísos (Artigas) and Valle Platón (Rivera). Argentina: Estación San José (Buenos Aires), Árraga (Santiago del Estero) and Nogoyá (Entre Ríos). Thus, the present study includes 12 localities from Argentina, 6 from Paraguay, 1 from Bolivia, 6 from Uruguay and 1 from Brazil (Table 1 and Fig. 1).
Table 1
Field identification code or collection numbers of the specimens from which tissues were obtained. Location of the sampling sites is showed in Fig. 1 (in parenthesis, the provinces or departments), geographic coordinates, GenBank accession numbers and Museums or Collections where the voucher specimens are deposited.


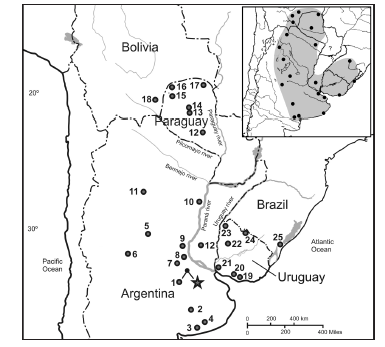
Fig. 1. Map of geographical locations of individuals listed in Table 1. Circles represent localities sampled in the present study; the star corresponds to the type locality of the neotype designated herein for Calomys laucha. The inset depicts the distributional range of C. laucha based on marginal localities (modified from Salazar-Bravo, 2015).
DNA extractions were performed according to the standard CTAB protocol. The complete Cyt-b was amplified with the primer combination Mus14095/ Mus15398 according to the conditions described in González-Ittig et al. (2010). All sequencing reactions were performed by Macrogen USA Inc. (http://www.macrogenusa.com). Nucleotide alignments were produced using MAFFT v7 (Katoh & Standley 2013). We used different iterative refinement methods to detect changes in the Cyt-b alignment; the slow options L-INS-i and G-INS-I, with gap opening penalty: 1.53, gap extension penalty: 0.1, were tested. Regardless of the method used, the alignment was always the same, since no gap was detected.
The number of haplotypes was obtained with the program DnaSP v5 (Rozas et al. 2003). We used Network v5.0.0.1 (Bandelt et al. 1999), with the maximum parsimony post-processing analysis, to draw a median-joining network and to analyze the relationships among the haplotypes detected. To compare with other studies, we used the Kimura 2 parameters (K2P) algorithm to estimate the levels of genetic distances and sequence divergence within and between clades.
Nomenclature used to describe the anatomy of the skull follows Carleton & Musser (1989) and Steppan (1995), and that of the molars follows Reig (1977). The measurements provided were taken with a digital caliper and recorded to the nearest 0.01 mm following Hershkovitz (1962) and Musser et al. (1998).
RESULTS
Thirty-three specimens of C. laucha were clustered into 23 haplotypes, which formed two haplogroups (Fig. 2). Haplogroup A included 20 haplotypes corresponding to 24 specimens from Argentina, Bolivia and Paraguay, and haplogroup B included 3 haplotypes corresponding to nine individuals from one locality in the Argentinean Mesopotamia (#12 in Fig. 1), and from all sampled localities of Brazil and Uruguay. A total of 36 substitutions separated the haplogroups (Fig. 2); the mean inter-sub-clade K2P genetic differentiation was 5.47% (4.74% - 6.53%). None of the haplotypes of sub-clade A were shared among individuals from different countries; the mean intra-sub-clade A differentiation was 1.11% (0.13% - 2.71%).
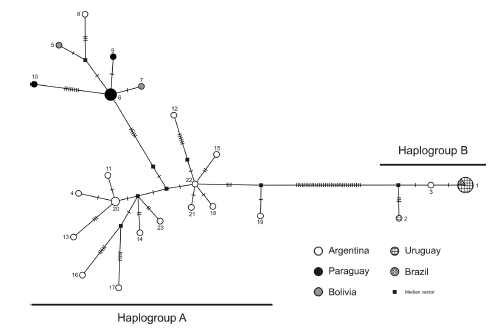
Fig. 2. Minimum spanning network showing the relationships and relative abundance of the Cyt-b haplotypes of C. laucha. The geographic origin of each haplotype is indicated with a different color or drawing pattern. Each bar through the solid line represents one nucleotide difference between haplotypes.
