Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Mastozoología neotropical
versão impressa ISSN 0327-9383versão On-line ISSN 1666-0536
Mastozool. neotrop. vol.25 no.1 Mendoza jun. 2018
ARTÍCULO
Diversity of cave bats in the brazilian tropical dry forest of Rio Grande do Norte State
Juan Carlos Vargas-Mena1, Eugenia Cordero-Schmidt1, Diego de Medeiros Bento2, Bernal Rodriguez-Herrera3, Rodrigo A. Medellín4, and Eduardo M. Venticinque1
1 Departamento de Ecologia, Centro de Biociências, Universidade Federal do Rio Grande do Norte, 59.072-970, Lagoa Nova, Natal, RN, Brasil [Correspondence: <jcvargasmena@gmail.com>].
2 Centro Nacional de Pesquisa e Conservação de Cavernas (CECAV) – Base Avançada Compartilhada no Rio Grande do Norte, Natal, RN, Brasil.
3 Escuela de Biología, Universidad de Costa Rica, 2060 Montes de Oca, San José, Costa Rica.
4 Instituto de Ecología, Universidad Nacional Autónoma de México, 70-275, 04510 México D. F., México.
Recibido 12 enero 2017.
Aceptado 26 septiembre 2017.
Editor asociado: M Weber
ABSTRACT.
Caves are important roosts for bats in karstic areas and play a critical role in the protection of bat populations. In the state of Rio Grande do Norte, in northeastern Brazil, cave-dwelling bats are poorly studied. Moreover, the state contains more than 900 caves, mainly in the Caatinga biome, that may offer important roosts for local bat populations. Thus, to gain a first insight into the richness, diversity, and colony size of cave-bats in the state, we sampled 13 caves through active search and captures with mist nets. Sixteen species from five families were recorded, and the biggest colonies belonged to Pteronotus gymnonotus and Phyllostomus discolor. Furna Feia cave was the richest, with 10 species. Our results showed that Rio Grande do Norte is home to a rich and abundant diversity of cave bats, including vulnerable species like Furipterus horrens, Natalus macrourus, and Lonchorhina aurita. This study is the first to determine the diversity of RN cave bats, providing useful fundamental data for future conservation actions.
RESUMO.
Morcegos cavernícolas na Caatinga do Rio Grande do Norte.
As cavernas são abrigos importantes para morcegos em ambientes cársticos e desempenham um papel fundamental para a proteção de suas populações. No estado do Rio Grande do Norte, os morcegos cavernícolas têm sido pouco estudos, no entanto, o estado contém um grande número de cavernas (~ 900) que poderiam abrigar uma grande diversidade de morcegos. A fim de determinar a riqueza, diversidade e tamanhos de colônias de morcegos cavernícolas no estado, foram amostradas 13 cavernas durante três dias consecutivos por cada caverna mediante busca ativa e capturas com redes de neblina. Foram capturadas 16 espécies pertencentes a cinco famílias onde as maiores colônias achadas pertenceram às espécies Pteronotus gymnonotus e Phyllostomus discolor. A caverna Furna Feia abrigou a maior riqueza com 10 espécies. Nossos resultados mostraram que o Rio Grande do Norte abriga uma rica e abundante diversidade de morcegos cavernícolas, incluindo espécies vulneráveis como Furipterus horrens, Natalus macrourus e Lonchorhina aurita. Este estudo é o primeiro a determinar a diversidade de morcegos cavernícolas no RN a fim de fornecer dados úteis para futuras ações de conservação.
Key words: Assemblage; Caatinga; Cave-dwelling bats; Colony size; Richness.
Palavras chaves: Assembleia; Caatinga; Morcegos cavernícolas; Riqueza; Tamanho de colônia.
INTRODUCTION
Caves and other underground cavities are optimal roosts for bats because they are thermally stable and humid environments that protect bats against weather and predators (Kunz 1982). Consequently, these sites are used by bats for social interactions, breeding, care of offspring, and as hibernacula in temperate zones (Humphrey 1975). Consequently, roosting caves play a fundamental role in protecting bat populations, particularly for those forming large colonies in a single cave (Arita 1993).
Bats are known to be important pollinators and seed dispersers of plants in tropical and subtropical habitats, including several plant species used by humans (Medellín & Gaona 1999; Lobova et al. 2009). Furthermore, bats are effective predators of vertebrates and invertebrates (Kalka et al. 2008). Likewise, cave bats are essential to the maintenance of cave ecosystems. They are responsible for the input of organic matter into the caves through guano deposition that sustains complex invertebrate communities (Gnaspini & Trajano 2000; Ferreira et al. 2010). However, such essential ecological services may be disrupted as a consequence of incidental disturbances, extractive industries, and uncontrolled visitation inside caves (Furey & Racey 2016).
In Brazil, at least 58 bat species have been recorded using caves as a main or alternative roost (Guimarães & Ferreira 2014) and from a speleological perspective, the country is also very rich, with more than 12 000 caves recorded to date (CECAV 2016). However, less than 5% of its underground cavities are known (Jansen et al. 2012). The available data of Brazilian cave-bat communities comprise surveys in only 269 caves, mainly on the Atlantic Forest and Cerrado biomes in the central and the southern regions, leaving the Amazon and the Caatinga in the north and northeastern region, respectively, undersampled and poorly known (Guimarães & Ferreira 2014).
