Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Mastozoología neotropical
Print version ISSN 0327-9383On-line version ISSN 1666-0536
Mastozool. neotrop. vol.25 no.1 Mendoza June 2018
ARTÍCULO
First record of Promops davisoni (Thomas, 1921) (Chiroptera, Molossidae) from Chile and a description of its echolocation calls
Gonzalo Ossa1, 2, Thomas M. Lilley3, Joaquín Ugarte-Núñez4, Lasse Ruokolainen5, Karol Vilches2, 6, Pablo Valladares-Faúndez2, 6 and Veronica Yung7
1 Conserbat EIRL, San Fabián, Región del Biobío, Chile. [Correspondence: Gonzalo Ossa <chalofoh@gmail.com>]
2 Programa para la Conservación de los Murciélagos de Chile PCMCh.
3 Biodiversity Unit, University of Turku, Turun Yliopisto, Finland.
4 Asociación para la Conservación y el Desarrollo Sostenible Sallqa, Lima, Perú.
5 Department of Biological and Environmental Sciences, University of Helsinki, Finland.
6 Laboratorio de Zoología Integrativa, Departamento de Biología, Facultad de Ciencias, Universidad de Tarapacá, Arica, Chile.
7 Laboratorio de la Sección Rabia, Subdepartamento de Virología, Instituto de Salud Pública, Santiago, Chile.
Recibido 11 julio 2017.
Aceptado 17 enero 2018.
Editor asociado: G D'Elía
ABSTRACT.
Few studies have been conducted on the bat fauna of the Arica and Parinacota region, northern Chile. The genus Promops (Gervais, 1855) is a poorly known group of molossid bats, with three species widely distributed in Central and South America. We report for the first time the species Promops davisoni in Chile. Identification was based on echolocation calls obtained at the Azapa and Lluta valleys and compared to reference calls from Tacna, Peru. We report the species as far as 127 km south of the previous published southernmost record in Pampa Alta, Peru. In addition we obtained an individual found at the Anzota caves, near the city of Arica. The species is found on the coast and in the fluvial valleys of Northern Chile, with altitudes ranging from sea level to 822 m a.s.l. We propose that the distribution of P. davisoni may extend further south, to the locality of Tana in the Tarapacá region, based on the capacity of the species to cross distances of over 100 km over large desert areas. With this species, we increase the known bat fauna of Chile to 14 species, and the bat fauna of the Arica and Parinacota region to nine species.
RESUMEN.
Primer registro de Promops davisoni (Thomas, 1921) (Chiroptera, Molossidae) en Chile, y descripción de sus llamadas de ecolocación.
Pocos estudios sobre la fauna de murciélagos se han realizado en el la región de Arica y Parinacota, extremo norte de Chile. El género Promops (Gerais, 1855) corresponde a un grupo poco conocido de especies de la familia molossidae, con tres especies ampliamente distribuidas en América Central y Sur. Reportamos por primera vez la especie Promops davisoni en Chile, basados en el análisis de sus llamadas de ecolocación obtenidas en los valles de Azapa y Lluta, y comparados con llamadas de referencia de individuos registrados en Tacna, Perú. Se registró la especie a 127 km al sur del registro publicado anteriormente en Pampa Alta, Perú. Además obtuvimos un individuo encontrado en las cuevas de Anzota, cerca de la ciudad de Arica. La especie utiliza la costa y los valles del norte de Chile, desde el nivel del mar hasta los 822 m s.n.m. Consideramos que la distribución de P. davisoni se puede extender hasta la localidad de Tana, en la región de Tarapacá, dada la capacidad de la especie de cruzar extensas áreas desérticas de más de 100 km de distancia. Con este nuevo registro, incrementamos la diversidad de quirópteros de Chile a 14 especies y la diversidad de quirópteros en la región de Arica y Parinacota a nueve especies.
Key words: Arica; Bioacoustics; Echolocation; Mastiff bat; Peru.
Palabras clave: Arica; Bioacústica; Ecolocación; Murciélago mastín; Perú.