The International Code of Zoological Nomenclature (ICZN 1999; see Article 75.3.1) justifies the designation of a neotype under “... the express purpose of clarifying the taxonomic status or the type locality [italics are ours] of a nominal taxon.” Based on the premises discussed above, we designate a neotype for Calomys laucha as follows:
Calomys laucha (Fischer, 1814)
Neotype.— MACN-Ma 18795 (original collector’s number 7317), adult scrotal male collected by James N. Mills on November 30th, 1988; preserved as skin and skull in good condition (Fig. 3).

Fig. 3. From top to bottom: right lateral, ventral and dorsal views of the skull and labial view of the mandible of the neotype of Calomys laucha (MACN 18795; Argentina, Buenos Aires, Estación Experimental Agropecuaria Pergamino). Scale = 5 mm.
Measurements of the neotype in mm (external measurements were taken from the label).— Total length = 140; tail length = 55; hindfoot length (with claw) = 17; ear length = 14; greatest length of skull = 22.21; condylo-incisive length = 20.53; greatest zygomatic breadth = 11.76; least interorbital breadth = 3.40; length of nasals along midline = 9.05; breadth of braincase = 9.45; breadth of the zygomatic plate = 2.37; length of incisive foramina = 5.10; maximum breadth across the incisive foramina = 1.65; length of palatal bridge = 3.55; bullar length less tube = 3.35; alveolar length of the maxillary toothrow = 3.04; alveolar length of the mandibular toothrow = 2.84.
Type locality.— Estación Experimental Agropecuaria Pergamino (33°55’S, 60°35’W; 65 m a.s.l.), Partido de Pergamino, Provincia de Buenos Aires, Argentina.
Distribution.— Natural grasslands, cultivate fields and forest fringes, up to an elevation of 1000 m, from northern Paraguay and southeastern Bolivia to southern Brazil, Uruguay and central Argentina (Salazar-Bravo 2015; see inset on Fig. 1).
Emended diagnosis (modified from Salazar-Bravo 2015).— A member of the tribe Phyllotini, subfam ily Sigmodontinae, characterized by the following combination of characters: size small (12-20 g; total length ~125 mm), overall dorsal coloration olive gray, with brown and golden hairs intermingled, darker at the midline and clearly separated from the white of the venter; ears with a white patch of hairs behind; tail bicolored, ~43% of the combined length of the head and body; skull small (GLS ~22 mm), with straight lateral profile, rostrum short and narrow; interorbital region wide, with posterior divergent supraorbital edges; braincase large and rounded; zygomatic plate broad, with free upper border enclosing a poorly developed zygomatic notch; palate long, wide and flat; upper incisors smooth, hyperopisthodont and faced with light orange enamel; molars small (upper molar series <3.3 mm), brachydont, trilophodont and crested; main lingual and labial cusps in alternate pairs; M1 subrectangular in outline; procingulum well developed deeply divided by an anteromedian flexus and with a single anteromedian style typically pres ent; M3 approximately equal to 50% of the M2 and rounded; stomach unilocular-hemiglandular; glans penis complex, with a tridigitate bacular cartilage; gall bladder present; 2n = 64; FN = 68-72.
Remarks.— The morphological distinction between C. laucha and the widely sympatric C. musculinus was explored by Massoia et al. (1968). Synonymy, description, distribution, geographical and karyotypic variation and natural history were synthesized by Salazar-Bravo (2015); stomach morphology was described by Carleton (1973); morphology of male accessory glands was documented by Voss & Linzey (1981). Ecology, diet, reproduction and habitat selection well characterized, especially for the pampean region of Buenos Aires and Córdoba provinces populations (see review in Polop & Busch 2010). The species C. laucha from Argentina and Uruguay has a 2N = 64, FN = 68-72 (Bonvicino et al. 2010). The karyotype with a 2N = 56; FN = 59, reported by Brum-Zorrilla (1962) for one specimen attributed to this species should dismissed as no voucher is available to confirm the identification (EG, pers. obs.).
