Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Mastozoología neotropical
versão impressa ISSN 0327-9383versão On-line ISSN 1666-0536
Mastozool. neotrop. vol.25 no.1 Mendoza jun. 2018
NOTA
Bat fly (Diptera: Streblidae) parasitism in degraded and preserved areas in a Neotropical savanna
Daniel F. Ramalho1, 2, Gustavo Graciolli3, and Ludmilla M. S. Aguiar1, 2, 4
1 Programa de Pós-Graduação em Ecologia (PGECL), Universidade de Brasília (UnB), Brasília, Brazil. [Correspondence: <daniel.f.ramalho@gmail.com>]
2 Laboratório de Biologia e Conservação de Morcegos, Universidade de Brasília (UnB), Brasília, Brazil.
3 Centro de Ciências Biológicas e da Saúde, Universidade Federal do Mato Grosso do Sul (UFMS), Campo Grande, Brazil.
4 Programa de Pós-Graduação em Zoologia, Universidade de Brasília (UnB), Brasília, Brazil.
Recibido: 13 septiembre 2017.
Aceptado: 14 marzo 2018.
Editor asociado: R Robles
ABSTRACT.
We assessed the relationship between bat fly parasitism and habitat degradation. We used mist nets to capture 522 individuals of Carollia perspicillata and 429 individuals from eight fly species in the Brazilian Cerrado. Specimens were captured in degraded areas surrounding protected areas and within the protected areas. Trichobius joblingi was the most frequent parasite of Carollia perspicillata; consequently, it was analyzed in greater detail. Sex and age were not critical factors for parasitism. A robust anthropogenic influence was observed; unexpectedly, a higher prevalence and abundance of bat flies were observed in preserved areas.
RESUMO.
Parasitismo por moscas ectoparasitas (Diptera: Streblidae) de morcegos em áreas degradadas e preservadas de uma savanna Neotropical.
Avaliamos a relação entre parasitismo de moscas ectoparasitas de morcegos e degradação do habitat. Com redes de neblina instaladas no Cerrado, capturamos 522 indivíduos de C. perspicillata e 429 indivíduos de oito espécies de Streblidae. As capturas foram realizadas em áreas protegidas e em áreas degradadas nos seus arredores. Trichobius joblingi era altamente representativa em Carollia perspicillata; consequentemente, esta espécie foi analisada com mais detalhe. Apesar de sexo e idade não serem fatores críticos para prevalência e intensidade de infestação, há uma forte influência antropogênica; inesperadamente, a maior prevalência e abundância de moscas está nas áreas preservadas.
Key words: Carollia perspicillata; Cerrado; Ectoparasite; Hippoboscoidea; Trichobius joblingi.
Palavras-chave: Carollia perspicillata; Cerrado; Ectoparasita; Hippoboscoidea; Trichobius joblingi.
Urbanization and habitat degradation may have detrimental effects and can alter species diversity and abundance patterns (Kurta & Teramino 1992). In urban areas, an increased abundance of generalist bat species that are well adapted to anthropogenic habitat alterations is observed, whereas the abundance of more specialized species tends to decrease (Willig et al. 2007). Neotropical bat species, such as Carollia perspicillata, Sturnira lilium and Artibeus lituratus are abundant in urban areas because of the high resource availability pres ent in human-modified environments (Fleming 1988; Willig et al. 2007). Environmental characteristics may also influence parasitism (Krasnov et al. 2007; Pilosof et al. 2012) for species that rely on roosts for pupae deposition, such as bat flies (Dick & Patterson 2007). Bat flies belong to the families Streblidae and Nycteribiidae and are exclusive ectoparasites of bats (Marshall 1981). Each of these species of flies usually parasitizes a single bat species or genus (Wenzel et al. 1966; Dick 2007).
Studies have suggested that habitat degradation increase rates of parasitism because habitat loss promotes higher concentration of hosts, which can increase stress and facilitate parasite transmission (Mbora & McPeek 2009). Conversely, other studies have shown that rates of parasitism are higher in preserved areas (Pilosof et al. 2012; Frank et al. 2016). To date, few studies have assessed the influence of urbanization and environmental quality in bat fly parasitism. Furthermore, these limited studies have presented contrasting results (Pilosof et al. 2012; Saldaña-Vázquez et al. 2012; Frank et al. 2016; Bolívar-Cimé et al. 2017).
Therefore, we aim to evaluate the relationship between parasitism in degraded versus preserved habitats and explore intraspecific variation in parasitism in relation to the sex and age of the bat. We focus on Seba's short-tailed bat Carollia perspicillata in the city of Brasília, Federal District of Brazil. Brasília is in the central region of the country at an altitude of 1160 m, and it is located in the core area of the Cerrado biome (Fig. 1). The Cerrado is considered one of the world's hotspots of biodiversity. Sixty percent of the original Cerrado area has been deforested at a rate that has reached 30 000 km² per year (Machado et al. 2004). The study area is in a tropical savanna (Aw) according to Köppen–Geiger classification.