The Brazilian tropical dry forest, or Caatinga, is a semi-arid ecoregion endemic to northeastern Brazil (MMA/IBAMA 2011). The Caatinga is one of the less studied ecoregions in Brazil, and that includes its bat fauna (Oliveira et al. 2003). Furthermore, the ecoregion is experiencing rapid land degradation and desertification, as a result of human activities, such as deforestation and agriculture (Leal et al. 2003).
Located in the northeastern region of Brazil, the state of Rio Grande do Norte (RN) occupies the northeasternmost area of South America, and 95% (49 714 km2) of its territory is covered by Caatinga vegetation. Additionally, the state has a high speleological potential, with more than 900 underground cavities registered so far (CECAV 2016). To date, only 26.5% of those cavities are situated in areas under integral protection, while the remaining cavities are prone to human disturbances (Bento et al. 2013).
The bat fauna of RN is one of the most significant data gaps of Brazil (Bernard et al. 2011) and the available data of cave-dwelling bats in RN comes from bio-speleological inventories, recording just nine species for the state (Coelho 2006; Ferreira et al. 2010). Therefore, we report the results of a series of surveys in caves in the Caatinga of RN, which allowed us to assess their species richness, magnitudes of colony size, species-cave distribution, and their conservation panorama in the state.
MATERIALS AND METHODS
Site description
This study was developed in three areas within the Caatinga in the state of RN, in northeastern Brazil (Fig. 1). The Caatinga is a seasonal, deciduous, tropical, dry forest characterized by plant species with thorns and small leaves (microphyllous), with some xerophytic characteristics. Leaves and flowers are produced during a short rainy season and the dry season is leafless and dormant for much of the year (Leal et al. 2003). The rainy season extends from February to April, with an average annual precipitation ranging from 240-1500 mm (Leal et al. 2003), although long-lasting droughts are frequent and subjected to annual variations (Leal et al. 2005).

Fig. 1. Map of study area in the Caatinga of Rio Grande do Norte, northeastern Brazil, showing political limits of the states. The patch of pale gray corresponds to the distribution of the Caatinga ecoregion, and the dark gray area to other ecoregions. Location of caves in the Furna Feia National Park (A) and in the Felipe Guerra Cave System (B) are shown in the circles. Geographical coordinates of surveyed caves are found in Table 1.
The studied areas are located about 330 km from the state capital, Natal, on the west portion of the state (Fig. 1). The surveys were developed in 13 calcareous caves (Table 1) distributed into two caves systems and in one locality with an isolated cave. The first cave system is located in Furna Feia National Park (FFNP), in the municipalities of Mossoró and Baraúna. The FFNP was created in June 2012, be ing the first national park established in the state, with an area of 8494 ha that integrally protects 248 underground cavities (Bento et al. 2013).
Table 1 Caves surveyed in the Caatinga of Rio Grande do Norte, northeastern Brazil, from June to October of 2015. For each cave data of horizontal development (HD) in meters, area (A) in squared meters, volume (V) in cubic meters and coordinates are given.

The second cave system, hereafter Felipe Guerra Cave System (FGCS), is located 58 km south of FFNP and distributed into three municipalities (Felipe Guerra, Governador Dix-sept Rosado, and Caraúbas). This karstic area contains ~ 37.8% (341) of the known underground cavities of RN (CECAV 2016). However, none are formally protected (Bento et al. 2015). The third study site is the Gruta dos Trinta, an isolated unprotected cave, situated in the municipality of Mossoró.
Data collection
From June 8 to October 23 of 2015, we surveyed the caves to determine their bat species richness, composition, and estimation of colony sizes. Each cave was surveyed during three consecutive days, except for the Gruta dos Trinta, which was surveyed for two days. On each of the 3 days, active searches and bat captures were conducted. The active search consisted of an exhaustive search inside the cave, by illuminating colonies and individuals during daylight, which ensures that all the bats that were found at that moment were using the cave as a day roost (Kunz et al. 2009). Binoculars and cameras were used to assist in the identification of individuals.
The colony size was estimated for each species in each cave. When the number of clustered bats was small (< 30), we were usually able to count all individuals. The bat counting was done exclusively by the same two observers (authors J. C. V. M. and E. C. S.) in all caves. When medium-sized colonies were found (< 100−200) (e.g., Desmodus rotundus, Glossophaga soricina) we took photos and counted later, from either or both the camera screen and computer. However, the direct counting method may be acceptable for small and compact colonies, but for large clustered active colonies, estimation of colony size through direct observation may not be reliable (Kunz et al. 2009). Considering that we encountered large-sized colonies (> 200) (Phyllostomus discolor, G. soricina, and Pteronotus gymnonotus) on only three occasions, the estimation of such colonies sizes was assessed by one of the authors (R. A. M) with about 25 years of experience studying cave bats.
From the quantitative data of the direct bat counting, we determined the colony size by assigning them into categories. Despite the possible bias, given that this method may lead to an over- or underestimation of the colony sizes by the observers, the current study aimed to provide a preliminary insight into the bat colonies, in order of magnitude. Also, we did not attempt to estimate the absolute population size or census the bat colonies. The sizes of the colonies were divided into the magnitude categories used by Arita (1996): 1 (Night visitor); 2 (< 10 individuals); 3 (1-100); 4 (101-1000); 5 (1001-10 000); 6 (> 10 000). We added category "Night visitor," corresponding to individuals that were captured entering the cave at night but no colony was spotted during the diurnal active search, suggesting the use of the cave as a night roost (probably as feeding perches).
