Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Mastozoología neotropical
versão impressa ISSN 0327-9383versão On-line ISSN 1666-0536
Mastozool. neotrop. vol.25 no.1 Mendoza jun. 2018
ARTÍCULO
The Genus Dasyprocta Illiger, 1811 (Mammalia: Rodentia) in Colombia
Héctor E. Ramírez-Chaves1, María C. Calderón-Capote2, and Andrés Felipe Suárez-Castro2,3
1 Departamento de Ciencias Biológicas, Facultad de Ciencias Exactas y Naturales, Manizales, Colombia. [Correspondencia: Héctor E. Ramírez-Chaves <hector.ramirez@ucaldas.edu.co>]
2 Grupo de Mastozoología, Grupo de Conservación y Manejo en Vida Silvestre, Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Bogotá, Colombia.
3 Centre for Biodiversity and Conservation Science, The University of Queensland, Brisbane, Qld 4072, Australia.
Recibido 13 febrero 2017.
Aceptado 12 agosto 2017.
Editor asociado: S Solari
ABSTRACT.
Two species of Dasyprocta are currently recognized in Colombia. However, the taxonomic history of this genus is complex, with several available names for different populations. Here, we explore intra and interspecific variation of the two species currently recorded in Colombia, D. fuliginosa y D. punctata, and evaluate whether morphological characters allow the separation between specimens from different geographical regions. In addition, we clarify the application of subspecific names to different populations in the country and the distribution patterns of D. punctata subspecies. We show that D. fuliginosa and D. punctata are allopatric in Colombia. D. fuliginosa is distributed in the Amazon, Orinoco and part of the North Andean regions (eastern foothills of the Eastern Cordillera), whereas D. punctata is found in the Caribbean, Chocó and western foothills of North Andean regions of the country. Although there are no clear morphometric or morphological differences in the skull between most taxa, different coat colorations are associated with different geographical regions and contrasting ecosystems. D. punctata candelensis is distributed in the High Magdalena River Basin, North-Andean Province, instead of the Chocó Region as suggested by other authors. D. punctata chocoensis is found in the Colombian Pacific coast and western foothills of the Western Cordillera; D. punctata colombiana is found in the northern part of Colombia in the Caribbean Region. We also found specimens matching the description of D. punctata zuliae from the North-Andean Province in the Eastern Cordillera of Colombia. Despite the present geographic differences in coloration, further revisionary work—including genetic approaches—is needed to further understand the diversity of the genus in Colombia.
RESUMEN.
El género Dasyprocta Illiger, 1811 (Mammalia: Rodentia) en Colombia.
Actualmente se reconocen dos especies del género Dasyprocta en Colombia. Sin embargo, la historia taxonómica del género es compleja, con varios nombres disponibles para diferentes poblaciones. En este trabajo exploramos la variación intra e interespecífica de las dos especies reconocidas en Colombia, D. fuliginosa y D. punctata, y evaluamos los caracteres morfológicos que permiten la separación entre ejemplares procedentes de diferentes regiones geográficas del país. Además, clarificamos la asignación de los nombres subespecíficos a diferentes poblaciones y los patrones de distribución de las subespecies de D. punctata. Dasyprocta fuliginosa y D. punctata son alopátricas en Colombia. Dasyprocta fuliginosa se distribuye en las regiones de la Amazonía, la Orinoquía y parte de la región Norandina (estribaciones orientales de la cordillera Oriental), mientras que D. punctata se encuentra en las regiones Caribe, Chocó y al occidente de la cordillera Oriental en la región Norandina. Aunque no hay variación morfométrica o morfológica craneal entre la mayoría de los taxones, diferentes coloraciones están asociadas con diferentes regiones geográficas y ecosistemas contrastantes. D. punctata candelensis se distribuye en la cuenca alta del río Magdalena, en la provincia Norandina, en lugar de la región del Chocó como ha sido sugerido por otros autores. D. punctata chocoensis se encuentra en la región Pacífica de Colombia y las estribaciones occidentales de la cordillera Occidental; D. punctata colombiana se encuentra en el norte de Colombia en la región Caribe. Además, encontramos ejemplares que se ajustan a la descripción de D. punctata zuliae de la Provincia Norandina en la cordillera Oriental de Colombia. A pesar de las diferencias geográficas de coloración, un trabajo de revisión adicional, que incluya aproximaciones genéticas, es necesario para entender mejor la diversidad de este género en Colombia.
Key words: Amazon; Andes; Chocó; Coat pattern; Distribution.
Palabras clave: Amazonia; Andes; Chocó; Distribución; Patrones de coloración.
