Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Mastozoología neotropical
Print version ISSN 0327-9383On-line version ISSN 1666-0536
Mastozool. neotrop. vol.25 no.2 Mendoza Dec. 2018
ARTÍCULO
First coprological survey of helminths in a wild population of black capuchin monkeys (Sapajus nigritus) in Northeastern Argentina
Ilaria Agostini1, 2, Ezequiel Vanderhoeven2, 3, Pablo M. Beldomenico4, Romina Pfoh1, 2 and Juliana Notarnicola1
1 Grupo de Ecología y Conservación de Mamíferos (GECOMA), Instituto de Biología Subtropical (IBS), sede Iguazú, CONICET - UNaM, Puerto Iguazú, Misiones, Argentina [Correspondence: <agostini.ilaria@gmail.com>].
2 Asociación Civil Centro de Investigaciones del Bosque Atlántico (CeIBA), Puerto Iguazú, Misiones, Argentina.
3 Instituto Nacional de Medicina Tropical (INMeT), Puerto Iguazú, Misiones, Argentina.
4 Laboratorio de Ecología de Enfermedades, ICIVET LITORAL, CONICET - UNL, Esperanza, Santa Fe, Argentina.
ABSTRACT
Parasites play an important role in primate ecology. Although gastrointestinal parasites have been surveyed in several primate taxa, there is still a substantial paucity of data for some species. Here we report the first coprological survey of helminths in a primate species, the wild black capuchin monkey (Sapajus nigritus), which is endemic to the Atlantic Forest. During three winters and one summer, we collected 665 faecal samples from 44 identified individuals of two capuchin groups in Iguazú National Park, NE Argentina, for parasitological analysis. Overall, we identified eight helminths: Filariopsis sp., Strongyloides sp., Trichuris sp., Ascaris sp., a Subuluridae, a Physalopteridae, a Hymenolepididae cestode, and an undetermined Trematode. The Hymenolepididae (25-42%), Filariopsis sp. (15-42%), and Strongyloides sp. (11-27%) were the most prevalent parasites regardless of the survey year, group and season. On average, infected capuchins harboured 1.12-1.26 parasite taxa. The parasite community observed in the black capuchin monkeys resembled those found in other Cebidae.
RESUMEN
Primer estudio coprológico de helmintos en una población silvestre de monos caí (Sapajus nigritus) en el Nordeste de Argentina.
Los parásitos juegan un rol importante en la ecología de los primates. Aunque los parásitos gastrointestinales han sido investigados en varios taxa de primates, todavía existe un vacío de información para algunas especies. Aquí reportamos los resultados del primer estudio coprológico de helmintos en una población silvestre de monos caí (Sapajus nigritus), una especie endémica del Bosque Atlántico. Durante tres inviernos y un verano, colectamos 665 muestras fecales de 44 individuos identificados pertenecientes a dos grupos de monos caí en el Parque Nacional Iguazú en el Nordeste de Argentina, para realizar análisis parasitológicos. En total identificamos ocho helmintos: Filariopsis sp., Strongyloides sp., Trichuris sp., Ascaris sp., un Subuluridae, un Spiruridae, un cestode Hymenolepididae y un Trematode indeterminado. Hymenolepididae (25-42%), Filariopsis sp. (15-42%) y Strongyloides sp. (11-27%) fueron los parásitos más prevalentes independientemente del año y la estación. En promedio, los monos caí infectados presentaron entre 1.12-1.26 taxa de helmintos. La comunidad de parásitos que encontramos presenta similitudes con la de otros Cebidae.
Key words: Atlantic Forest; Parasite prevalence; Parasite richness; Primates.
Palabras clave: Bosque Atlántico; Prevalencia de parásitos; Primates; Riqueza parasitaria.
Recibido 27 noviembre 2017.
Aceptado 5 marzo 2018.
Editor asociado: P. Teta.