Mist-nets were set at the cave entrance, or entrances, from 17:30 to 24:00 h, to capture emerging bats and, thereby, confirm species identification and detect other roosting species overlooked on the day active search. The number and size (3-12 m long) of the mist-nets used, depended on the size of the cave entrance. In multiple entrance caves, entrances were covered with a plastic tarp and any other possible exit holes or cracks were blocked to persuade bats to emerge into the mist-net sites at selected entrances.
Using the available natural history information about the primary diet of the bats in Brazil (Reis et al. 2007, 2013), the recorded species were classified into trophic guilds based on Hill & Smith (1984). However, we substituted the foliage-gleaning insectivorous and carnivorous guilds for gleaning animalivorous guild, given that bats belonging to these guilds can have both an insectivore and carnivore diet (e.g., Tonatia bidens, Trachops cirrhosus, and Chrotopterus auritus) (Gardner 2007). Guilds were aerial insectivores, gleaning animalivores, piscivores, frugivores, nectarivores, omnivores, and sanguinivores.
Bat capture and handle protocols followed the guidelines of the American Society of Mammalogists for the use of wild mammals in research by Sikes et al. (2011). Bats were identified using a combination of information from books, field guides, identification keys, and articles with descriptions of species occurring in Brazil (Willig 1983; Gardner 2007; Reis et al. 2007, 2013). Collected individuals were deposited in the collection of mammals of the Universidade Federal do Rio Grande do Norte (UFRN). The survey and collection of specimens were approved by the Brazilian environmental agency (SISBIO license number 48325-2 MMA, IBAMA, and ICMBio). Taxonomy and nomenclature followed Gardner (2007).
Statistical analysis
We computed species accumulation curves to measure the inventory efficacy for each cave system. We did not compute a curve for the Gruta dos Trinta Cave because a 2-day survey did not give us sufficient data to build a curve for this cave. The curves were obtained by using the number of days as a measure of sampling effort. The sample order was randomized 100 times, to eliminate the influence of the order in which days were added to the total surveyed days.
The bat distribution patterns among the surveyed caves were determined using the nestedness temperature calculator (NTC), based on Atmar & Patterson (1995) and Rodríguez-Gironés & Santamaria (2006). The NTC calculates a matrix temperature T, which is a measure of species presences and absences or system 'disorder,' where 0 corresponds to a perfectly nested matrix and 100 indicates a random species distribution pattern. We empirically assessed its significance using Monte Carlo techniques, with 5000 null models generated by the swap algorithm (Gotelli & Entsminger 2003). The NTC (function nestedtemp), null model tests (function oecosimu), and species accumulation curves (function specaccum) were performed using the vegan package (Oksanen et al. 2016) in R software (R Development Core Team, 2016).
RESULTS
We recorded 16 bat species of five families using the caves as day or night roost in the 13 sampled caves (Fig. 2). The mean richness was 6 ± 2 species, ranging from two species (Caverna do Arapuá in FGCS) to 10 species (Furna Feia cave in FFNP) (Table 2). The FGCS had the highest richness, with 14 species, followed by the FFNP, with 10 species and the Gruta dos Trinta, with five species. Phyllostomidae was the most common family with 12 species, followed by Emballonuridae, Mormoopidae, Furipteridae, and Natalidae, all with one species each.

Fig. 2. Species of bats captured in thirteen caves during June to November 2015 in the Caatinga of Rio Grande do Norte, Brazil. Aerial Insectivores are: a) Peropteryx macrotis, b) Pteronotus gymnonotus, c) Natalus macrourus, d) Furipterus horrens. Gleaning Animalivorous are: e) Tonatia bidens, f) Lonchorhina aurita, g) Micronycteris sp., h) Chrotopterus auritus, i) Trachops cirrhosus. Sanguinivorous are: j) Diphylla ecaudata, k) Desmodus rotundus. Nectarivorous are: l) Glossophaga soricina, m) Lonchophylla mordax. Frugivorous are n) Artibeus lituratus, o) Artibeus planirostris; and Omnivorous are: p) Phyllostomus discolor. Species with a yellow star are vulnerable species in Brazil.
Table 2 Community composition, colony sizes (rank categories) and occurrence of cave bats in thirteen caves in the Caatinga of Rio Grande do Norte from June to October of 2015. Colony size categories in ranges of number of individuals: Night visitor (1); <10 (2); 11-100 (3); 101-1000 (4); 1001-10 000 (5); >10000 (6). Trophic guilds are: Aerial Insectivore (AI); Gleaning Animalivore (GA); Frugivore (F); Nectarivore (N); Omnivore (O); Sanguinivore (S). Caves are: Furna do Urubu (FU); Caverna Boa (CB); Gruta da Carrapateira (FC); Gruta dos Três Lagos (GTL); Caverna do Arapuá (CA); Caverna Lajedo Grande (CLG); Gruta Capoeira do João Carlos (CJC); Gruta Casa de Homens (GCH); Furna Feia (FF); Furna Nova (FN); Caverna da Pedra Lisa (CPL); Caverna Porco do Mato (CPM); and Gruta dos Trinta (GT). Occurrence per cave corresponds to the number of caves where each species occurred. Percentage of occurrence is in relation to the 13 sampled caves.


The species accumulation curve suggested that we performed a good sampling effort for each of the caves system (Fig. 3). The curve for FFNP almost reached an asymptote after 8-10 days, while the curve for FGCS almost stabilized at the end of the survey. Such curves suggest that a small number of species (1-3) will be added with a few more survey days for each cave system.

Fig. 3. Species accumulation curves found at (a) Felipe Guerra Cave System and (b) Furna Feia National Park of cave-dwelling bats in the Caatinga of Rio Grande do Norte, Brazil. The curves were obtained by using the number of days as sampling effort. The sample order was randomized 100 times to eliminate the influence of the order in which nights were added to the total surveyed nights.