Fig. 1. Study areas and sampling sites in Brasília, Federal District, Brazil. 1. PNB, National Park of Brasília; 2. APA GCV, Environmental Protection Area Gama-Cabeça-de-Veado; and 3. ESECAE, Ecological Station of Águas Emendadas. Closed squares represent preserved areas, and open squares represent degraded areas.
We collected bats and flies in a typical cerrado sensu strictu phytophysiognomy within three protected areas (PAs) in the city of Brasília: the National Park of Brasília - PNB (42 389 ha, 15°41′42″ S/48°08′10″ W), the Environmental Protection Area Gama-Cabeça de Veado - APA GCV (25 000 ha, 15°52′29″ S/47°50′48″ W), and the Ecological Station of Águas Emendadas - ESECAE (10,547 ha, 15°36′32″ S/47°33′03″ W) (Fig. 1). Brasília is the fourth most populated city in Brazil and has approximately three million inhabitants; thus, the three sampling locations are within a highly urbanized landscape. Bats and bat flies were collected at 24 capture sites in continuous fragments of cerrado located inside the protected areas (preserved habitats) and in small fragments surrounding the protected areas within the urban matrix (degraded habitats).
From April 2012 to August 2013, we sampled during 96 trap nights using ten mist nets (12 × 2.5 m), which were opened from 18:00 to 24:00 h. The total sampling effort was 5760 net/hours, equally distributed among sampling sites (240 net/hours in each site). Captured bats were identified following Diaz et al. (2016). We removed ectoparasites from each bat with tweezers and brushes and fixed them in 70% alcohol. Bat flies were identified to the species level following Guerrero (1993, 1994a; b, 1995a; b, 1996). We deposited two individuals (one male and one female) of each bat species and all Diptera into the Collection of Chiroptera at the University of Brasilia (CCUnB) as voucher material (Carollia perspicillata: CCUnB 0722 ♂ and CCUnB 0629 ♀; Trichobius joblingi: CCUnBd 0634 – CCUnBd 0821). We used two indicators of parasitism (Bush et al. 1997) to describe bat fly infestation: prevalence (number of parasitized hosts/number of hosts examined × 10) and mean intensity of infestation (number of ectoparasites/number of parasitized host ± standard deviation). Because data were not independent, we used Pearson's Chi-squared tests with Yates' continuity correction to evaluate possible differences in prevalence between sexes, age classes, and habitat types. To describe the relationship of the intensity of infestation (number of parasites per individual) with sex, age, habitat, as well as with the interactions between these variables, we used a Multivariate Analysis of Variance (MANOVA). We conducted all tests on the R 3.2.2 platform (R Core Team 2015), and the significance level was set at p<0.05.
We examined 522 Carollia perspicillata individuals, of which 187 were parasitized with a total of 429 ectoparasites, resulting in a total prevalence of 35.82% and a mean intensity of 2.29 (±1.7) flies/host. We identified eight species of ectoparasites: Aspidoptera falcata, Megistopoda proxima, Paratrichobius longicrus, Speiseria ambigua, Stebla guajiro, Trichobius lonchophyllae, Trichobius tiptoni and Trichobius joblingi. Because the latter species was present in 90.91% of host individuals, we analyzed the relationship between parasitism rates and the habitat type, sex, and age for this species.
Carollia perspicillata was more abundant in degraded habitats (343 individuals) than in preserved habitats (179 specimens). Prevalence was only related to the habitat type, and it was higher in preserved areas (χ² = 14.174, p = 0.0002). Sex and age were not related to prevalence (χ² = 1.926, p = 0.1652 and χ² = 0.144, p = 0.7048, respectively) (Table 1). The intensity of infestation was also significantly higher in preserved areas (p = 0.000526). Sex and age as well as all interactions among the three factors were not related to the mean intensity of infestation (Table 2).
Table 1 Prevalence and mean intensity of Trichobius joblingi on Carollia perspicillata in sampling points in the city of Brasília, Federal District, Brazil (p = Pearson's Chi-squared and Multivariate Analysis of Variance [MANOVA]).
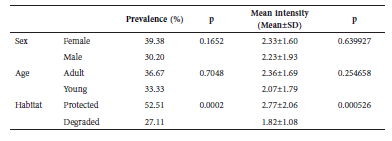
Table 2 Values derived from the Multivariate Analysis of Variance (MANOVA) between the mean intensity and predictable variables (sex, age, habitat type, and all interactions) of Trichobius joblingi isolated from Carollia perspicillata at sampling points in the city of Brasília, Federal District, Brazil. Mean square, Mean Sq.; F values, F; and significant values, p.