INTRODUCTION
The genus Dasyprocta Illiger, 1811 comprises at least 10 species of medium-size rodents commonly known as agoutis, distributed from southern Mexico to northern Argentina (Patton & Emmons 2015). Although agoutis are easily identified externally from other Neotropical medium-sized rodents (e.g., Myoprocta, Coendou, Cuniculus), the number of species, their distinctive morphological characters and distributions are poorly understood (Patton & Emmons 2015). As many of the species are questionable and based only on geographic distribution (Voss et al. 2001; Woods & Kilpatrick 2005), the genus is in need of a modern revision along its entire range (Voss et al. 2001; Patton & Emmons 2015).
In Colombia, two species are currently recognized: D. punctata Gray, 1842, distributed in the Andes, Caribbean, and Pacific regions (Solari et al. 2013; Patton & Emmons 2015), and Dasyprocta fuliginosa Wagler, 1832, distributed in the Amazon and Orinoco regions. In addition, four taxa have type localities in the country. Bangs (1898) described Dasyprocta colombiana based on specimens from the Santa Marta District in northern Colombia. Allen (1915) described Dasyprocta fuliginosa candelensis, with the type locality in the Department of Huila, and mentioned that this subspecies was distributed northward along the eastern slope of the Eastern Andes to the vicinity of Bogotá. Allen (1915) also described Dasyprocta variegata chocoensis for the Pacific Region of Colombia. Finally, Thomas (1917) described Dasyprocta pandora based on a specimen collected in Gorgona Island. Currently, these four taxa are considered subspecies or junior synonyms of D. punctata Gray, 1842 (Patton & Emmons 2015), with pandora being probably extinct (Alberico 1986), and only known from two specimens. There are no subspecies recognized within D. fuliginosa (Patton & Emmons 2015).
Although species and subspecies of Dasyprocta in Colombia are tentatively recognized following geographic distribution and coloration patterns (Allen 1915; Patton & Emmons 2015), there are no detailed reviews of color and morphological variation among populations from different regions, complicating the assignment of some populations to specific taxonomic units. In this sense, dissimilarities between the recognition of the identity of subspecies have led to contradictions about the sympatric or allopatric nature of both species. Some authors (e.g. Eisenberg 1989; Patton & Emmons 2015) suggested D. fuliginosa and D. punctata are allopatric species; however, previous checklists of mammals of Colombia (Cuervo Díaz et al. 1986; Alberico et al. 2000; Solari et al. 2013) suggested that both species are sympatric at least in the Upper Magdalena River Basin. This lack of resolution complicates the progress of ecological studies and the development and implementation of conservation plans for these species (Smythe 1978; Merrit 1983; Aliaga- Rossel et al. 2008; Jorge 2008).
Here, we reviewed coat coloration and qualitative and quantitative cranial characters of specimens of Dasyprocta from different regions of Colombia aiming to explore intra and interspecific variation of the taxa currently recorded in the country. In addition, we clarify whether both taxa present allopatric distributions using morphometrics and the analysis of discrete characters. We also explore whether certain forms are restricted to different geographic regions and provinces of the country (Table 1; Hernández Camacho et al. 1992), and propose their assignment to the available subspecific epithets.
Table 1 Distribution of the genus Dasyprocta in seven geographical provinces of Colombia: AM, Amazonia; CA, Peri-Caribbean Arid Belt; CH-MG, Chocó-Magdalena; GU, Guyana; SN, Sierra Nevada de Santa Marta; NA, North Andean; OR, Orinoco. Question marks denote the expected presence of the species based on records from neighboring provinces. Elevation range (E) in meters. Other abbreviations are: EEE: Eastern slopes of the Eastern Cordillera. HMR: High Magdalena River Basin. WEW: Western slopes of the Western Cordillera.

MATERIAL AND METHODS
We reviewed coat coloration, and cranial characters of 132 specimens of the genus Dasyprocta from Colombia (Appendix) housed at the following collections: American Museum of Natural History, New York (AMNH), Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Bogotá (ICN), Instituto de Investigaciones Biológicas, Alexander von Humboldt, Villa de Leyva (IAvH), Museo de Historia Natural, Universidad de Caldas, Manizales (MHN-UCa), and Museo de Historia Natural, Universidad del Cauca, Popayán (MHNUC). External characters, including total length (TL), length of tail (LT), and length of hindfoot (HF), were obtained from the original labels.
Additionally, we took 15 cranio-dental measurements using digital calipers to the nearest 0.01 for morphometric analyses following Ojasti (1972) and Voss et al. (2001): Condyle-incisive Length (CIL), Length of Diastema (LD), Maxillary Toothrow (MTR), Length of Molars (LM), Breadth of M1 (BM1), Breadth of P4 (BP4), Breadth of Palatal Bridge (BPB), Length of Nasals (LN), Least Interorbital Breadth (LIB), Breadth of Braincase (BB), Zygomatic Breadth (ZB), Zygomatic Length (ZL), Maximum Postpalatal Width (MPW), Maximum Anterorbital Width (MAW), Width of the Middle Part of the Rostrum (WMPR).