In both cave systems, we recorded bats of all trophic guilds except piscivores (Fig. 4). The Gruta dos Trinta cave contained only three guilds (Table 2). The most common species were Peropteryx macrotis and Diphylla ecaudata, which occurred in 84% of the surveyed caves, followed by D. rotundus (69%), and Furipterus horrens and T. bidens (61%). Species, such as Lonchophylla mordax, C. auritus, Artibeus lituratus, and P. gymnonotus were recorded roosting in just one cave. Lonchorhina aurita, Micronycteris sp., and T. cirrhosus were only found in the Gruta dos Três Lagos.

Fig. 4. Taxonomic (a) and trophic guild (b) composition of cave bat assemblages in the Caatinga of Rio Grande do Norte State in northeastern Brazil.
Most of the encountered species (11 of 16 species) formed small colonies (categories 2 and 3) (Table 2). The largest colony belonged to the mormoopid P. gymnonotus, with ca. 10 000 individuals (category 6) in one of the two big rooms of the Furna do Urubu cave in FGCS. A colony of about 1000 individuals (category 5) of P. discolor was found in the lower rooms of the Furna Feia cave (Table 2) in the FFNP. In the Caverna Boa in the FGCS, we found the largest colony of D. rotundus of about 100 to 150 individuals (category 4) and a large colony of G. soricina, with ca. 300-400 individuals (category 4) in the Gruta dos Trinta cave in Mossoró.
We recorded four species (D. ecaudata, T. bidens, Artibeus planirostris, and A. lituratus) using caves as night roost (category 1) (Table 2). The most common species using caves as night-roosts was T. bidens, which was captured entering into five different caves (in two caves it was captured carrying cerambycid beetles), but no roosting colonies of this species were found during this study.
The matrix community temperature presented an observed temperature (23.64º) that was not significantly different from that expected by the 5000 simulations of Monte Carlos's null models (mean = 24.57º, confidence interval = 21.85−29.34°, p = 0.6829), indicating that the cave bat assemblages in the studied caves do not have a nested distribution pattern (Fig. 5).

Fig. 5. Distribution of bat species among caves in the Caatinga of Rio Grande do Norte, Brazil. Filled squares shows the presence of a given species in each cave. The matrix community temperature was 23.64º not significantly different (p = 0.6829) from Monte Carlos's null models (mean = 24.57º). Cave are: (A) Furna Feia, (B) Gruta dos Três Lagos, (C) Furna Nova, (D) Furna da Carrapateira, (E) Caverna Boa, (F) Gruta dos Trinta, (G) Furna do Urubu, (H) Gruta Capoeira de João Carlos, (I) Caverna Porco do Mato, (J) Caverna da Pedra Lisa, (K) Gruta Casa de Homens, (L) Caverna do Lajedo Grande, and (M) Caverna do Arapuá.
Finally, we collected 15 specimens, representing 9 species sampled through the captures, as evidence material and deposited in the Mammal Collection Prof. Adalberto Varela (CMAV) of the UFRN. These nine species included D. ecaudata (CMAV135), F. horrens (CMAV 137), G. soricina (CMAV147), L. N. macrourus (CMAV126, CMAV148), P. macrotis (CMAV129; 130; 131; 133), P. gymnonotus (CMAV125, CMAV127), and T. bidens (CMAV138).
DISCUSSION
Bat diversity
This study is the first to provide details about the diversity, assemblages and colony sizes of the cave bats of the state of RN. The record of F. horrens, N. macrourus, and L. aurita in the studied caves reveals that the caves in RN are refugia of three (43%) of the seven endangered species of bats in Brazil (MMA/ICMBio 2014). Also, we found that the bat richness at the surveyed caves (n = 16) is higher than the records of Coelho (2006) (n = 9) and Ferreira et al. (2010) (n = 8). All bat species reported in those studies were found in the present study. Additionally, however, we recorded seven new species using RN's caves as a day or night roost, or both, including C. auritus, P. discolor, A. lituratus, P. gymnonotus, F. horrens, L. mordax, and Micronycteris sp. (Table 2).
The recorded cave bat assemblages are dominated by members of the Phyllostomidae family (75%), particularly by gleaning-animalivorous bats (subfamily Phyllostominae), followed by a few species of nectarivore, frugivore and sanguinivore bats (Fig. 4). Aerial insectivorous bats were also common members and were found in all the surveyed caves (Table 2). Such bat composition patterns seem to be common in Brazilian cave bat assemblages (Trajano 1995).
The two studied cave systems presented bat richness (FGCS = 14 spp. and FFNP = 10 spp.) similar to other cave systems found in Brazil (Table 3). However, higher richness (> 15 spp.) has been found in other cave systems in the Cerrado (Bredt et al. 1999; Esbérard et al. 2005; Félix et al. 2016), Atlantic Forest (Trajano 1985) and Amazon (Zortéa et al. 2015) ecoregions. Within the context of Caatinga, our findings show that the FGCS is one of the cave systems with the highest bat diversity in the ecoregion, surpassed only by the Chapada Diamantina National Park with records on 15 species (Gregorín & Mendes 1999; Oliveira & Pessôa 2005; Sbragia & Cardoso 2008).
Table 3 Studies on cave bat assemblages in karstic areas and cave systems in Brazil. For each cave system is presented data on: locality and state; ecoregions that corresponds to Amazon (Am), Atlantic Forest (AF), Cerrado (Ce) and Caatinga (Caa); richness of bat species per locality; sampling effort that corresponds to number of sampled caves in each locality; and studies references.