INTRODUCTION
To date, 13 bat species have been recorded in Chile (Ossa et al. 2014; Ossa & Diaz 2014; Lapapiat et al. 2016; Rodriguez-San Pedro et al. 2016) with the highest species richness found in the north of the country (Mann 1950, 1978; Bonacic et al. 2016; Rodríguez-San Pedro et al. 2016). This is consistent with the latitudinal gradient of mammalian diversity in Chile, with peaks at 18° S (Puna highland and Atacama Desert), and 37° S (Mediterranean and Temperate forest) (Samaniego & Marquet 2009). Northern Chile comprises a desert matrix, where vegetation is practically absent (Luebert & Pliscoff 2006), except for the fluvial valleys, where vegetation and human activities are developed.
Few studies on bats have been conducted in the Arica and Parinacota region, resulting in a lack of information on the order in the region. The only study conducted so far in the Azapa valley resulted in the addition of the long snouted bat Platalina genovensium (Thomas 1928) to the known bat fauna of Chile (Galaz et al. 1999). Recent studies confirm the presence of P. genovensium in Chile after 19 years of uncertainty (Ossa et al. 2016). Other species recorded in the region are Myotis atacamensis (Lataste 1982), Histiotus montanus (Phillipi & Landbeck 1861), Histiotus macrotus (Poeppig 1835), Tadarida brasiliensis (I. Geoffroy 1824), Mormopterus kalinowskii (Thomas 1893), Amorphochillus schnablii (Peters 1877), and Desmodus rotundus (E. Geoffroy 1810), totalizing eight species for the region (Rodríguez-San Pedro et al. 2016).
Promops is a poorly known genus of molossid bats with three species widely distributed in Central and South America; P. centralis (Thomas 1915) ranges from Mexico to Nicaragua, and from French Guyana to Paraguay and northern Argentina (Marinkelle & Cadena 1973; Genoways & Williams 1979; Kennedy et al. 1984); P. nasutus (Spix 1823) has a wide distribution in South America, from Guyana, Venezuela, and Colombia to northern Argentina and southern Brazil (Genoways & Williams 1979; Siles et al. 2005; Sandoval et al. 2010; PaQui et al. 2016); and P. davisoni (Thomas 1921) is found in the coastal Peruvian desert province (sensu Morrone 2006), from Manabi, Ecuador to Tacna, Peru. Promops davisoni was recently recognized as a distinct species (Gregorin & Chiquito 2010; Flores et al. 2015).
All species of Promops have a convex face and a very deep palate, characteristics that differentiate this genus from other molossid genera (Gregorin & Taddei 2002; Pari et al. 2015; Diaz et al. 2016). According to Gregorin & Chiquito (2010), P. davisoni is smaller than P. centralis and bigger than P. nasutus, with a forearm length ranging from 47.6 to 52.0 mm; dorsal pelage is light brown or cinnamon brown, being lighter than P. centralis and darker than P. nasutus. Promops davisoni is distinguished by the whitish bands on its back, which cover up to half of the total length of the individuals (Diaz et al. 2016).
Ultrasound detectors are widely used to study the distribution of bat species (Zsebők et al. 2012; Briones-Salas et al. 2013; Winkler et al. 2014); especially for those species that can be distinguished by their echolocation calls or are difficult to capture (O'Farrell & Gannon 1999). Even though bats of the family Molossidae display very high plasticity in their echolocation calls, those of the genus Promops are easily recognizable because of their structure (pulses rising or falling in frequency), which differ from those of other genera such as Tadarida, Eumops and Cynomops (Jung et al. 2014).
Here we report the first record of P. davisoni for Chile, in the Arica and Parinacota region (Fig. 1), extending its distribution in the region as far as 127 km S of the previous published record in Peru (Flores et al. 2015), comparing the echolocation calls of identified individuals from Peru and random records from Chile. An individual captured 102 km S of the previous record (Flores et al. 2015) is also reported. We suggest that the species is commonly distributed in the Azapa and Lluta Valleys. With this species, we increase the known bat fauna of Chile to 14 species, and the bat fauna of the Arica and Parinacota region to nine species.
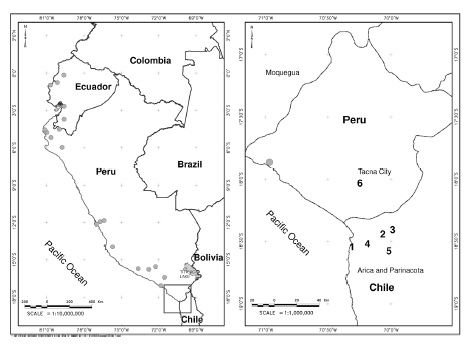
Fig. 1. Recording localities of Promops davisoni (grey circles). New records in Chile are numbered: 1. Anzota caves; 2. Linderos; 3. El Tambo; 4. San Miguel de Azapa; 5. Casa Grande, and 6. Reference recordings from Tacna. See Table 1 for coordinates of these sites. Other recording localities were taken from Ortiz de la Puente (1951); Zeballos et al. (2001); Pacheco et al. (2009); Gregorin & Chiquito (2010); Velazco et al. (2013); and Flores et al. (2015).