INTRODUCTION
Parasites of mammals can be detrimental to their fitness and are an important ecological driver in the evolution of behaviour and social organization (Nunn & Altizer 2006). The opportunity of surveying at least some groups of parasites, such as those inhabiting the gastrointestinal tract, using non-invasive faecal sample analyses, has contributed to the remarkable increase of studies documenting primate parasites in the last decade. However, at least in the Neotropical region, there is still a significant bias towards sampling some primate genera over others. So far, most surveys and longitudinal studies have focused on howler monkeys (Alouatta spp.; e.g. Stoner 1994; Stuart et al. 1998; Vitazkova & Wade 2006; Trejo-Macías et al. 2007; Kowalewski et al. 2011). Although capuchin monkeys (Cebus spp. or gracile capuchins, and Sapajus spp. or robust capuchins; Lynch Alfaro et al. 2012) have been studied deeply in many aspects of their ecology and behaviour (Fragaszy et al. 2004), their parasite community is still almost unknown. The only systematic survey on gastrointestinal parasites published on Cebus capucinus is from Parr et al. (2013), while very few reports exist on parasites of Sapajus spp. (in captivity: Santa Cruz et al. 1998, 2000; in the wild: a one-point-in-time survey on a group of 10 individuals in Peru, Phillips et al. 2004) none of them based on systematic sampling. In all cases, analyses indicate that Strongyloidessp. and Filariopsissp. are among the most common parasite taxa recovered.
Robust capuchins of genus Sapajus rely on an omnivorous diet including fruits, arthropods and small birds and mammals (Robinson & Janson 1987; Brown & Zunino 1990), and they use low-canopy levels, as well as the ground for foraging, drinking and playing (Terborgh 1983; Siemers 2000). They have a complex social structure and interact frequently with other individuals of their own group (Fragaszy et al. 2004). These characteristics are likely to expose them to high levels of parasitism (Nunn & Altizer 2006). Black capuchins (Sapajus nigritus), in particular, are endemic to the Atlantic Forest (Lynch Alfaro et al. 2012). Given the high degrees of heterogeneity and the outstanding levels of endemism, the Atlantic Forest is one of the world’s top biodiversity hotspots, harbouring 26 species of primates, 73% of which are endemic (Pinto et al. 2009). This biome has experienced a long lasting destruction and degradation and is currently reduced to 12% of the original surface (Ribeiro et al. 2011). Largely because of this habitat loss, 65% of Atlantic Forest’s primate species are threatened (Pinto et al. 2009). Within this context, baseline data regarding patterns of parasitic infections in wild primate populations endemic to the Atlantic Forest are critical to providing indicators of population health and to facilitate longitudinal or cross-sectional studies of populations to assess and manage disease risks. In this study, we present the first coprological survey on helminths of wild black capuchins in Iguazú National Park, Argentina.
MATERIALS AND METHODS
Study site and subjects
We carried out the field work in Iguazú National Park, Northeastern Argentina (25° 40´S; 54° 30´W), in the southwestern section of the South American Atlantic Forest. This semi-deciduous forest is characterised by a humid subtropical climate with marked seasonality in day length and temperature (Crespo 1982). Mean annual rainfall varies between 1500 and 2000 mm without a marked dry season (Brown & Zunino 1990). There is seasonal variation in the production of fleshy fruits and arthropods, which is lower during winter months (June-August) and reaches its maximum between October and January (Janson 1996). Due to this seasonal pattern, winter is a critical time during which black capuchins depend mostly on bamboo shoots, meristems and leaf bases of epiphytes (Di Bitetti 1997). Black capuchins live in polygamous multi-male, multi-female groups of 7-30 individuals (Di Bitetti 1997). Individuals can be identified based on physical characteristics, such as facial colour patterns, body size and shape of tufts (Di Bitetti 2001).
We conducted the study on two groups, known as Macuco (N=23-27) and Spot (N=14-21), whose composition changed across the three years (Table1). Macuco was exposed to a relatively high degree of contact with tourists, with interactions occasionally including physical contact and occurring during 6%-13% of the observation days in 2013 and 2014. In addition, individuals of Macuco occasionally had access to human food. In contrast, the Spot group displayed very limited and sporadic interactions with tourists in the park, occurring between 3 and 6% of the observation days in 2013 and 2014 (Agostini unpublished data).