DISCUSSION
The current concept of C. laucha includes two main clades, one encompassing sampled populations in southern Brazil, Uruguay and 1 locality in Argentina and another including those from the remainder of the distribution in Argentina, Bolivia and Paraguay. The karyotype of individuals assigned to both of these haplogroups is the same and includes a diploid count of 2N = 64 (Gardenal et al. 1977; Brum-Zorrilla et al. 1990; Mattevi et al. 2005; Badzinski et al. 2012). No metric or detailed qualitative or quantitative morphological studies encompassing populations from throughout the range of this species have been published to date. According to González-Ittig et al. (2014), if the two main clades represent distinct, cryptic species, the available names for them would be C. bimaculatus Waterhouse, 1837 (type locality “Maldonado,” Maldonado, Uruguay [Waterhouse, 1837: 18]) and C. laucha. However, these authors overlooked the fact that Mus (?) dubius Fischer, 1829, with type locality restricted to “São Gabriel (30°19’S, 54°19’W, 118 m, Rio Grande do Sul, Brazil” (Pardiñas et al. 2007: 401) is also an available name for Uruguayan and Brazilian populations. If dubius proves to be a synonym of bimaculatus, then this name should correspond by priority, at species or subspecies level, to those populations of Brazil and Uruguay. Mus (?) dubius Fischer, 1829 was based on the “blanco debaxo,” described also by Azara (1802). Cabrera (1961: 477) used the name dubius as valid and included into its synonymy, at subespecific level, the nominal forms bimaculatus and bonariensis Osgood, 1933 (type locality “Torrecita, province of Buenos Aires, Argentina;” Osgood 1933: 14).
Regarding the type locality of C. laucha, when discussing his “rat septième ou rat laucha,” Azara indicated that his servant Francisco had taken two animals from a “paille de maïs d’un Chacarra of Buenos-Ayres, et un autre dans les Pampas, par les vingt-cinq degrés de latitude” (freely translated as “from a maize straw at a farm in Buenos Aires, and another from the Pampas, by the twenty-five degrees of latitude”). Olfers (1818: 209) referred the origin of laucha as “Paraguay” and Hershkovitz (1962: 153), following Olfers (1818) and invoking his right as first reviser, restricted the type locality to “neighborhood of Asuncion [sic], Paraguay.” Cabrera (1961: 479) instead, referred the type locality of this species to “…las quintas de Buenos Aires.” Mistakenly, Hershkovitz (1962: 153) concluded that in the late XVIII century, Buenos Aires included the current areas of Uruguay and the Argentinean provinces of Misiones, Corrientes and Entre Rios, electing to restrict the type locality to the vicinity of Asunción, Paraguay, in reference to the “vingt-cinq ... degres of latitude” from Azara’s statement. Part of the ideas of Hershkovitz (1962: 153) were based on the assumption that Azara had not worked in the area of the present province of Buenos Aires, an inference widely refuted by historic documents (e.g., Mones & Klappenbach 1997; Contreras Roqué 2010). Clearly, the specimens that Azara (1801) used in his description correspond to animals caught at two different localities, one in the vicinity of Buenos Aires (“paille de maïs d’un Chacarra of Buenos-Ayres,..”) and another in Paraguay (“… dans les Pampas, par les vingt-cinq degrés de latitude”). The restriction made by Hershkovitz (1962) to Paraguay is not convenient; based on our current knowledge, only C. tener is confidently reported in the eastern portion of this country (see González-Ittig et al., 2014). Thus, conceivably, Azara’s “rat septième ou rat laucha” could be a composite between these two, externally very similar, taxa. As the designation of a neotype implies a redefinition of the type locality, we chose this action to correct the restriction made by Hershkovitz (1962), restoring part of the original reference of Azara (1801) to northern Buenos Aires, central-eastern Argentina. The choice of a neotype from central Argentina has the additional benefit that populations of northern Buenos Aires province have been pivotal in defining the current concept of C. laucha (e.g., Massoia & Fornes 1965; Massoia et al. 1968). Other sigmodontines and didelphids described by Azara (1801, 1802) have similar taxonomic histories, where the need to avoid confusion has led to the designation of neotypes; among others, Oligoryzomys nigripes (Myers & Carleton 1981), Oxymycterus rufus (Oliveira & Gonçalves 2015), Sooretamys angouya (Musser et al. 1998), or Thylamys pusilla (Voss et al. 2009).