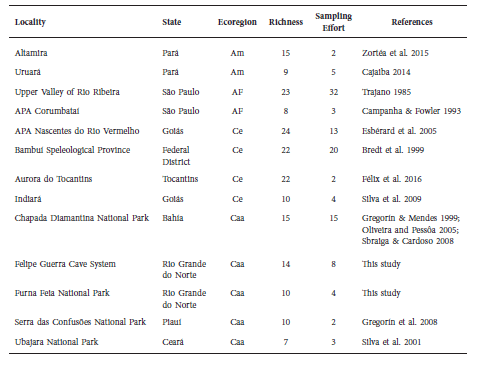
Nevertheless, studies and inventories on cave-dwelling bats in multiple caves at karstic areas in Brazil are scarce, considering the large number of underground cavities registered in the country (Janzen et al. 2012). Additionally, the abovementioned studies contain differences with our study, as they have differentiated sampling efforts and number of sampled caves (Table 3). Thus, our comparison across studies is in a general perspective.
Regarding the Caatinga, it is noteworthy that the set of studies on its cave bat assemblages, including ours, have minimally sampled the totality of the underground cavities in such an ecoregion. Indeed, only 2% of registered underground cavities in the Caatinga (ca. 1800, see Jansen & Pereira 2014) have been sampled for bat inventories (Guimarães & Ferreira 2014) and is likely that in this ecoregion, the diversity of cave bats is larger.
Species distribution
The species distribution between bats and cave roosts on our studied cave systems did not present a nested pattern, unlike that found by Arita (1993, 1996) in Mexico and Colombia (Cardona-Ramírez 2012) where cave bat assemblages presented a nested structure. This measure of structure in ecological systems in the Neotropics regarding cave bats has been poorly explored and is difficult to determine if nested or non-nested patterns are common in these assemblages. However, our observed temperature matrix (t = 23.64ºC) presented closer values to a nested structure (t = 0 °C) than to a random one (t = 100 °C), even though it was not significantly different from that of the null models. Given the high number of caves in the state, we encourage the bat inventories in more caves in the state, to verify if the cave bat assemblages in the region follow a nested pattern or remain non-nested.
Conservation concerns
Although only 2% of the caves registered in Brazil have been surveyed for bats, more than a quarter of the bat species recorded in Brazil (58 of the 178) have been found roosting in caves and other underground cavities (Guimarães & Ferreira 2014). However, after the establishment of the Decree 6640 (see Instrução Normativa MMA No-2 20/08/2009), the protection of Brazilian caves was drastically reduced, threatening the rich underground ecosystems, bat populations, and other cave fauna (Bernard et al. 2012).
In RN, underground cavities suffer from illegal mining, exploration and extraction of oil (petroleum), unorganized rural settlements, agriculture expansion, and disorderly cave visitation that causes negative effects on the rich cave fauna of the state (Cruz et al. 2010). Such disturbances are known to be major causes of cave bat population declines around the world (Furey & Racey 2016), and due to their low annual reproductive rates, bat populations are unable to recover quickly after a population decline associated with human activities (Racey & Entwistle 2000).
During our surveys, we found trace amounts of heavy, uncontrolled visitation (waste, ropes, clothes, candles, debris) in two caves, including Gruta dos Trinta in Mossoró and Gruta dos Três Lagos in the FGCS. Such caves presented unique bat assemblages by harboring vulnerable species and large uncommon colonies that were not recorded in the FFNP or any other cave under protection. Therefore, their conservation panorama is of concern.
For instance, the Gruta dos Trinta cave is regularly visited according to the local people, because it is easily accessed and is closely located (~5 km) to the metropolitan area of Mossoró city, the second largest city in RN. The bat community in this cave is particular, is intensively used by colonies of the nectarivores Glossophaga soricina and Lonchophylla mordax (Table 2). Such colonies are likely providing a valuable ecological service in the area since nectar-eating bats are important pollinators of plants of economic and ecological importance in semi-arid environments (e.g., Agavaceae, Cactaceae) (Kunz et al. 2011). Moreover, the cave roosts vulnerable species like Natalus macrourus and the near threatened L. mordax (IUCN 2016), whose distribution has been restricted as a consequence of a taxonomic revision (see Moratelli & Dias 2015).
The Gruta do Três Lagos in FGCS is in a similar scenario to Gruta dos Trinta cave. The cave is located about 1 km from the Felipe Guerra town and contains three year-round water pools visited by the residents on weekends, according to the local consulted people. Even though the cave is not intensively used by bats, it had a relatively high richness (Table 2) and harbored the only known colony in the state of L. aurita, another vulnerable species in Brazil. We encourage a deeper monitoring of L. aurita and L. mordax in the abovementioned caves, to better understand the roosting natural history (e.g., cave use, mating systems) of these poorly known species.
From the cave system perspective, it is evident that bat diversity of the FGCS is under no current protection. According to our data, the cave system is an important area for RN's cave bats. It harbors colonies of three national vulnerable species of bats (F. horrens, N. macrourus, and L. aurita) (Table 2) and recorded the highest diversity of bats among cave systems (FFNP = 10 species) with 14 species (Fig. 3), namely, 87% of the total diversity of cave bats in RN.