Table 1
Age composition of Macuco (MAC) and Spot (SPO) black capuchin groups from Iguazu National Park, Misiones, Argentina, along the study seasons. We considered adults males (ADM) > 6 years old; adult females (ADF) > 5 years old, subadult males (SUBM) 5-6 years old; subadult females (SUBF) 4-5 years old, juvenile (JUV) males 1-4 and females 1-3 years old, and infants (INF) < 1 years old.

Faecal sampling and analysis
To obtain data on parasitism, we collected faecal samples of individuals belonging to Macuco and Spot groups during three winters and one summer: 52 days between June and August 2012; 108 days between May and August 2013 and 107 days between May and August 2014; 83 days between December 2013 and March 2014 (the latter only from Macuco group). Faecal samples were collected opportunistically from 44 identified individuals (26 from Macuco group, and 18 from Spot), and within each study period, most individuals were sampled repeatedly. We collected a total of 97 faecal samples from 33 individuals in winter 2012 (meanñSE samples per individual: 2.94ñ0.21); 164 samples from 35 individuals during winter 2013 (3.90ñ0.56); and 352 samples from 36 individuals during winter 2014 (8.38ñ1.29) from both Macuco and Spot groups. In addition, we gathered 52 samples from 16 individuals from Macuco during summer 2013-2014 (3.25ñ0.59). Approximately one half the subjects of each group were sampled along the study period (58% and 50% for Macuco and Spot, respectively). We focused our sampling on adult and subadult individuals rather than on juveniles, and infants were discarded from the analyses given the difficulty of collecting their faeces. During the winters of 2013 and 2014, we ran a parallel experimental study that involved deworming of some selected individuals in both groups (Agostini et al. 2017). However, in order to describe the natural parasite community hosted by wild black capuchins, in the present survey we considered only faecal samples from untreated individuals and samples collected during the pre-treatment phase for those who were treated later in each field season.
For the analysis of gastrointestinal parasites, an aliquot of approximately 5 g of each faecal sample was stored in 15 ml of 10% formalin. Then, in the Laboratory of Parasitology of the National Institute of Tropical Medicine (INMeT) in Puerto Iguazú, Argentina, samples were processed using a semi-quantitative flotation method with a saturated sugar solution (Cox & Todd 1962). The technique consisted of weighing 3 g of each faecal sample, adding 43ml of tap water and homogenizing it. Then, using a strainer and a funnel, the solution was poured filling a 15-ml tube, and centrifuged at 1500 rpm for 2 minutes. After discarding the supernatant, the volume of the tube was filled with a supersaturated sugar solution until forming a meniscus. Then, a cover slip of 18x8 mm was placed on top and the tube was further centrifuged at 1000 rpm during 2 minutes. Finally, the cover slip was put on a slide and examined with the microscope. We identified eggs and larvae of helminths and took pictures using a microscope with a camera using 10x and 40x magnifiers. We counted and identified eggs and larvae at the genus or family level based on colour, shape, content and size.
Data analysis
We described parasitic infections in terms of prevalence (number of individuals infected with a given parasite/total individuals sampled) and richness (number of taxa infecting the same host) (see Bush et al. 1997). For each surveyed period, we considered an individual as infected by a given type of parasite when at least one of the faecal samples collected were positive for that parasite.
We compared prevalence of the three most common parasites between the two groups (Macuco and Spot), using the Fisher-Freeman-Halton exact test for 2x3 contingency tables (Freeman & Halton 1951), stratifying by sampling period. To evaluate if there was an inter-annual variation in prevalence of the most common parasites, we compared the frequencies of infected individuals among three consecutive winters, separately for each parasite taxon. Similarly, to determine whether parasitic prevalence varied between seasons, we compared the frequencies of infected individuals in summer vs. previous and following winter, separately for each of the most common parasites. Due to the fact that most of the capuchin individuals were repeatedly sampled along different seasons and years, to evaluate inter-annual as well as seasonal differences, we performed a Cochran’s Q test for related samples (Siegel & Castellan 1988) considering only the subjects that stayed in the study groups across all study periods. This test is particularly suitable when the variables are categorical and dichotomous as in our case (presence of infection: yes/no). In case of significant difference obtained by Cochran’s Q test, we evaluated specific sample pairs using post hoc Dunn’s (1964) with Bonferroni adjustment. Since we had no replicates of summer sampling, the comparison between seasons should be taken with caution and cannot be generalised. All statistical tests were two tailed with ɑ set at 0.05, and were performed with software R (v. 3.3.0; R Development Core Team 2016). To run Cochran’s Q test, we used the R package nonpar (Sweet 2017).