MATERIALS AND METHODS
We used mist nets (Ecotone, Poland) and recorded bat ultrasounds during the months of October 2015 and January 2016 in the Azapa and Luta valleys (Table 1). We deployed 100 linear meters of mist nets every night; six nights in October 2015 and six nights in January 2016 at different sites in both valleys, from 200 to 800 m of altitude. We selected humid sites near crops and livestock. In addition, we used a Song Meter SM3Bat recorder (Wildlife Acoustics Inc., Maynard, MA, USA), set to record bats from sunset to sunrise. Recordings were conducted using a sampling frequency of 384 kHz and saved as .WAV files. Files were analysed manually using the software Avisoft SAS Lab Pro 5.2.07 (Avisoft Bioacoustics, Berlin, Germany) using a FFT length of 256 kHz, Frame size 75% and Overlap 50%. We measured start frequency, end frequency, peak frequency, interval and duration of the pulses for each file obtained in the field (Fig. 2), as those measures are the most useful for the identification of bat species (Jung et al. 2014; Ossa 2010).
Table 1 Characterization of the Chilean sites where Promops davisoni was acoustically recorded.
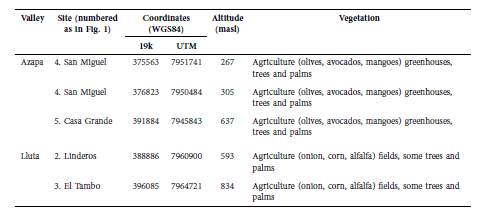
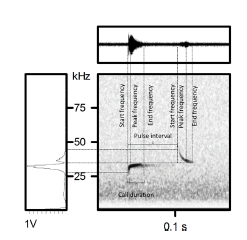
Fig. 2. Spectrogram of Promops davisoni, with upward and downward modulated echolocation calls in search phase, illustrating the measured points. The illustrated call was obtained at the Arica and Parinacota region, Chile.
To define which calls belong to P. davisoni, we analysed 36 bat passes (284 pulses) obtained in the city of Tacna from previously captured and morphologically identified individuals leaving their roost. We compared the measured parameters from Tacna to the measured parameters obtained at five different locations at the Arica and Parinacota region (309 bat passes, 1044 pulses). The locations sampled in Chile closely resemble the environmental and spatial conditions of the site in Peru. Three additional sites were also monitored in Chile, but the confined environment of these sites heavily affected the call parameters, and they were therefore omitted from the analyses.
Between-country differences in acoustic parameters were visualised using redundancy analysis (RDA). We tested the RDA-axis (constrained for the country of origin) against the country of origin using a linear mixed model, setting individual nested within locality as a random factor. We also individually tested the different echolocation variables against country. The mixed models were fitted with function lme in the nlme package in R (Pinheiro & Bates 2017).
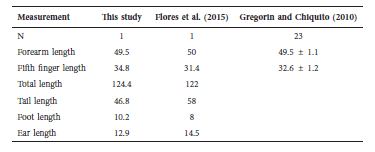
We obtained a male individual of P. davisoni (#16- 431) captured in March 2016 at the Anzota caves in the Arica and Parinacota region, northernmost Chile (18.5° S-70.3° W) by the Rabies Laboratory of the National Health Service (ISP). Using a 0.1 mm calliper we obtained external morphological measures (Table 2). However, no weights are presented because the liver and brain were extracted for rabies diagnostic studies by ISP previous to our examination. We used identification keys to identify the specimen to specific level (Gregorin & Taddei 2002; Diaz et al. 2016).
Table 2 External measurements of a Chilean specimen of Promops davisoni, the nearest specimen captured in Peru (Flores et al. 2015), and specimens reviewed by Gregorin & Chiquito (2010).