To define morphological groups and assign available subspecific names, we followed the descriptions provided by Allen (1915), Thomas (1917), Ojasti (1972) and Patton & Emmons (2015). In addition, we evaluated coloration patterns of 47 adult and subadult specimens and explored three discrete cranial characters for morphological group separation in 74 skulls: nasofrontal suture shape, presence/absence of the posterior medial process of the palatine, and the orbital surface of the maxilla interrupted or not by the lacrimal bone (Teta & Lucero 2016). We associated each detected morphological group to different geographic provinces of the country according to Hernández Camacho et al. (1992).
A principal component analysis (PCA) was performed on log transformed cranio-dental variables to evaluate whether the nominal taxa were morphometrically distinct. To keep a low ratio of variables to specimens for the analysis and account for the relatively small sample sizes within groups (Zuur et al. 2007), we selected just a subset of the available measurements. To this end, we used a Spearman correlation analysis and retained one variable from each of the pairs coefficients > 0.8. In addition, we only used variables that were available for more than 90 % of the specimens. The final variables selected were CIL, MTR, BPB, LN, LIB, and BB. Previous work had identified ZB as an important measurement to differentiate some Dasyprocta species (Teta & Lucero 2016), so we also included this variable in the multivariate analyses.
In addition, we used a stepwise canonical discriminant function analysis to investigate whether specimens identified by color differences could be assigned to geographic location using craniometric variables and to identify which variables most effectively separated geographic groups. Correct group classification was verified using leave-one-out cross validation. For this analysis, variables were size adjusted using a geometric mean procedure, where raw values for each individual were divided by the geometric mean of all measurements from that individual and then transformed using natural logarithms (Jungers et al. 1995; Dumont 2004). Specimens identified as D. punctata chocoensis from two different natural regions (Chocó and Andes) were included as independent subgroups of the same taxon to explore whether the contrasting geographic origin of both populations can be informative for this analysis. The significance level was set at P < 0.05 to evaluate what variables significantly discriminate between the different groups. We only used adult specimens for all the multivariate analyses (n = 35). For the age classification (juveniles, subadults, adults and old adults), we used maxillary tooth eruption and cranial suture closure following Voss & da Silva (2001). Analyses were performed in R version 3.1.3 (R Core Team 2015) using the packages MASS (Venables & Ripley 2002) and klaR (Weihs et al. 2005).
RESULTS
Coat coloration patterns
Based on coat coloration (Fig. 1, Table 2), we identified discrete groups distributed in different geographic regions. Reddish and yellowish patterns appear in the Peri-Caribbean Arid Belt, North-Andean and Chocó-Magdalena regions. Specimens from the Low Magdalena basin and nearby locations (south of Córdoba and Bolivar departments; n = 5) exhibited a dorsal black/ brown coat with yellowish tips and a black middle line giving a yellowish to reddish coloration pattern (Fig. 1a). Specimens from the Caribbean Region and the Peri-Caribbean Arid Belt Province (n = 8) exhibited a light yellowish dorsum with a faint dark middle line. These specimens exhibit the external pattern of the type series of D. punctata colombiana. A second group with a yellowish coloration pattern but smaller size than D. punctata colombiana (Table 2) was found in the western (Barrancabermeja/Sabana de Torres) and central parts (Encino) of the Department of Santander and is here identified as D. punctata zuliae (Fig. 1b), based on coat coloration and size measurements available in literature (Ojasti 1972). In contrast, specimens of D. punctata chocoensis (n = 15) had orange to reddish tones, stronger in the flanks, whereas the dorsal surface can either have or not a black conspicuous middle line. This coloration is also observed in specimens from the western slopes of the Western Cordillera (Cauca, El Tambo, 1800 m; MHNUC 276), in the Andean Region adjacent to the biogeographic Chocó (Fig. 1c).

Fig. 1. Distribution map of Dasyprocta in Colombia showing the typical coloration patterns in each region. a) Filled circles: D. punctata colombiana; b) Star: D. punctata zuliae; c) Triangles: D. punctata chocoensis; d) Filled stars: D. punctata candelensis; e) Squares: D. fuliginosa.
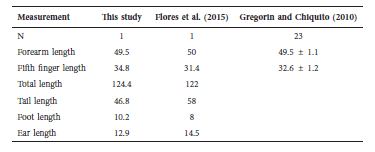
Table 2 Coloration patterns and measurements (Mean [range] and n) of the species and subspecies of Dasyprocta from Colombia. Abbreviations of the measurements in the text.; n = total of specimens analyzed.
Dark coat colorations with yellowish tips were observed in specimens from the High Magdalena River Basin (n = 7), an inter- Andean valley located between the Central and Eastern cordilleras (Fig. 1d). This pattern has been previously assigned to individuals of D. punctata candelensis. Finally, dark coat coloration, varying from dark brown to blackish, is characteristic of specimens from the Amazonia and Orinoco regions (n = 11), that are usually identified as D. fuliginosa (Fig. 1e). Specimens from the Amazonia exhibited more scarcely white tips in the dorsum when compared to those from the Guyana and Orinoco, but there are not strong differences between specimens of these regions.