RESULTS
We identified eight taxa of helminths corresponding to six nematodes (Strongyloides sp. [Family Strongyloididae], Trichuris sp. [Trichuridae], Filariopsis sp. [Filaroididae], Ascaris sp. [Ascarididae], an undetermined Subuluridae, an undetermined Physalopteridae), one cestode (undetermined Hymenolepididae), and one unidentified trematode (Fig. 1). Macuco presented eight parasite taxa, whereas Spot displayed six taxa (Table 2). This difference was due to the finding of Trichuris sp. and a trematode in a single individual of Macuco. We also recovered some protozoan oocysts; however, since our fixation and analysis techniques were not adequate for protozoan identification, we could not determine to a further level the parasites belonging to this taxon in our capuchin study groups. Table 3 shows the measurements and a brief description of the morphology of eggs and larvae.
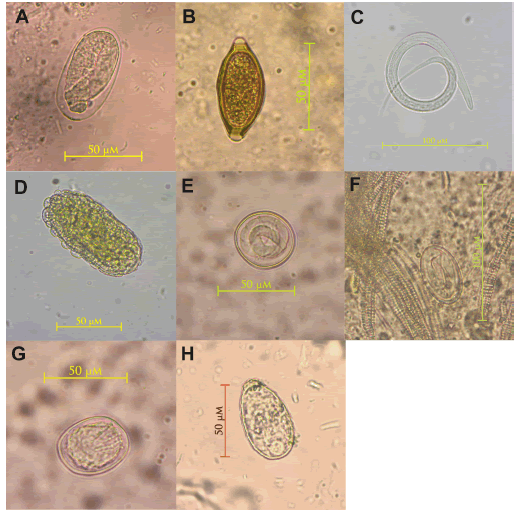
Fig. 1. Parasites from wild black capuchin monkeys in Northeastern Argentina: a) Strongyloides sp., b) Trichuris sp., c)Filariopsis sp., d) Ascaris sp., e) undetermined Subuluridae, f) undetermined Physalopteridae, g) undetermined Hymenolepididae, h) undetermined Trematode.
Table 2
Prevalence of helminths across survey periods in two black capuchin groups, Macuco (MAC) and Spot (SPO), or as a total pooling both groups at Iguazú National Park, Misiones, Argentina. Transmission mode and intermediate host (when applicable) are indicated.

Table 3
Measurements and morphological characters of larvae and eggs obtained from 665 faecal samples from two black capuchin groups at Iguazú National Park, Misiones, Argentina (n=number of eggs/larvae used for obtaining measurements).

Parasite prevalence and richness
The overall proportion of individuals infected with at least one parasite across all survey periods ranged between 50% and 82% for Macuco and between 45% and 62% for Spot. In winter and summer, the undetermined Hymenolepididae and Filariopsis sp. were the most prevalent parasites, followed by Strongyloides sp. The other identified parasite taxa were scarcely represented (Table 2). The mean individual parasite richness ranged between 1.12 and 1.26 and modal richness was 1. In the 17 samples in which we found two parasite taxa at the same time, the most frequent combination was the pair Filariopsis sp.-Hymenolepididae (6/17), followed by Filariopsis sp.-Strongyloides sp. (4/17).