RESULTS
Here, we present the first record of Promops davisoni in Chile, increasing the bat fauna of the country to 14 species. Despite the capture effort using mist nets, we did not capture any bat species other than those already reported for the region. We obtained 284 echolocation pulses from a known roost of P. davisoni from Tacna, Peru, and 1044 echolocation pulses from individuals recorded in Chile. We analysed the Peruvian recordings of P. davisoni to obtain the echolocation parameter values of the species. P. davisoni emits two different pulses, upward and downward (Fig. 2, Table 3). The most common pulses are upward, with start frequency of 31.3 ± 0.7 kHz; end frequency of 32.3 ± 0.6 kHz; peak frequency of 32.2 ± 0.7 kHz; duration of the calls of 9.8 ± 3.3 ms; and interval between pulses of 196.7 ± 80.9 ms for recordings obtained in Peru (n = 36); and start frequency of 31.8 ± 2.9 kHz; end frequency of 32.5 ± 1.2 kHz; peak frequency of 32.2 ± 1.5 kHz; duration of the calls of 10.5 ± 3.0 ms; and interval between pulses of 196.7 ± 99.2 ms for recordings obtained in Chile (n = 305) (Table 3). An RDA model explaining the echolocation variables with country of origin was significant (P < 0.001, tested with 999 permutations), even though the difference was relatively small; the RDA-axis accounted only for 1.6% of the total variance (Fig. 3). However, this estimate was confounded by non-independent observations from the same localities and individuals. When accounting for the residual dependence structure, the between-country difference no longer remained significant (P = 0.84). While there was a tendency for bats from Peru to have higher maxima (Fig. 3), these differences were also not significant (P = 0.42).
Table 3 Measured parameters of the upward and downward echolocation calls of Promops davisoni obtained in Peru and Chile. Frequencies are measured in kilohertz's (kHz) and pulse duration and interval in milliseconds (ms).
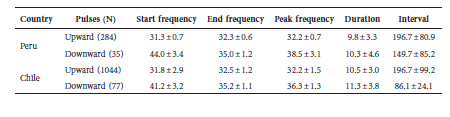
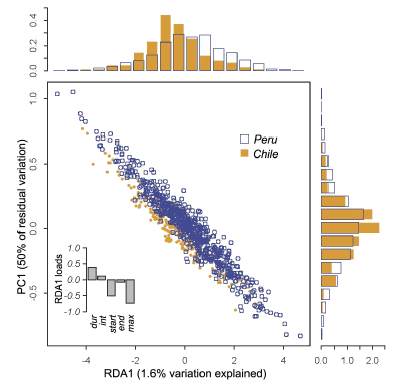
Fig. 3. The main panel shows the dispersion of recorded bat calls, according to the RDA axis, constrained by country (RDA1), and the first residual, unconstrained axis (PC1). Countries are slightly segregated along the RDA-axis, but this differentiation is not statistically significant when accounting for the residual dependence structure (P = 0.84). The inlet barplot shows the loading of the echolocation variables on the RDA-axis. None of these independently differ between countries. The histograms show the distribution of observations along the RDA-axis (top) and the PC-axis (right).
We observed the known characteristics of the species P. davisoni (Gregorin & Taddei 2002; Diaz et al. 2016) from the individual brought to ISP: upper incisors pointed, curved and separated at the tip (Fig. 4A); palate markedly deep; minute, hard, and spoon-like hairs under the nostrils, resulting in a distinctive area (Fig. 4B); very convex rostrum and oval and detached antitragus from lower border of the ear (Fig. 4C); presence of fur at the base of the fifth metacarpal (Fig. 4D); and long calcars that arrives almost until the tip of the tail (Fig. 4E).