Cranial analyses
We failed to detect any discrete cranial character that allows separation of morphological groups within Dasyprocta (Table 3). In D. fuliginosa (n = 27 of skulls available for review), D. punctata chocoensis (n = 3), and D. punctata zuliae (n = 10), the nasofrontal suture (NSF) was either U or W-shaped (W-shaped in 63 %, 33%, and 80 %, respectively). In D. punctata candelensis (n = 11) the NSF was U-shaped in only one subadult specimen. In three specimens (100 %) of D. punctata colombiana the NSF was W- shaped. The posterior medial process of the palatine (PMP) was only observed in 75 % of individuals of D. punctata chocoensis (n = 4), and slightly projected in 25 % of the specimens of D. punctata zuliae (n = 20), 14 % of the specimens of D. punctata candelensis (n = 14), 40 % of D. punctata colombiana (n = 5), and 3.6 % of specimens of D. fuliginosa (n = 28). The orbital surface of the maxilla (OSM) was not interrupted by the lacrimal in 66.7 % of the specimens of D. punctata candelensis (n = 9), 52.6 % of specimens of D. punctata zuliae (n = 19), and in 68 % of specimens of D. fuliginosa (n = 22). Only one sub-adult specimen of D. punctata candelensis presented the OSM not interrupted by the lacrimal; thus, this character seems to be affected by age.
Table 3 Occurrence of three qualitative skull characters in Dasyprocta from Colombia. PMP: Posterior medial process of the palatine (A, absent; P, present). NSF: Nasofrontal suture (U or W-shaped). OSM: Orbital surface of the maxilla (Int, interrupted by the lacrimal; Ni, not interrupted by the lacrimal). -: No data.

Multivariate analyses using craniometric variables showed no clear morphometric gap between D. fuliginosa and D. punctata. In contrast, there was a trend in size for the subspecies of D. punctata. The first three axes of the PCA explained 83 % of the craniometrical variation and all variables area positively loaded in the principal component 1 (Fig. 2, Table 4), which suggests that this component represents size. Specimens identified as D. punctata colombiana from the Peri-Caribbean Arid Belt Province clustered together in the PCA plot, representing the largest subspecies of D. punctata, whereas those from the north part of the North-Andean Province (D. punctata zuliae) were the smallest. In addition, specimens of D. punctata candelensis overlapped with specimens of D. punctata chocoensis, although they can be separated based on coat coloration (Fig. 1, Table 2). The second principal component (PC2) carried high positive loadings for BPB and a high negative loading for LN (Table 4). In general, specimens of D. fuliginosa tended to have large values for BPB and low for LN when compared to specimens of D. punctata (Table 2). However, there was not a clear separation of groups along this component (Fig. 2).
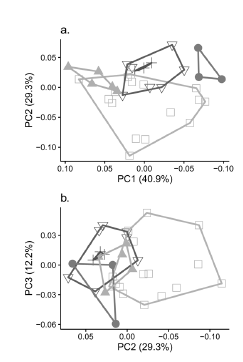
Fig. 2. Results of the PCA for 35 adult specimens of five taxa of Dasyprocta in Colombia: a) first and second principal components, b) second and third principal components. Squares: D. fuliginosa; triangles: D. punctata zuliae; inverted triangles: D. punctata chocoensis; crosses: D. punctata candelensis; filled circles: D. punctata colombiana.
Table 4 Loadings of the first three components of the PCA for Dasyprocta species in Colombia. See abbreviations in text.

When individuals were grouped based on coloration pattern and geographic origin, the discriminant function analysis showed a clearer segregation of individuals and correctly classified 28 of the 35 specimens (i.e. 80 %) (Fig. 3, Table 5). For this analysis, the first two axes accumulated 85.2 % of the variance. With the exception of two specimens from the eastern foothills of the North-Andean Region in the Meta and Boyacá departments (AMNH 136307 and ICN 2998 respectively), D. fuliginosa formed a homogeneous group, well separated from D. punctata. All the individuals of D. punctata candelensis were grouped with specimens of D. punctata chocoensis from the Chocó Region. Combining the discriminant functions for LN, ZB and BB allowed to predict all but 0.139 of the variation among groups (F = 4.150, P = < 0.0005; Table 5).

Fig. 3. Canonical axes of the discriminant analysis for 35 adult specimens of five taxa of Dasyprocta in Colombia. D. fuliginosa (Amazonia and Orinoco regions); triangles: D. punctata zuliae (North Andean Region); crosses: D. punctata candelensis (North Andean Region); filled circles: D. punctata colombiana (Peri-Caribbean Arid Belt Region). Filled squares: D. punctata chocoensis (El Tambo, Cauca; North Andean Region); Diamonds: D. punctata chocoensis (Chocó Region). Specimens identified as D. punctata chocoensis were included as independent subgroups considering their contrasting geographic origin.