Moreover, the FGCS harbors the large colony (> 10 000) of the aerial insectivore P. gymnonotus found at the Furna do Urubu cave. This cave can be considered a "hot cave" (see Ladle et al. 2012) and was the only cave of this type registered in the state. Bats that roost in hot caves (e.g., mormoopids, natalids and phyllostomids) tend to have limited geographic distribution, and some species that only occur in hot caves environments (genera Mormoops, Pteronotus, Natalus, Monophyllus, Erophylla and Phyllonycteris) maintain unique communities of cave arthropods through guano deposition (Ladle et al. 2012). Furthermore, the presence of the colony in the area is likely to provide benefit to local agricultural activities, given aerial insectivore bats are known to be important controllers of arthropod populations including those considered as crop pests (Boyles et al. 2011).
Efforts to establish a conservation unit in the FGCS are ongoing. Given that the cave bat assemblages in RN presented a non-nested pattern, the future conservation strategies in the area should contemplate the protection of multiple caves. Such an approach is necessary because the protection of solely the richest species caves will not fully include the totality of the bat species, including vulnerable species, in the cave system. However, a more deeply and multidisciplinary approach for the prioritization of cave protection in FGCS in needed urgently, given the chiropterological and speleological relevance that the cave system possesses. We expect that the caves discussed as conservation concerns in this study will be considered for future conservation actions.
We found at the FFNP an important cave bat fauna integrally protected. According to our data, the park contains the cave with the highest richness of cave bats in the state—the Furna Feia cave—with 10 recorded species including the biggest known colony of P. discolor in the state. Besides harboring the richest diversity of bats in a single cave, the Furna Feia cave has a potential use as a tourist attraction, due to its large size (Table 1) and scenic beauty. Moreover, the national park also harbors vulnerable species, such as F. horrens and N. macrourus (Table 2). Being the only national park in RN, the park protects 248 underground cavities, a significant number for the integral conservation of the speleological heritage in the state (Bento et al. 2013).
This study provided the first step to unravel the great diversity of cave bats in RN. We encourage the exploration of more caves for bat inventories and, furthermore, the mid- and long-term monitoring of the caves presented herein, to understand the ecological dynamics of the bat assemblages in response to the seasonality of the Caatinga. If this is done, it will be possible to generate more accurate data, enabling better conservation actions for the cave bats in the state.
ACKNOWLEDGMENTS
Major thanks to CNPq (Pesquisador Visitante Especial- PVE "Ecologia e Conservação de Morcegos na Caatinga Potiguar" Project: 401467/2014-7) for the financial support and CNPq (309458/2013-7) professorships provided to EMC. To WCS Brazil for the key support in field logistics. Special thanks to Valtenci Santana and Iatagan Mendes for their patience and great help in the field and to Helder Mateus Viana Espírito-Santo for his valuable contribution on data analysis.
LITERATURE CITED
1. Arita, H. T. 1993. Conservation biology of the cave bats of Mexico. Journal of Mammalogy 74:693-702. [ Links ]
2. Arita, H. T. 1996. The conservation of cave bats of Yucatan, Mexico. Biological Conservation 76:177-185. [ Links ]
3. Atmar, W., & B. D. Patterson. 1995. The nestedness temperature calculator: a visual basic program, including 294 presence–absence matrices. AICS Research, Inc., University Park, NM and The Field Museum, Chicago.
4. Bento, D. M., J. C. Brandão, D. J. Dos Santos, J. I. M. Freitas, U. P. Campos, & R. F. R. Souza. 2013. Parque Nacional da Furna Feia: o parque nacional com a maior quantidade de cavernas do brasil. Anais do 32° Congresso Brasileiro de Espeleologia, Barreiras, Bahia (M. A. Rasteiro & L. Morato, eds.). Sociedade Brasileira de Espeleologia, Campinas, São Paulo. [ Links ]
5. Bento, D. M., J. C. Brandão, J. I. M. Freitas, & U. P. Campos. 2015. Área de Proteção Ambiental Pedra de Abelha: proposta para a conservação da maior concentração de cavernas do Rio Grande do Norte. Anais do 33vo Congresso Brasileiro de Espeleologia (M. A. Rasteiro & W. Sallun-Filho, eds.). Sociedade Brasileira de Espeleologia, Campinas, São Paulo. [ Links ]
6. Bernard, E., L. Aguiar, & R. B. Machado. 2011. Discovering the Brazilian bat fauna: A task for two centuries? Mammal Review 41:23-39. [ Links ]
7. Bernard, E. et al. 2012. Uma análise de horizontes sobre a conservação de morcegos no Brasil. Mamíferos do Brasil: Genética, Sistemática, Ecologia e Conservação (T. R. O. Freitas & E. M. Vieira, eds.). Sociedade Brasileira de Mastozoología, Rio de Janeiro. [ Links ]
8. Boyles, J. G., P. M. Cryan, G. F. Mccracken, & T. H. Kunz. 2011. Economic importance of bats in agriculture. Science 332:41-42. [ Links ]
9. Bredt, A., W. Uieda, & E. D. Magalhães. 1999. Morcegos cavernícolas da região do Distrito Federal, centro-oeste do Brasil. Revista Brasileira de Zoologia 16:731-770. [ Links ]
10. Cajaiba, R. L. 2014. Morcegos (Mammalia, Chiroptera) em cavernas no município de Uruará, Pará, norte do Brasil. Biota Amazônia 4:81-86. [ Links ]
11. Campanha, R. A. D. C, & H. G. Fowler. 1993. Roosting assemblages of bats in arenitic caves in remnant fragments of Atlantic Forest in Southeastern Brazil. Biotropica 362-365. [ Links ]