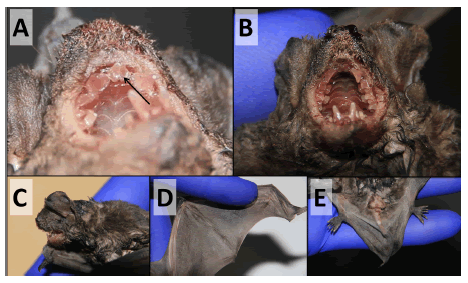
Fig. 4. Views of some external characteristics of Promops davisoni. A) Upper incisors pointed, curved and separated at the tip; B) Palate markedly deep. Minute, hard, and spoon-like hairs under nostrils resulting in a distinct area; C) Very convex rostrum and oval and detached antitragus from lower border of the ear; D) Fur at the base of the fifth metacarpal; E) Long calcars. See also Gregorin & Taddei (2002) and Diaz et al. (2016)
DISCUSSION
We report for the first time the presence of the species P. davisoni in Chile, extending its known distribution 127 km southward from Pampa Alta (Tacna department, Peru) to the Azapa valley (Arica and Parinacota region, Chile) by utilizing echolocation calls of identified individuals from Peru as a reference. We obtained records in the region at the Azapa and Lluta valleys, from sea level to altitudes of 822 and 634 m a.s.l., respectively. According to the multivariate analysis of echolocation parameters, recordings from Chile do not differ significantly from those obtained in Peru (Fig. 3). We also obtained an individual stored at the ISP from the Anzota caves, eight kilometers southward the city of Arica.
Three survey sites were omitted from the statistical analysis because of marked differences in spatial structure compared to the other sites. The omitted sites were more closed by buildings or because they were in a ravine. Otherwise, the studied sites in Chile and Peru were comparable agricultural or open urban spaces. Bats adjust call parameters according to the structure of the surroundings (Barclay et al. 1999; Gillam et al. 2009); in particular, closed sites result in higher peak frequencies that allow bats to better detect obstacles in the environment.
The previous southernmost record of P. davisoni (Flores et al. 2015) from Pampa Alta, Tacna is 125 km south from the earlier record, in Arequipa. As in southern Peru, in Northern Chile the valleys are separated by wide extensions of desert (Morrone 2006). It seems that P. davisoni is capable of crossing these barriers when the distance is smaller than 50-100 km. As such the species could be found as south as the Tana Valley, Tarapacá Region, Chile (19.5° S); surveys at that and intermediate valleys are needed to test this possibility.
Promops davisoni is difficult to capture using mist nets, although it is a common species in Arequipa, Peru (Pari et al. 2015). However, during our field work in Chile, utilizing more than 100 linear meters of mist-nets during 12 nights, we were not able to catch any individual of P. davisoni. This species forages at a high altitude, which is common for molossid bats, but can also fly at low altitude near light spots (J. Ugarte-Nuñez pers. comm.). The flight behaviour makes them difficult to capture in open spaces such as the principal valleys in the region. Known roost sites for this species are building roofs and rock crevices (Pari et al. 2015). The description of the echolocation calls of P. davisoni is an interesting output of this study for further surveys in the country of this difficult to collect species.
Promops davisoni is a recently revalidated species (Gregorin & Chiquito 2010) and is listed in the IUCN RedList as Data Deficient (Solari 2016), given that several key aspects of its biology and ecology are unknown. It seems that populations of the species are rare at the localities where it has been described (Solari 2016), nevertheless this statement is based on the low number of captured individuals. Because of the high abundance of recordings obtained at every sampled site in Chile, we agree with Pari et al. (2015) in that it is a common species.
As a consequence of this new species record, the known bat diversity of the Arica and Parinacota region increases to nine species, making it the most diverse Chilean region for bats. Chile has 14 bat species, including three molossids. Promops davisoni is the largest insectivorous bat recorded in the country, and it would be interesting to know its insect preys. It is urgent to understand the ecology of this species at a local scale and include it to the classification lists of the country, because of the rapid development of onshore wind farms in northern Chile, which represent a threat for high altitude bats (Jana & Pogacnik 2008; Ossa et al. 2015).
ACKNOWLEDGEMENTS
This study was financed by the University of Tarapacá (Undergraduate grants 4713-15 and 4715-16, and UTA Mayor 4710-15). We would like to thank SAG Arica and Parinacota region for providing authorization to capture bats (Resolución Exenta N°7547/2015); Nicole Alvarez for her help during the field work; Michelle Lineros and Tania Gatica from the ISP for their help to obtain and analyze the captured individual; Dr. Renato Gregorin for his help to identify the individual and to the editor Dr. Guillermo D'Elía, the reviewer Dr. Luis F. Aguirre and one anonymous reviewer for their input on a previous version of this work.