The course of action taken above has implications on many fronts, especially for the taxonomy of small-bodied Calomys present in the provinces of Corrientes and Entre Ríos, between the Paraná and the Uruguay rivers; this region, known as the Argentinean Mesopotamia, covers upwards of 167 000 km2. In the present study we included one specimen from Entre Ríos Province, which clustered within the haplogroup B. If this is the only form present in Mesopotamia, then the Paraná River might potentially be acting as a geographic barrier separating individuals of these distinct clades. This is an important issue, since both the Paraná and Uruguay rivers appear to be barriers for other species’ distributions; for example, Scapteromys (in which eastern and western clades are separated by the Uruguay River; see D’Elía & Pardiñas 2004) and Reithrodon (in which the clades referred to the species typicus and auritus are apparently separated by the Paraná River; see Pardiñas et al. 2015). Nevertheless, we sequenced only one individual from Entre Ríos, and many other populations of small-sized Calomys are known from this region (e.g., Hershkovitz 1962; Massa et al. 2014). In fact, we cannot exclude the possibility that C. tener could also be present in the northern portion of the Mesopotamia, since C. tener and C. laucha clade B are nearly sympatric at similar latitudes in southern Brazil (Quintela et al. 2014). Calomys tener is present in eastern Paraguay and in the Argentinean province of Misiones (González-Ittig et al. 2014), and the southern limit of its distribution, as well as those of C. laucha in this region, are currently unclear. In fact, although we lack Cyt-b confirmation about the presence of C. laucha in eastern Paraguay and northeastern Argentina, the species has been mentioned several times for these territories (see Salazar-Bravo 2015). C. laucha was first mentioned for Misiones province by Massoia (1980, 1983), based on materials derived from owl pellets recovered in the locality of El Cruce, about 7 km NE Apósteles. Later, Massoia (1988) introduced the first reference to C. tener based on animals from Campo Ramón, a forested region near Oberá. In his synthesis on the rodents from Misiones, Massoia (1993) stated the presence of both species of Calomys in this province, referring the distribution of C. laucha to the southern unforested portion and restricting C. tener to central forested localities. The occurrence of C. tener in Misiones was summarily dismissed by Pardiñas & Teta (2006) and the sylvan populations of Calomys were referred to C. laucha. Clearly, Calomys is an important component of the small mammal assemblages in central and southern Misiones as well as in northeastern Corrientes (Teta & Pardiñas 2007) and its alpha-taxonomy in this large territory deserves further inspection. The same is true for the eastern half of Paraguay, an area of fragmented forests intermixed with agricultural lands and open grasslands, in which both species appear as sympatric (cf. Salazar-Bravo 2015).
In summary, additional data are still needed to better address the taxonomy of C. laucha and to delimit with accuracy its geographical distribution, especially in the areas in which C. laucha and C. tener could be sympatric (e.g., northeastern Argentina). Additional qualitative and quantitative morphological approaches are needed, as they could add substantial data, together with molecular markers, to the recognition of eastern and western clades as different entities (i.e., species or subspecies). In addition, the analysis of DNA sequences of more specimens from the Argentinean Mesopotamia is necessary to solve their taxonomic status, and consequently, to add a new piece in the biogeographical puzzle of the mammals of this part of the La Plata River Basin.
ACKNOWLEDGEMENTS
Sergio Lucero (MACN) helped us at the first stages of this work. James Mills shared with us some field notes about the individual here selected as neotype. We thank Silvana Levis, Gladys Calderón and María Laura Martín from INEVH (Pergamino, Argentina) for providing some of the specimens and sequences used in this study. The following institutions supported this study: Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, PIP 11220150100474CO to Cristina N. Gardenal), Secretaría de Ciencia y Tecnología (SECYT) of the Universidad Nacional de Córdoba, and Agencia Nacional de Promoción Científica y Tecnológica (PICT 2012-1275 to Cristina N. Gardenal and PICT 2014-1039 to UFJP). JSB acknowledges the support of the American Philosophical Society, a grant from the Systematics Research Fund of the Systematics Association and Faculty Research Award from Texas Tech University.
LITERATURE CITED
1. Azara, F. 1801. Essais sur l’histoire naturelle des quadrupeds de la province du Paraguay. Paris: Charles Pougens 2:1-499.