Table 5 Summary of the stepwise discriminant analysis of six linear, size-adjusted, cranial shape variables comparing Dasyprocta specimens from different regions in Colombia. See abbreviations in text.

DISCUSSION
Our analyses show that D. fuliginosa and D. punctata have allopatric distributions in Colombia, with D. fuliginosa distributed east of the Andes (including eastern slopes of the Eastern Cordillera), in the Amazon and Orinoco regions of the country, and D. punctata distributed in the North-Andean (except the eastern slopes of the Eastern Cordillera), Caribbean, and Chocó regions. These results contrast with previous checklists of mammals of Colombia (Cuervo Díaz et al. 1986; Alberico et al. 2000; Solari et al. 2013) that reported sympatry of these species in the Magdalena River Basin of the North Andean Region.
Based on the yellowish coloration pattern and the smaller size when compared to D. punctata colombiana (Ojasti 1972), we assigned specimens from the western (municipality of Barrancabermeja/Sabana de Torres) and south-central Santander (municipalities of Bolivar and Encino) in the North-Andean Region of the eastern Cordillera of Colombia to D. punctata zuliae (Fig. 1b). The distribution of this subspecies also encompasses areas in northwestern Venezuela from lowlands to the Sierra de Perijá in Colombia (Ojasti 1972).
Based on geographic distributions, we agree with Eisenberg (1989) and Patton & Emmons (2015) in regarding the name candelensis as a subspecies of D. punctata rather than of D. fuliginosa. However, D. punctata candelensis is distributed in the High Magdalena River Basin, North-Andean Province instead of the Chocó Region as suggested by these authors (Fig. 1d). The information provided by Patton & Emmons (2015) regarding the origin of candelensis is contradictory, considering that Allen (1915) defined the type locality as "La Candela (altitude 6500 feet [1981 m]), Huila, Colombia". In fact, La Candela is located in the western slopes of the Eastern Cordillera, approximately 170 km far from the Pacific Region, and separated from the latter by the Central and Western cordilleras (Fig. 1). In addition, the Department of Huila is not adjacent to the Pacific Region. Therefore, candelensis is not the earliest name applicable to the Pacific coast populations in South America, as stated by Patton & Emmons (2015). In this context, D. punctata chocoensis is the name applicable to the populations of the Colombian Pacific coast and western slopes of Western Cordillera.
In general, coat coloration is the main trait to differentiate subspecies within the genus Dasyprocta. Coloration patterns have been used as a major basis for the delimitation of species within Dasyprocta and to emphasize the general allopatric nature of populations (Ojasti 1972; Emmons & Feer 1997; Iack- Ximenes 1999; Feijó & Langguth 2013; Teta & Lucero 2016). In contrast, craniometrics analyses showed no clear morphometric gap separating specimens of different subspecies as discrete groups, but there was a trend in size for the subspecies of D. punctata. For example, specimens of D. punctata colombiana from the Caribbean Region and D. punctaca zuliae from the North-Andean Region that are larger and smaller respectively when compared with the other subspecies of D. punctata (Table 2). A larger sample size is needed to clarify whether or not clinal variation bridges the apparent gap in coloration between both taxa.
Considering that different coat colorations are associated with geographical regions and contrasting ecosystems, it is necessary to evaluate whether D. punctata comprises a species complex with slight morphological variation. If this is the case, there are several available names for these groups in Colombia (chocoensis, candelensis, colombiana); however, additional names from Panama and Venezuela (e.g. isthmica, apurensis) should be also considered (Ojasti 1972; Patton & Emmons 2015). To address these questions, genetic information could play an import role by providing additional, independent data to confirm or reject the suggested allopatry between species of Dasyprocta and seek for cryptic genetic diversity within the genus. As suggested for other medium sized rodents in Colombia (Ramírez-Chaves & Solari 2014; Ramírez-Chaves et al. 2014), we highlight the need of further revisionary work based on additional specimens, aimed to understand the intra and inter-specific variation and ontogenetic changes in cranial characters. This information is crucial to categorize and implement actions to preserve these heavily hunted mammals, which play important roles across a high variety of ecosystems (e. g., Salm 2006).
ACKNOWLEDGMENTS
Hugo López (ICN), Pilar Rivas and Hernando Vergara (MHNUC), Claudia Medina and Socorro Sierra (IAvH), and Paúl Velazco (AMNH) facilitated access to museum specimens. AF Suárez-Castro is supported by a PhD Scholarship from COLCIENCIAS (call 529). H. E. Ramírez- Chaves thanks Rufford Small Grants (Grant 23710-1) and Vicerrectoría de Investigaciones de la Universidad de Caldas (project 0223418) for support.