LITERATURE CITED
1. Barclay, R. M. R., J. H. Fullard, & D.S. Jacobs. 1999. Variation in the echolocation calls of the hoary bat (Lasiurus cinereus): influence of body size, habitat structure, and geographic location. Cannadian Journal of Zoology 77:530-534. [ Links ]
2. Bonacic, C., G. Ossa, L. M. Forero-Rozo, & J. Leichtle. 2016. Guía de campo: micromamíferos de la Región de Tarapacá. Serie Fauna Australis, Facultad de Agronomía e Ingeniería Forestal, Pontificia Universidad Católica de Chile, Santiago de Chile. [ Links ]
3. Briones-Salas, M., M. Peralta-Pérez, & M. García- Luis. 2013. Acoustic characterization of new species of bats for the State of Oaxaca, Mexico. Therya 4:15-32. [ Links ]
4. Diaz, M. M., S. Solari, L. F. Aguirre, L. M. S. Aguiar, & R. M. Barquez. 2016. Clave de identificación de los murciélagos de Sudamérica. PCMA (Programa de Conservación de los Murciélagos de Argentina), Tucumán. [ Links ]
5. Flores, M. G., G. C. Mamani, V. Pacheco, & G.A. Alvarado. 2015. Distribution of Promops davisoni Thomas, 1921 (Chiroptera: Molossidae) in Peru with a new record and southward range extension. Check List 11:1573. [ Links ]
6. Galaz, J. L., J. Torres-Murra, & J. Yañez. 1999. Platalina genovensium (Thomas, 1928), un quiróptero nuevo para la fauna de Chile (Phyyllostomatidae: Glossophaginae). Noticiario Mensual del Museo Nacional de Historia Natural 337:6-12. [ Links ]
7. Genoways, H. H., & S. L. Williams. 1979. Records of Bats Mammalia (Chiroptera) From Suriname. Annual Carnegie Museum 4818:323-335. [ Links ]
8. Gillam, E. H., G. F. Mccracken, J. K. Westbrook, Y. Lee, M. L. Jensen, & B. B. Balsey. 2009. Bats aloft: variability in echolocation call structure at high altitudes. Behavioral Ecology and Sociobiology 64:69-79. [ Links ]
9. Gregorin, R., & E. A. Chiquito. 2010. Revalidation of Promops davisoni Thomas (Molossidae). Chiroptera Neotropical 16:648-659. [ Links ]
10. Gregorin, R., & V. A. Taddei. 2002. Chave artificial para a identificacao de molossideos brasileiros (Mammalia, Chiroptera). Mastozoología Neotropical 9:13-32. [ Links ]
11. Jana, S., & M. Pogacnik. 2008. The impacts of wind farms on animal species. Acta Veterinaria Brno 58:615-632. [ Links ]
12. Jung, K., J. Molinari, & E. K. Kalko. 2014. Driving factors for the evolution of species-specific echolocation call design in New World free-tailed bats (Molossidae). PLoS One 9:e85279. [ Links ]
13. Kennedy, M. L., T. L. Best, & M. J. Harvey. 1984. Bats of Colima, Mexico. Mammalia 48:397-408. [ Links ]
14. Lapapiat, I., J. Yañez, J. L. Allendes, G. Ossa, & A. Rodríguez-San Pedro. 2016. Murciélagos de Chile: lineamientos para su estudio. Gestión Ambiental 32:5-18. [ Links ]
15. Luebert, F., & P. Pliscoff. 2006. Sinopsis bioclimática y vegetacional de Chile. Editorial Universitaria, Santiago de Chile. [ Links ]
16. Mann, G. 1950. Nuevos mamíferos de Tarapacá. Investigaciones Zoológicas Chilenas 1:4-6. [ Links ]
17. Mann, G. 1978. Los pequeños mamíferos de Chile. Gayana Concepción 40:1-342. [ Links ]
18. Marinkelle, C. J., & A. Cadena. 1973. Notes on bats new to the fauna of Colombia. Mammalia 36:49-58. [ Links ]
19. Morrone, J. J. 2006. Biogeographic areas and transition zones of Latin America and the Caribbean islands based on panbiogeographic and cladistics analyses of the entomofauna. Annual Review of Entomology 51:467-494. [ Links ]
20. O'farrell, M. J., & W. L. Gannon. 1999. A comparison of acoustic versus capture techniques for the inventory of bats. Journal of Mammalogy 80:24-30. [ Links ]