12. Cardona-Ramírez, D. M. 2012. Patrón anidado de distribución de murciélagos en un conjunto de cuevas del enclave seco de Chicamocha (Santander-Colombia). Master thesis. Universidad Internacional Menéndez Pelayo, Madrid, España. [ Links ]
13. CECAV - Centro Nacional de Pesquisa e Conservação de Cavernas. 2016. Base de Dados Geoespacializados de Cavidades Naturais Subterrâneas do CECAV, situação em 30/11/2016. <http://www.icmbio.gov.br/cecav/downloads/mapas.html> [ Links ]
14. Coelho, D. C. 2006. Levantamento da fauna de morcegos no Carste de Felipe Guerra, RN. Diagnóstico espeleológico do Rio Grande do Norte (J. Cruz, D. M. Bento, U. P. Campos & J. I. M. Freitas, eds.). Instituto Brasileiro de Meio Ambiente e dos Recursos Naturais Renováveis, Centro Nacional de Estudo, Proteção e Manejo de Cavernas. Brasília, DF. [ Links ]
15. Cruz, J. B., D. M. Bento, F. H. R. Bezerra, J. I. Freitas, & U. P. Campos. 2010. Diagnóstico espeleológico do Rio Grande do Norte. Revista Brasileira de Espeleologia 1:1-24. [ Links ]
16. Esbérard, C. E., J. A. Motta, & C. Perigo. 2005. Morcegos cavernícolas da Área de Proteção Ambiental (APA) Nascentes do Rio vermelho, Goiás. Revista Brasileira de Zoociências 7:311-325. [ Links ]
17. Felix, S., R. L. M. Novaes, R. F. Souza, & L. S. Avilla. 2016. Bat assemblage in a karstic area from northern Brazil: seven new occurrences for Tocantins state, including the first record of Glyphonycteris sylvestris Thomas, 1896 for the Cerrado. Check List 12:1999. [ Links ]
18. Ferreira, R. P., X. Prous, L. O. F. Bernardi, & M. Souza- Silva. 2010. Fauna subterrânea do estado do Rio Grande do Norte: caracterização e impactos. Revista Brasileira de Espeleologia 1:1. [ Links ]
19. Furey, N., & P. A. Racey. 2016. Conservation ecology of cave bats. Bats in the Anthropocene: Conservation of bats in a changing world (C. C. Voigt & T. kingston, eds.). Springer, New York. [ Links ]
20. Gardner, A. L. 2007. Mammals of South America, volume 1: marsupials, xenarthrans, shrews, and bats (Vol. 1). University of Chicago Press, Chicago. [ Links ]
21. Gnaspini, P., & E. Trajano. 2000. Guano communities in tropical caves. Ecosystems of the World. Ecosystems of the world. Subterranean ecosystems (H. Wilkens, D. C. Culver & W. F. Humphreys, eds.). Elsevier, Amsterdam. [ Links ]
22. Gotelli, N. J., & G. L. Entsminger. 2003. Swap algorithms in null model analysis. Ecology 84:532-535. [ Links ]
23. Gregorín, R., & L. D. F. Mendes. 1999. Sobre quirópteros (Emballonuridae, Phyllostomidae, Natalidae) de duas cavernas da Chapada Diamantina, Bahia, Brasil. Iheringia Série Zoologia 86:121-124. [ Links ]
24. Gregorin, R., A. P. Carmignotto, & A. R. Percequillo. 2008. Quirópteros do Parque Nacional da Serra das Confusões, Piauí, nordeste do Brasil. Chiroptera Neotropical 14:366-383. [ Links ]
25. Guimarães, M. M., & R. L. Ferreira. 2014. Morcegos cavernícolas do Brasil: novos registros e desafios para conservação. Revista Brasileira de Espeleologia 2:1-33. [ Links ]
26. Hill, J. E., & J. D. Smith. 1984. Bats: a natural history. University of Texas Press, Austin. [ Links ]
27. Humphrey, S. R. 1975. Nursery roosts and community diversity of Nearctic bats. Journal of Mammalogy 56:321-346. [ Links ]
28. IUCN – International Union for Conservation of Nature. 2016. The IUCN Red List of Threatened Species. Version 2016–3. <http://www.iucnredlist.org>
29. Jansen, D. C., L. F. Cavalcanti, & H. S. Lamblém. 2012. Mapa de potencialidade de ocorrência de cavernas no Brasil, na escala de 1:2.500.000. Revista Brasileira de Espeleologia 1:42-57. [ Links ]
30. Jansen, D. C., & K. N. Pereira. 2015. Distribuição e caracterização das cavernas brasileiras segundo a base de dados do CECAV. Revista Brasileira de Espeleologia 2:47-70. [ Links ]
31. Kalka, M. B., A. R. Smith, & E. K. Kalko. 2008. Bats limit arthropods and herbivory in a tropical forest. Science 320:71-71. [ Links ]
32. Kunz, T. H. 1982. Roosting ecology of bats. Ecology of bats (T. H. Kunz, eds.) Springer Science & Business Media, New York. [ Links ]
33. Kunz, T. H., M. Betke, N. I. Hristov, & M. J. Vonhof. 2009. Methods for assessing colony size, population size, and relative abundance of bats. Ecological and behavioral methods for the study of bats (T. H. Kunz & S. Parsons, eds.). Johns Hopkins University Press, Baltimore. [ Links ]
34. Kunz, T. H., E. Braun De Torrez, D. Bauer, T. Lobova, & T. H. Fleming. 2011. Ecosystem services provided by bats. Annals of the New York Academy of Sciences 1223:1-38. [ Links ]
35. Ladle, R. J., J. V. Firmino, A. Malhado, & A. Rodríguez‐ Durán. 2012. Unexplored diversity and conservation potential of Neotropical hot caves. Conservation Biology 26:978-982. [ Links ]
36. Leal, I. R., M. Tabarelli, & J. M. C. Silva. 2003. Ecologia e conservação da Caatinga. Editora Universitária da Universidade Federal de Pernambuco, Recife, Pernambuco. [ Links ]
37. Leal, I. R., J. Da Silva, M. Cardoso, M. Tabarelli, & T. E. Lacher. 2005. Changing the course of biodiversity conservation in the Caatinga of northeastern Brazil. Conservation Biology 19:701-706. [ Links ]
38. Lobova, T. A., C. K. Geiselman, & A. S. Mori. 2009. Seed dispersal by bats in the Neotropics. New York Botanical Garden, The Bronx. [ Links ]
39. Medellin, R. A., & O. Gaona. 1999. Seed dispersal by bats and birds in forest and disturbed habitats of Chiapas, Mexico. Biotropica 31:478-485. [ Links ]
40. MMA/IBAMA – Ministério do Meio Ambiente/ Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis. 2011. Monitoramento do bioma Caatinga 2008-2009. Monitoramento do desmatamento nos biomas brasileiros por satélite. Acordo de cooperação técnica MMA/IBAMA, Brasília.