2. Azara, F. 1802. Apuntamientos para la historia natural de los quadrúpedos del Paraguay y Río de la Plata. La Imprenta de la Viuda de Ibarra, Madrid. [ Links ]
3. Badzinski, C., D. Galiano, & J. R. Marinho. 2012. Mammalia, Rodentia, Cricetidae, Calomys laucha (Fischer, 1814): Distribution extension in southern Brazil. Check List 8:264-266. [ Links ]
4. Bandelt, H-J., P. Forster, & A. Röhl. 1999. Median-joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution 16:37-48. [ Links ]
5. Bonvicino, C. R., & F. C. Almeida. 2000. Karyotype, morphology and taxonomic status of Calomys expulsus (Rodentia: Sigmodontinae). Mammalia 64:339-351. [ Links ]
6. Bonvicino, C. R., J. A. de Oliveira, & R. Gentile. 2010. A new species of Calomys (Rodentia: Sigmodontinae) from eastern Brazil. Zootaxa 2336:19-25. [ Links ]
7. Brum-Zorrilla, N. 1962. Investigaciones citogenéticas sobre algunas especies de Cricetidae (Rodentia) del Uruguay. Anales del 2do Congreso Latinoamericano de Zoología 2:315-320. [ Links ]
8. Brum-Zorrilla, N., G. Hurtado de Catalfo, C. Degiovanangelo, R. L. Wainberg, & T. Gentile de Fronza. 1990. Calomys laucha chromosome (Rodentia, Cricetidae) from Uruguay and Argentina. Caryologia 43:65-77. [ Links ]
9. Cabrera, A. 1961. Catálogo de los mamíferos de America del Sur. Revista del Museo Argentino de Ciencias Naturales “Bernardino Rivadavia,” Ciencias Zoológicas 4: xxii + 309-732.
10. Carleton, M. D. 1973. A survey of gross stomach morphology in New World Cricetinae (Rodentia, Nuroidea), with comments on functional interpretations. Miscellaneous Publications, Museum of Zoology, University of Michigan 146:1-43. [ Links ]
11. Carleton, M. D., & G. G. Musser. 1989. Systematic studies of oryzomyine rodents (Muridae, Sigmodontinae): a synopsis of Microryzomys. Bulletin of the American Museum of Natural History 191:1-83. [ Links ]
12. Contreras Roqué, J. R. 2010. Félix de Azara: su vida y su época. Tomo segundo. El despertar de un naturalista: la etapa paraguaya y rioplatense. (1782-1801). Diputación de Huesca, Aragón, España. [ Links ]
13. D’Elía, G., & U. F. J. Pardiñas. 2004. Systematics of Argentinean, Paraguayan, and Uruguayan swamp rats of the genus Scapteromys (Rodentia, Cricetidae, Sigmodontinae). Journal of Mammalogy 85:897-910.
14. Fischer, G. 1814. Zoognosia tabulis synopticis illustrata. Volumen tertium. Quadrupedum reliquorum, cetorum et montrymatum descriptionem continens. Nicolai Sergeidis Vsevolozsky Moscow, Mosquae. [ Links ]
15. Gardenal, C. N., N. T. Juarez, M. Gutierrez, & M. S. Sabbatini. 1977. Contribución al conocimiento de tres especies del genero Calomys (Rodentia, Cricetidae). Physis 36:169-178. [ Links ]
16. González-Ittig, R. E., K. Narayan, S. Levis, G. Calderón, J. Salazar-Bravo, & C. N. Gardenal. 2014. Molecular systematics of the South American rodent Calomys laucha (Cricetidae: Sigmodontinae), a reservoir of the Laguna Negra hantavirus. Canadian Journal of Zoology 92:1093-1098. [ Links ]
17. González-Ittig, R. E., J. Salazar-Bravo, R. M. Barquez, & C. N. Gardenal. 2010. Phylogenetic relationships among species of the genus Oligoryzomys (Rodentia, Cricetidae) from Central and South America. Zoologica Scripta 39:511-526. [ Links ]
18. Hershkovitz, P. 1962. Evolution of Neotropical cricetine rodents (Muridae) with special reference to the phyllotine group. Fieldiana: Zoology 46:1-524. [ Links ]
19. International Commission on Zoological Nomenclature. 1999. International code of zoological nomenclature. 4th edition. The International Trust for Zoological Nomenclature, London. [ Links ]
20. Katoh, K., & D. M. Standley. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30:772-780. [ Links ]