21. Ortiz de la Puente, D. J. 1951. Estudio monográfico de los quirópteros de Lima y alrededores. Publicaciones del Museo de Historia Natural Javier Prado 7:1-48. [ Links ]
22. Ossa, G. 2010. Métodos bioacústicos: una aproximación a la ecología de comunidades de murciélagos en las eco-regiones mediterránea y el bosque templado de Chile. Pontificia Universidad Católica de Chile, Santiago de Chile. [ Links ]
23. Ossa, G., C. Bonacic, & R. M. Barquez. 2014. First record of Histiotus laephotis (Thomas, 1916) from Chile and new distributional information for Histiotus montanus (Phillipi and Landbeck, 1861) (Chiroptera, Vespertilionidae). Mammalia 79:457-461. [ Links ]
24. Ossa, G., & F. Díaz. 2014. Histiotus magellanicus (Philippi 1866), un ignorado dentro de la mastofauna chilena. La Chiricoca 17:4-6. [ Links ]
25. Ossa, G., C. Juarez, & R, Vargas-Rodríguez. 2015. La conservación de los murciélagos y el desarrollo eólico: el caso de Chile. Boletín de la Red para la Conservación de los Murciélagos 6:3-4. [ Links ]
26. Ossa, G., K. Vilchez, & P. Valladares. 2016. New record of the rare long-snouted bat Platalina genovensium (Thomas, 1928) (Chiroptera, Phyllostomidae) in the Azapa valley, northern Chile. Check List 12:1850. [ Links ]
27. Pacheco, V., R. Cadenillas, E. Salas, C. Tello, & H. Zeballos. 2009. Diversidad y endemismo de los mamíferos del Perú. Revista Peruana de Biología 16:5-32. [ Links ]
28. Paqui, M. F., J. Muñoz-Garay, H. Mantilla-Meluk, & F. Sánchez. 2016. First record of Promops nasutus (Spix, 1823) (Chiroptera: Molossidae) from Colombia. Check List 12:1915. [ Links ]
29. Pari, A., K. Pino, C. Medina, E. Lopez, & H. Ceballos. 2015. Murciélagos de Arequipa, Historia Natural y Conservación. Arequipa, Perú [ Links ].
30. Pinheiro, J., & D. Bates. 2017. Linear and nonlinear mixed effects model. [ Links ]
31. Rodríguez-San Pedro, A., J.L. Allendes, & G. Ossa. 2016. Lista actualizada de los murciélagos de Chile con comentarios sobre taxonomía, ecología, y distribución. Biodiversity and Natural History 2:18-41. [ Links ]
32. Samaniego, H., & P.A. Marquet. 2009. Mammal and butterfly species richness in Chile: taxonomic covariation and history. Revista Chilena de Historia Natural 82:135-151. [ Links ]
33. Sandoval, M. L., M. S. Sánchez, & R. M. Barquez. 2010. Mammalia, Chiroptera Blumenbach, 1779: new locality records, filling gaps, and geographic distribution maps from Northern Argentina. Check List 6:64-70. [ Links ]
34. Siles, L., D. Peñaranda, J. C. Pérez-Zubieta, & K. Barboza. 2005. Los murciélagos de la ciudad de Cochabamba. Revista Boliviana de Ecología 18:51-84. [ Links ]
35. Solari, S. 2016. Promops davisoni. IUCN Red List of Threatened Species. Revised on July 10th 2017. [ Links ]
36. Velazco, P. M., R. Cadenillas, O. Centty, H. Liz, & H. Zamora. 2013. New records of Platalina genovensium (Chiroptera, Phyllostomidae) and Tomopeas ravus (Chiroptera, Molossidae). Mastozoologia Neotropical 20:425-434. [ Links ]
37. Winkler, D., A. Erdő, J. Mille & H. Kovács. 2014. New data on the distribution of the barbastelle bat (Barbastella barbastellus) in Western Hungary. Natura Somogyiensis 25:213-218. [ Links ]
38. Zeballos, H., V. Pacheco, & L. E. Baraybar. 2001. Diversidad y conservación de los mamíferos de Arequipa, Perú. Revista Peruana de Biología 8:94-104. [ Links ]
39. Zsebők, S., P. Estók & T. Görföl. 2012. Acoustic discrimination of Pipistrellus kuhlii and Pipistrellus nathusii (Chiroptera: Vespertilionidae) and its application to assess changes in species distribution. Acta Zoologica Academiae Scientiarum Hungaricae 58:199-209. [ Links ]