41. MMA/ICMBio - Ministério de Meio Ambiente/ Instituto Chico Mendes de Conservação da Biodiversidade. 2014. Lista Nacional Oficial de Espécies da Fauna Ameaçadas de Extinção. Diário Oficial da União, Portaria MMA nº 444, de 17 de dezembro de 2014. [ Links ]
42. Moratelli, R., & D. Dias. 2015. A new species of nectar-feeding bat, genus Lonchophylla, from the Caatinga of Brazil (Chiroptera, Phyllostomidae). ZooKeys 514:73–91.
43. Oksanen, J. et al. 2016. vegan: community ecology package. R package version 2.3-4. <http://CRAN.R-project.org/package=vegan> [ Links ].
44. Oliveira, J. D., & L. M. Pessôa. 2005. Mamíferos. Biodiversidade e conservação da Chapada Diamantina. Ministério do Meio Ambiente, Brasília. [ Links ]
45. Oliveira, J. A., P. Rodriguez Gonçalves, & C. R. Bonvicino. 2003. Mamíferos da Caatinga. Ecologia e conservação da Caatinga (I. R. Leal, M. Tabarelli & J. M. Cardoso Da Silva, eds.). Editora Universitária da Universidade Federal de Pernambuco, Recife, Pernambuco. [ Links ]
46. R Core Team. 2016. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. <http://www.R-project.org/> [ Links ]
47. Racey, P. A., & A. E. Entwistle. 2000. Life history and reproductive strategies of bats. Reproductive biology of bats (E. G. Crichton & P. H. Krutzsch, eds.). Academic Press, San Diego, [ Links ]
48. Reis, N. R., A. L. Peracchi, W. A. Pedro, & I. P. De Lima. 2007. Morcegos do Brasil. Universidade Estadual de Londrina, Londrina. [ Links ]
49. Reis, N. R., M. N. Fregonezi, A. L. Peracchi, & A. O. Shibatta. 2013. Morcegos do Brasil: guia de campo. Technical Books, Rio de Janeiro. [ Links ]
50. Rodríguez-Gironés, M. A., & L. Santamaria. 2006. A new algorithm to calculate the nestedness temperature of presence-absence matrices. Journal of Biogeography 33:924–935.
51. Sbragia, I. A., & A. Cardoso. 2008. Quiropterofauna (Mammalia: Chiroptera) cavernícola da Chapada Diamantina, Bahia, Brasil. Chiroptera Neotropical 14:360-365. [ Links ]
52. Sikes, R. S., W. L. Gannon, & The Animal Care and Use Committee of the American Society of Mammalogists. 2011. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. Journal of Mammalogy 92:235-253. [ Links ]
53. Silva, S. S. P., P. G. Guedes, & A. L. Peracchi. 2001. Levantamento preliminar dos morcegos do Parque Nacional de Ubajara (Mammalia, Chiroptera), Ceará, Brasil. Revista Brasileira de Zoologia 18:139-144. [ Links ]
54. Silva, J. P. A., A. R. Carvalho, & J. A. Oliveira-Motta. 2009. Fauna de morcegos (Mammalia, Chiroptera) em cavernas do bioma Cerrado na região de Indiara (Goiás). Revista Brasileira de Zoociências, 11:209-2017 [ Links ]
55. Trajano, E. 1985. Ecologia de populações de morcegos cavernícolas em uma região cárstica do sudeste do Brasil. Revista Brasileira de Zoologia 2:255-320 [ Links ]
56. Trajano, E. 1995. Protecting caves for bats or bats for caves? Chiroptera Neotropical 1:19-21. [ Links ]
57. Willig, M. R. 1983. Composition, microgeographic variation and sexual dimorphism in Caatingas and Cerrado bat communities from northeast Brazil. Bulletin of Carnegie Museum of Natural History 23:1-131. [ Links ]
58. Zortéa, M., N. A. Bastos, & T. C. Acioli. 2015. The bat fauna of the Kararaô and Kararaô Novo caves in the area under the influence of the Belo Monte hydroelectric dam, in Pará, Brazil. Brazilian Journal of Biology 75:168-173. [ Links ]