Our results show that populations of C. perspicillata found in preserved areas are more frequently parasitized by Trichobius joblingi than populations found in degraded areas. This result is similar to the one observed for Sturnira ludovici in Mexico, where populations found in natural forest fragments had a higher prevalence than those found in coffee plantations (Saldaña-Vázquez et al. 2012). A high incidence of parasitism has also been observed in Artibeus jamaicensis in preserved areas in Mexico, where prevalence was positively correlated with the percentage of forest cover and landscape connectivity (Bolívar-Cimé et al. 2017).
Regarding the intensity of infestation, our results corroborate previous studies in which higher parasite abundance was observed in preserved areas. For example, in Costa Rica, male bats in preserved areas hosted more parasites than bats situated in species-poor areas (Frank et al. 2016). In a study in Venezuela, Pilosof et al. (2012) found that the parasitism of Artibeus planirostris and Pteronotus parnellii was inversely proportional to the human population density.
In contrast, the relationship between habitat degradation and Carollia perspicillata parasitism found in this study was inconsistent with findings of a study of the same species in Venezuela where the mean abundance of bat flies was positively related to human density and increased in densely occupied areas (Pilosof et al. 2012). The same relationship was observed by Frank et al. (2016) for female bats in Costa Rica, where a higher number of parasites was observed in poorer areas than in preserved areas.
In our study area, degraded sites had a higher diversity and abundance of hosts, which suggests that a dilution effect could be causing the observed difference in parasitism. Frank et al. (2016) observed that areas with higher bat species richness had lower parasite loads, indicating the presence of a dilution effect in the bat-fly relationship.
Moreover, habitat quality can directly influence the reproductive rates and survival of ectoparasites (Marshall 1981; Patterson et al. 2007). Fragmented areas tend to present significant changes in environmental characteristics, such as temperature and humidity (Matlack 1993), which could be detrimental to parasites.
In the present study, sex and age were not predictors of parasitism rates; however, previous studies have shown that females carried more parasites than males (Fritz 1983; Patterson et al. 2008). These studies suggest that the formation of nursing groups during the reproductive period may facilitate horizontal transmission, thereby increasing parasite prevalence. However, Carollia perspicillata is not known to form nursing groups (Cloutier & Thomas 1992), which may explain why we did not observe differences in parasite loads related to sex or age.
Variation among studies suggests that future investigations should be performed to determine the factors that influence parasitism across habitats and species. The effect of human interference on patterns of bat-fly interactions remains unclear. However, our results indicate that the anthropogenic influence is robust and the prevalence and abundance of bat flies is high in preserved areas.
Acknowledgments.
We thank SISBIOTA/ICMBIO for permission (27719-28) to study in the research area and CNPq for providing funding to DFR (130876/2013-5) and a fellow research grant to LMSA (309299/2016-0) and GG (304616/2015-0). We also thank the anonymous reviewers and the editors for their comments and suggestions that helped us improve our paper.
LITERATURE CITED
1. Bolívar-Cimé, B., A. Cuxim-Koyoc, E. Reyes-Novelo, J. B. Morales-Malacara, J. Laborde, & R. Flores- Peredo. 2017. Habitat fragmentation and the prevalence of parasites (Diptera, Streblidae) on three Phyllostomid bat species. Biotropica 50:90-97. [ Links ]
2. Bush, A. O., K. D. Lafferty, J. M. Lotz, & A. W. Shostak. 1997. Parasitology meets ecology on its own terms: Margolis et al. revisited. Journal of Parasitology 83:575-583. [ Links ]
3. Cloutier, D., & D. W. Thomas. 1992. Carollia perspicillata. Mammalian species 417:1-9. [ Links ]
4. Diaz, M. M., S. Solari, L. F. Aguirre, L. M. S. Aguiar, & R. M. Barquez. 2016. Clave de identificacion de los murciélagos de sudamérica. Publicacion Especial no 2, PCMA (Programa de Conservacion de los Murciélagos de Argentina), Tucumán, Argentina. [ Links ]
5. Dick, C. W. 2007. High host specificity of obligate ectoparasites. Ecological Entomology 32:446-450. [ Links ]