LITERATURE CITED
1. Alberico, M. 1986. Los mamíferos. Isla de Gorgona (H. von Prahl & M. Alberico, eds.). Universidad del Valle, Biblioteca Banco Popular, Cali. [ Links ]
2. Alberico, M., A. Cadena, J. I. Hernández-Camacho, & Y. Muñoz-Saba. 2000. Mamíferos (Synapsida: Theria) de Colombia. Biota Colombiana 1:43-75. [ Links ]
3. Aliaga-Rossel, E., R. W. Kays, & J. M. V. Fragoso. 2008. Home-range use by the Central American agouti (Dasyprocta punctata) on Barro Colorado Island, Panama. Journal of Tropical Ecology 24:367-374. [ Links ]
4. Allen, J. A. 1915. New South American mammals. Bulletin of the American Museum of Natural History 34:625-634. [ Links ]
5. Bangs, O. 1898. Description of some new mammals from The Sierra Nevada de Santa Marta, Colombia. Proceedings of the Biological Society of Washington 12:161-165. [ Links ]
6. Cuervo Díaz, A., J. Hernández Camacho, & A. Cadena G. 1986. Lista actualizada de los mamíferos de Colombia: anotaciones sobre su distribución. Caldasia 15:471-501. [ Links ]
7. Dumont, E. R. 2004. Patterns of diversity in cranial shape among plant-visiting bats. Acta Chiropterologica 6:59-74. [ Links ]
8. Eisenberg, J. F. 1989. Mammals of the Neotropics: the northern Neotropics. Volume 1. Panama, Colombia, Venezuela, Guyana, Suriname, French Guiana. The University of Chicago Press, Chicago. [ Links ]
9. Emmons, L. H., & F. Feer. 1997. Neotropical Rainforest Mammals: a field guide. The University of Chicago Press, Chicago, Illinois. [ Links ]
10. Feijó, A., & A. Langguth. 2013. Mamíferos de médio e grande porte do nordeste do Brasil: distribuição e taxonomia, com descrição de novas espécies. Revista Nordestina de Biologia 22:3-225. [ Links ]
11. Hernández Camacho, J., A. Hurtado-Guerra, R. Ortiz-Quijano, & T. Walschburger. 1992. Unidades biogeográficas de Colombia. La diversidad biológica de Iberoamérica. Vol. I (G. Halffter, ed.). Acta Zoológica Mexicana, Nueva Serie, Vol. Especial, México, D.F. [ Links ]
12. Jorge, M. L. S. P. 2008. Effects of forest fragmentation on two sister genera of Amazonian rodents (Myoprocta acouchy and Dasyprocta leporina). Biological Conservation 141:617-23. [ Links ]
13. Jungers, W. L., A. B. Falsetti, & C. E. Wall. 1995. Shape, relative size, and size-adjustments in morphometrics. American Journal of Physical Anthropology 38:137-161. [ Links ]
14. Iack-Ximenes, G. E. 1999. Sistemática da família Dasyproctidae Bonaparte, 1838 (Rodentia, Hystricognathi) no Brasil. Dissertação de Mestrado, Universidade de São Paulo, São Paulo. [ Links ]
15. Merrit Jr, D. A. 1983. Preliminary observations on reproduction in the Central American agouti, Dasyprocta punctata. Zoobiology 2:127-131. [ Links ]
16. Ojasti, J. 1972. Revisión preliminar de los picures o agutíes de Venezuela (Rodentia, Dasyproctidae). Memorias de la Sociedad de Ciencias Naturales La Salle 32:159-204. [ Links ]
17. Patton, J. L., & L. H. Emmons. 2015. Family Dasyproctidae Bonaparte, 1838. Mammals of South America, Volume 2. Rodents (J. L. Patton, U. F. J. Pardiñas & G. D'Elía, eds.). The University of Chicago Press, Chicago and London. [ Links ]