21. Leigh, J., & D. Bryant. 2015. PopArt: full-feature software for haplotype network construction. Methods in Ecology and Evolution, 6:1110-1116. [ Links ]
22. Massa, C., P. Teta, & G. Cueto. 2014. Effects of regional context and landscape composition on diversity and composition of small rodent assemblages in temperate grasslands and wetlands. Mammalia 78:371-382. [ Links ]
23. Massoia, E. 1980. Mammalia de Argentina -I- Los mamíferos silvestres de la provincia de Misiones. Asociación para la Conservación de la Naturaleza Argentina, Iguazú 1:15-43. [ Links ]
24. Massoia, E. 1983. La alimentación de algunas aves del orden Strigiformes en la Argentina. El Hornero (Número Extra.):125-148. [ Links ]
25. Massoia, E. 1988. Presas de Tyto alba en Campo Ramón, Departamento Oberá, provincia de Misiones - I. Boletín Científico de la Asociación para la Protección de la Naturaleza 7:4-16. [ Links ]
26. Massoia, E. 1993. Los roedores misioneros -1- Lista sistemática comentada y geonemia provincial conocida. Boletín Científico de la Asociación para la Protección de la Naturaleza 25:42-51. [ Links ]
27. Massoia, E., & A. Fornes. 1965. Contribución al conocimiento de los roedores miomorfos argentinos vinculados con la Fiebre Hemorrágica (Rodentia: Cricetidae y Muridae). Comisión Nacional Coordinadora para el Estudio y Lucha contra la Fiebre Hemorrágica Argentina, Ministerio de Asistencia Social y Salud Pública, Buenos Aires. [ Links ]
28. Massoia, E., A. Fornes, R. Wainberg, & T. G. Fronza. 1968. Nuevos aportes al conocimiento de las especies bonaerenses del género Calomys (Rodentia - Cricetidae). Revista de Investigaciones Agropecuarias, INTA. Serie Biología y Producción Animal 5:63-92. [ Links ]
29. Mattevi, M. S., T. Haag, L. F. B. de Oliveira, & A. R. Langguth. 2005. Chromosome characterization of Brazilian species of Calomys Waterhouse, 1837 from Amazon, Cerrado and Pampa domains (Rodentia, Sigmodontinae). Arquivos do Museo Nacional (Rio de Janeiro) 63:175-181. [ Links ]
30. Mones, A., & M. A. Klappenbach. 1997. Un ilustrado aragonés en el virreinato del río de La Plata: Félix de Azara 1742 1821: estudios sobre su vida, su obra, y su pensamiento. Anales del Museo Nacional de Historia Natural, Montevideo, ser. 2, 9: i-viii, 1-231. [ Links ]
31. Musser, G. G., M. D. Carleton, E. Brothers, & A. L. Gardner. 1998. Systematic studies of Oryzomyine rodents (Muridae, Sigmodontinae): diagnoses and distributions of species formerly assigned to Oryzomys “capito.” Bulletin American Museum of Natural History 236:1-376.
32. Musser, G. G., & M. D. Carleton. 2005. Superfamily Muroidea. Mammal Species of the World: A Taxonomic and Geographic Reference. 3rd edition. (D. E. Wilson & D. A. M. Reeder, eds.). Johns Hopkins University Press, Baltimore, MD. [ Links ]
33. Myers, P., & M. D. Carleton. 1981. The species of Oryzomys (Oligoryzomys) in Paraguay and the identity of Azara’s “rat sixième ou rat à tarse noir.” Miscellaneous Publications, Museum of Zoology, University of Michigan 161:1-41.
34. Olds, N. 1988. A revision of the genus Calomys (Rodentia: Muridae). Ph.D. Dissertation. City University of New York, New York, USA. [ Links ]
35. Olfers, I. V. 1818. Bemerkungen zu Illiger’s Ueberblick der Säugthiere nach ihrer Vertheilung über die Welttheile, rücksichtich der Südamericanischen Arten (Species). Abhandlung X in W. L. von Eschwege, Journal von Brasilien, odor vermischte Nachrichten aus Brasilien, auf wissenschaftlichen Reisen gesammelt. Heft 2 in F. Bertuch (ed.), Neue Bibliothek der wichtigsten Reisebeschreibungen zur Erweiterung der Erd- und Völkerkunde, Band 15:192-237. Weimar.
36. Oliveira, J.A., & P. R. Gonçalves. 2015. Genus Oxymycterus Waterhouse, 1837. Mammals of South America. Volume 2 - Rodentia (J. Patton, U. F. J. Pardiñas & G. D’Elía, eds.). University of Chicago Press.