6. Dick, C. W., & B. D. Patterson. 2007. Against all odds: explaining high host specificity in dispersal-prone parasites. International Journal of Parasitology 37:871-876. [ Links ]
7. Fleming, T. H. 1988. The short-tailed fruit bat: A study in plant-animal interactions. University of Chicago Press, Chicago, Illinois. [ Links ]
8. Frank, H. K., C. D. Mendenhall, S. D. Judson, G. C. Daily, & E. A. Hadly. 2016. Anthropogenic impacts on Costa Rican bat parasitism are sex specific. Ecology and Evolution 6:4898-4909. [ Links ]
9. Fritz, G. N. 1983. Biology and ecology of bat flies (Diptera: Streblidae) on bats in the genus Carollia. Journal of Medical Entomology 20:1-10. [ Links ]
10. Guerrero, R. 1993. Catalogo de los Streblidae (Diptera: Pupipara) parásitos de murciélagos (Mammalia: Chiroptera) del Nuevo Mundo. I. Clave para los géneros y Nycterophillinae. Acta Biologica Venezuelica 14:61-75. [ Links ]
11. Guerrero, R. 1994a. Catalogo de los Streblidae (Diptera: Pupipara) parásitos de murciélagos (Mammalia: Chiroptera) del Nuevo Mundo. II. Los grupos: pallidus, caecus, major, uniformis y longipes del género Trichobius Gervais, 1844. Acta Biologica Venezuelica 5:1-18. [ Links ]
12. Guerrero, R. 1994b. Catalogo de los Streblidae (Diptera: Pupipara) parásitos de murciélagos (Mammalia: Chiroptera) del Nuevo Mundo. IV. Trichobiinae con alas desarrolladas. Boletín de Entomología Venezolana 9:161-192. [ Links ]
13. Guerrero, R. 1995a. Catalogo de los Streblidae (Diptera: Pupipara) parásitos de murciélagos (Mammalia: Chiroptera) del Nuevo Mundo. III. Los gupos: dugesii, dunni y phyllostomae del género Trichobius Gervais, 1844. Acta Biologica Venezuelica 5:1-27. [ Links ]
14. Guerrero, R. 1995b. Catalogo de los Streblidae (Diptera: Pupipara) parásitos de murciélagos (Mammalia: Chiroptera) del Nuevo Mundo. V. Trichobiinae con alas reducidas o ausentes y misceláneos. Boletín de Entomología Venezolana 10:135-160. [ Links ]
15. Guerrero, R. 1996. Catalogo de los Streblidae (Diptera: Pupipara) parásitos de murciélagos (Mammalia: Chiroptera) del Nuevo Mundo. VI. Streblinae. Acta Biologica Venezuelica 16:1-25. [ Links ]
16. Krasnov, B. R., M. Stanko, & S. Morand. 2007. Host community structure and infestation by ixodid ticks: repeatability, dilution effect and ecological specialization. Oecologia 154:185-194. [ Links ]
17. Kurta, A., & J. A. Teramino. 1992. Bat community structure in an urban park. Ecography 15:257-261. [ Links ]
18. Machado, R. B. et al. 2004. Estimativas de perda da área do Cerrado brasileiro. Relatório técnico não publicado. Conservação Internacional, Brasília. [ Links ]
19. Marshall, A. G. 1981. The ecology of ectoparasitic insects. Academic Press, London [ Links ]
20. Matlack, G. R. 1993. Microenvironment variation within and among forest edge sites in the eastern United States. Biological Conservation 66:185-194. [ Links ]
21. Mbora, D. N. M., & M. A. McPeek. 2009. Host density and human activities mediate increased parasite prevalence and richness in primates threatened by habitat loss and fragmentation. Journal of Animal Ecology 78:210-218. [ Links ]
22. Patterson, B. D., C. W. Dick, & K. Dittmar. 2007. Roosting habits of bats affect their parasitism by bat flies (Diptera: Streblidae). Journal of Tropical Ecology 23:177-189. [ Links ]
23. Patterson, B. D., C. W. Dick, & K. Dittmar. 2008. Sex biases in parasitism of neotropical bats by bat flies (Diptera: Streblidae). Journal of Tropical Ecology 24:387-396. [ Links ]
24. Pilosof, S., C. W. Dick, C. Korine, B. D. Patterson, & B. R. Krasnov. 2012. Effects of anthropogenic disturbance and climate on patterns of bat fly parasitism. PLoS ONE 7:e41487. [ Links ]
25. R Core Team. 2015. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. <https://www.R-project.org/> [ Links ].
26. Saldaña-Vázquez, R. A., A. A. Castro-Luna, C. A. Sandoval-Ruiz, J. R. Hernández-Montero, & K. E. Stoner. 2012. Population composition and ectoparasite prevalence on bats (Sturnira ludovici; Phyllostomidae) in forest fragments and coffee plantations of central Veracruz, Mexico. Biotropica 45:351-356. [ Links ]
27. Wenzel, R. L., V. J. Tipton, & A. Kiewlicz. 1966. The streblid bat flies of Panama (Diptera: Calyptera: Streblidae). Ectoparasites of Panama (R. L. Wenzel & V. J. Tipton, eds.). Field Museum of Natural History, Chicago, Illinois. [ Links ]
28. Willig, M. R. et al. 2007. Phyllostomid bats of lowland Amazonia: effects of habitat alteration on abundance. Biotropica 39:737-746. [ Links ]