18. R Core Team. 2015. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. <http://www.R-project.org/> [ Links ].
19. Ramírez-Chaves, H. E., & S. Solari. 2014. Sobre la disponibilidad del nombre Cuniculus hernandezi Castro, López y Becerra, 2010 (Rodentia: Cuniculidae). Actualidades Biológicas 36:59-62. [ Links ]
20. Ramírez-Chaves, H. E., A. F. Suárez-Castro, & B. D. Patterson. 2014. Re-examining the hypothesis of allopatric distribution of Myoprocta acouchy and M. pratti (Mammalia: Dasyproctidae) in South America. Papéis Avulsos de Zoologia 54:447-456. [ Links ]
21. Salm, R. 2006. Invertebrate and vertebrate seed predation in the Amazonian palm Attalea maripa. Biotropica 38:558-560. [ Links ]
22. Smythe, N. 1978. The natural history of the Central American agouti (Dasyprocta punctata). Smithsonian institutional Press, Washington D.C. [ Links ]
23. Solari, S., Y. Muñoz-Saba, J. V. Rodríguez-Mahecha, T. R. Defler, H. E. Ramírez-Chaves, & F. Trujillo. 2013. Riqueza, endemismo y conservación de los mamíferos de Colombia. Mastozoología Neotropical 20:301-365. [ Links ]
24. Teta, P., & S. O. Lucero. 2016. ¿Cuántas especies del género Dasyprocta (Rodentia, Dasyproctidae) hay en la Argentina? Mastozoología Neotropical 23:193-199. [ Links ]
25. Thomas, O. 1917. Notes on agoutis with descriptions of new forms. Annals and Magazine of Natural History 20:310-313. [ Links ]
26. Venables, W. N., & B. D. Ripley. 2002. Modern applied statistics with S. Fourth Edition. Springer, New York. [ Links ]
27. Voss, R. S., & M. N. F. da Silva. 2001. Revisionary notes on Neotropical Porcupines (Rodentia: Erethizontidae). 2. A review of the Coendou vestitus group with descriptions of two new species from Amazonia. American Museum Novitates 3351:24-32. [ Links ]
28. Voss, R. S., D. P. Lunde, & N. B. Simmons. 2001. The mammals of Paracou, French Guiana: a Neotropical lowland rainforest fauna Part 2. Nonvolant species. Bulletin of the American Museum of Natural History 263:1-236. [ Links ]
29. Weihs, C., U. Ligges, K. Luebke, & N, Raabe. 2005. klaR Analyzing German Business Cycles. Data Analysis and Decision Support (D. Baier, R. Decker & L. Schmidt- Thieme, eds.). Springer-Verlag, Berlin. [ Links ]
30. Woods, C. A., & C. W. Kilpatrick. 2005. Infraorder Hystricognathi Brandt, 1855. Mammal Species of the World: a taxonomic and geographic reference. 3a ed., vol. 1, 2 (D. E. Wilson & D. M. Reeder, eds.). The Johns Hopkins University Press, Baltimore, Maryland, USA. [ Links ]
31. Zuur, A. F., E. M. Ieno, & G. M. Smith. 2007. Analysing Ecological Data. Springer, New York. [ Links ]
Specimens reviewed. Localities are transcribed directly from specimens labels.
Dasyprocta fuliginosa (n = 57): Amazonas: Leticia, en la trocha de Tabatinga a Tacana, 8 km al este de Leticia (Brasil) (IAvH 385: skin + skull); Río Amacayacu, Puerto Mogue, cerca de las bocas de la Quebrada Cahuinas, PNN Amacayacu (IAvH 1870: skin + skull); Río Igará-Paraná, 50 km arriba de La Chorrera (IAvH 2322: skin + skull); Leticia, PNN Amacayacu (IAvH 6026: skin; IAvH 5528: skull; IAvH 5744: skull); Leticia, PNN Amacayacu, río Amacayacu (IAvH 5372: skull); Araracuara (IAvH 3846: skull; ICN 6461: skull). Boyacá: Pajarito (ICN 2995: skull; ICN 3000: skull); no additional data (ICN 2996, ICN 2997, ICN 2998, ICN 2999, ICN 3019: all skull). Boyacá: Miraflores (ICN 124: skull). Caquetá: Río Caquetá, Araracuara, zoocriadero (IAvH 4155: skin + skull; IAvH 5299: skull); Araracuara (IAvH 5248: skull); Río Cuemaní, a 30 km de su desembocadura sobre el río Caquetá, 290 m (IAvH 2331: skin + skull). Cauca: Santa Rosa, vereda La Planada, 1100 m (IAvH 6043: skull). Meta: Restrepo (AMNH 136303: skin + skull; AMNH 136307: skull; AMNH 136308: skull); Restrepo, El Retiro (AMNH 136304: skin + skull); Villavicencio - Restrepo, Caibe (AMNH 136305: skin + skull); Villavicencio (AMNH 136306: skin + skull; ICN 118: skull; ICN 2071: skull; ICN 3019: skull; ICN 3105: skull; ICN 3107: skull); raudal del caño Cafre, alrededores de la cabaña (IAvH 2191: skin + skull); Parque Nacional Natural - PNN La Macarena, 20 m oeste de cabaña Duda (IAvH 2540: skin + skull); no additional data (ICN 2069: skull; ICN 2894: skull). Putumayo: Umbría (ICN 10044: skin + skull). Vaupés: Yunipari (IAvH 1543: skin + skull); Río Apaporis (ICN 776; ICN 777; ICN 778: all skull). Vaupés: Laguna El Churuco (ICN 4437: skull). Vichada: El Tuparro, Río Tomo, Centro administrativo (IAvH 556: skin + skul); PNN El Tuparro, El Tapón (IAvH 2001: skin; IAvH 4079: skull); PNN El Tuparro, río Orinoco (IAvH 2002: skin); PNN El Tuparro, centro Administrativo (IAvH 3845: skin + skull; IAvH 5884: skull); Territorio Faunístico El Tuparro, Puerto Nuevo, Río Tomo, 14 km al norte del Tapón (IAvH 492: skull); Territorio Faunístico El Tuparro, encontrado en sabana entre maypunes y cabaña (IAvH 1023: skull); Territorio Faunístico Tuparro, bosque cerca del centro Administrativo (IAvH 1294: skull); El Tuparro, 500 m al sur del Centro administrativo (IAvH 1444: skull); a 30 millas de San José de Ocuné, Curaliunuatsia (IAvH 1530: skull); Sabanitas, orilla izquierda del río Guaviare (ICN 8500: skull); PNN El Tuparro (IAvH 3977: skull); PNN El Tuparro, Bocas del Tomo (IAvH 4078: skull); no additional data (ICN 119: skull; ICN 2759: skull).