37. Osgood, W. H. 1933. Two new rodents from Argentina. Field Museum Natural History, zoological series 20:11-14. [ Links ]
38. Pardiñas, U. F. J., & P. Teta. 2006. Roedores sigmodontinos de la provincia de Misiones: estado actual de su conocimiento nomenclatorial y taxonómico. Los Mamíferos Silvestres de la Provincia de Misiones (E. Massoia, J. C. Chebez & A. Bosso, eds.). Edición de los autores. Buenos Aires. [ Links ]
39. Pardiñas, U. F. J., P. Teta, G. D’Elía, S. Cirignoli, & P. E. Ortiz. 2007. Missing type localities of sigmodontine (Cricetidae, Sigmodontine) rodents: some solutions. The Quintessential Naturalist: Honoring the Life and Legacy of Oliver P. Pearson (D. A. Kelt, E. P. Lessa, J. A. Salazar-Bravo & J. L. Patton, eds.). University of California Publications in Zoology.
40. Pardiñas, U. F. J, C. A. Galliari, & P. Teta. 2015. Tribe Reithrodontini Vorontsov, 1959. Mammals of South America. Volume 2 - Rodentia (J. Patton, U. F. J. Pardiñas & G. D’Elía, eds.). University of Chicago Press.
41. Pérez-Zapata, A., A. D. Vitullo, & O. A. Reig. 1987. Karyotypic and sperm distinction of Calomys hummelincki from Calomys laucha (Rodentia: Cricetidae). Acta Científica Venezolana 38:90-93. [ Links ]
42. Polop, J. J., & M. Busch (eds.). 2010. Biología y ecología de pequeños roedores en la región pampeana de Argentina: enfoques y perspectivas. Universidad Nacional de Córdoba, Córdoba, Argentina. [ Links ]
43. Reig, O. A. 1977. A proposed unified nomenclature for the enamelled components of the molar teeth of the Cricetidae (Rodentia). Journal of Zoology London 181:227-241. [ Links ]
44. Rozas, J., J. C. Sánchez del Barrio, X. Messenguer, & R. Rozas. 2003. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19: 2496-2497. [ Links ]
45. Quintela, F. M., E. Conceição da Silveira, D. Gonçalves Dellagnese, & C. Vargas Cademartori. Calomys tener (Winge, 1887) (Rodentia: Cricetidae: Sigmodontinae): Filling gaps. Check List 10:650-654. [ Links ]
46. Salazar-Bravo, J. 2015. Genus Calomys Waterhouse, 1837. Mammals of South America. Volume 2 - Rodentia (J. Patton, U. F. J. Pardiñas & G. D’Elía, eds.). University of Chicago Press.
47. Salazar-Bravo, J., J. W. Dragoo, D. S. Tinnin, & T. L. Yates. 2001. Phylogeny and evolution of the neotropical rodent genus Calomys: inferences from mtDNA sequence data. Molecular Phylogenetics and Evolution 20:173-84. [ Links ]
48. Steppan, S. J. 1995. Revision of the tribe Phyllotini (Rodentia: Sigmodontinae), with a phylogenetic hypothesis for the Sigmodontinae. Fieldiana, Zoology, new series, 80:1-112. [ Links ]
49. Tate, G. H. H. 1932. The South American Cricetidae described by Felix Azara. American Museum Novitates 557:1-5. [ Links ]
50. Teta, P., & U. F. J. Pardiñas. 2007. Mammalia, Didelphimorphia, Didelphidae, Chacodelphys formosa (Shamel, 1930): range extension. Check List 3:333-335. [ Links ]
51. Voss, R. S., & A. V. Linzey. 1981. Comparative gross morphology of male accessory glands among Neotropical Muridae (Mammalia: Rodentia) with comments on systematic implications. Miscellaneous Publications, Museum of Zoology, University of Michigan 159:1-41. [ Links ]
52. Voss, R. S., P. Myers, F. Catzeflis, A. P. Carmignotto, & J. Barreiro. 2009. The six oppossums of Félix de Azara: identification, taxonomic history, neotype designations, and nomenclatural recommendations. Bulletin of the American Museum of Natural History 331:406-433. [ Links ]
53. Waterhouse, G. R. 1837. Characters of new species of the genus Mus, from the collection of Mr. Darwin. Proceedings of the Zoological Society of London 1837 (part V):15-21, 27-32. [ Links ]