Dasyprocta punctata: D. p. candelensis (n = 16): Boyacá, Toguí (ICN 15405, ICN 15408: all skull). Cauca, Tierradentro (ICN 185, ICN 196, ICN 197; all skull). Cundinamarca: Chaguaní, Valle Medio del Magdalena (IAvH 4108; skull); Medina (ICN 10185: skull). Huila: San Agustín, 5000 feet (AMNH 33901: skin + skull); Altamira, Andalucía, 3000 feet (AMNH 33903: skin + skull); Yacopí (ICN 15949; ICN 16362; ICN 16363; ICN 15949: all skull). Tolima: Icononzo (ICN 8879: skull); Río Chili (AMNH 69170: skin + skull); Mariquita, La Parroquia, 500-900 m (ICN 1770: skin); purchased at Bogotá (AMNH 34558: skin); no additional data (AMNH 33904: skin + skull). D. p. chocoensis (n = 18): Cauca: El Tambo, 1800 m (MHNUC 276: skin + skull); Sabanetas (AMNH 181598; skin + skull; AMNH 181509: skin + skull). Chocó: Riosucio, vereda El Tilupo, parte superior de la Quebrada El Tilupo, Salto El Tilupo (IAvH 3100; skin + skull; IAvH 3848: skin + skull; IAvH 3850: skin + skull; IAvH 3852: skin + skull; IAvH 3854: skin + skull); Riosucio, vereda Peye, PNN Los Katíos (IAvH 3849: skin; IAvH 3851: skin + skull); Riosucio, Hacienda Cacarica (IAvH 3853: skin + skul); PNN Ensenada de Utría (IAvH 7507: skin; IAvH 7508: skin; IAvH 7512: skin; IAvH 7366: skull); Reserva Forestal Las Teresitas (IAvH 3980: skull). Valle del Cauca: Cali, Río Oscuro (AMNH 14179: skin + skull); Los Cisneros (AMNH 32154: skin + skull). D. p. colombiana (n = 18): Antioquia: Fredonia, vereda Potosí (IAvH 379: skin; IAvH 3847: skull); Fredonia, Hacienda Potosí, Ex Inderena, regional occidental de Medellín, Programa Vida Silvestre 378 (IAvH 1020: skin + skull). Córdoba: SW Puerto Libertador, 75°29'40"N, 75°52´00"W (IAvH 7378: skull; IAvH 7379: skull). Magdalena: Santa Marta, Bonda (AMNH 14870: skin + skull; AMNH 14871: skin + skull; AMNH 15435: skin + skull; AMNH 15436: skin + skull; AMNH 15437: skin+ skull; AMNH 15438: skin + skull; AMNH 15439: skin; AMNH 15440: skin + skull); Santa Marta, Sierra Nevada de Santa Marta, vereda Alto Guachaca, valle del rio Guachaca (IAvH 7373: skull; IAvH 7374: skull); Santa Marta, Parque Nacional Natural Tayrona, sector Bahía Concha, sector Los Ciruelos, Ciruelos 1, 120 m (ICN CCG 012; skull). Sucre: Tolú, Hacienda La Eitanzuela, sector El Bobo, bosque La Culebra, 100 m (IAvH 4098: skull). D. p. zuliae (n = 20): Cesar: Perijá (ICN 18570, ICN 18571: all skull). Santander: Bolívar, vereda La Guinea, Serranía de Las Quinchas (MHN-UCa 879; MHN-UCa 881: all skull); El Encino (ICN 16411; ICN 16412, ICN 16413; ICN 16414; ICN 16415; ICN 16416; ICN 16417; ICN 16419; ICN 16420; ICN 16421: all skull); El Encino, Reserva Cachalú, límite SFF Guanetá - Alto Río Fonce, 1970 m (IAvH 6805: skull); no additional data (ICN 3832, ICN 3833, ICN 3834, ICN 3835; ICN 3836: all skull)
Dasyprocta sp.: Colombia: No additional data (IAvH 7417: skull; IAvH 7418: skull).