We did not find any significant differences in prevalence of the three most common parasites between groups in each of the three winters (Fisher-Freeman-Halton’s exact test; winter 2012: p=0.335; winter 2013: p=0.498; winter 2014: p=0.343). We found a significant inter-annual variation in prevalence only for Filariopsis infection (Cochran’s Q test: x22=10.71, p=0.005), mostly due to higher proportions of infected individuals in winter 2014 compared to winter 2012 (post-hoc Dunn’s test; z=-3.19, p=0.002), while the other pairwise comparisons of Filariopsis prevalence between years were not significantly different (winter 2012 vs. winter 2013: z=-1.74, p=0.123; winter 2013 vs. winter 2014: z=-1.45, p=0.221). We did not find any significant difference in the prevalence of the Hymenolepididae (c22=0.11, p=0.946) and Strongyloides (c22=4.00, p=0.135), across the three years. Finally, we found a significant difference between seasons (i.e. summer vs. previous winter vs. subsequent winter) only for Filariopsis prevalence (x22=6.22, p=0.044), and it was due to the higher prevalence shown during winter 2014 compared to the summer 2013-2014 (z=-2.18, p=0.044), while the other pairwise comparisons of Filariopsis prevalence between seasons were not significantly different (summer 2013-2014 vs. winter 2013: z=-0.73, p=0.701; winter 2013 vs. winter 2014: z=-1.45, p=0.221). Finally, we found no seasonal significant difference for the other two common parasites, the Hymenolepididae (x22=0.67, p=0.716) and Strongyloides (x22=5.25, p=0.072).
DISCUSSION
In our survey of the helminthic community of wild black capuchins, we recovered eight taxa. As most other parasitic assessments on New World primates, we relied on non-invasive sampling techniques (faecal sampling) for egg identification. Since we could not access adult parasites through necropsies or performed molecular analyses on eggs, we were not able to distinguish parasites at the species level (Solórzano-García & Pérez-Ponce de León 2017). Although this is a limitation of this study, the importance of our survey lies in the fact that it is the first report of a coprological study of helminths in a wild black capuchin population. All genera and families have already been recorded for capuchin monkeys, although mainly for Cebus spp. (Chinchilla et al. 2007, 2010; Notarnicola et al. 2008; Parr et al. 2013).
Our findings are similar to those reported by Santa Cruz et al. (2000) for a captive Sapajusspp. colony from Argentina, and by Parr et al. (2013) for wild Cebus capucinus from Costa Rica. In those studies, Filariopsissp. and Strongyloides sp. were among the most prevalent species. Since reports of infections with Strongyloides in New World primates commonly indicate Strongyloides cebus (Kaur & Singh 2009; Corrêa et al. 2016), this is likely to be the species infecting our capuchin study population. Other reports of the presence of Filariopsis in Sapajus or Cebus identify this nematode as Filariopsis barretoi (=F. arator), whose life cycle is still unknown (Rego & Schaeffer 1988; Santa Cruz et al. 1998; Parr et al. 2013). Considering the phylogenetic relatedness with Filaroides spp., which have a direct life cycle and immediately infective larvae shed in host faeces, it is likely that Filariopsis can also be directly transmitted through consumption of contaminated soil or food (Parr et al. 2013).
Another prevalent parasite found herein is the Hymenolepididae cestode. Although we cannot have certainty about the identification without molecular analyses or access to adult worms, egg morphology resembles to some Hymenolepis species (Yamaguti 1959; Thienpont et al. 1979). These eggs are circular to slightly oval, possess a thin wall, an embriophora without striations (in contrast to Taenia spp.) and the oncosphera holds six hooks displayed in fan. They are similar in shape and size to those found in wild Cebus capucinus by Parr et al. (2013), who classified them as belonging to the Anoplocephalidae family. However, these authors probably misinterpreted the egg morphology and erroneously classified them. In fact, eggs of Anoplocephalidae are different in shape and size. They are oval, with an oncosphera presenting either a pyriform apparatus like in Bertiella sp. or parallel hooks as is the case for Raillietina sp. and Mathevotaenia sp. Furthermore, measurements of eggs of Anoplocephalidae differ from those reported in Parr et al. (2013), which are similar to ours (Navone 1988; Ezquiaga et al. 2009; Mollericona et al. 2013). Our eggs are morphologically similar to those of Hymenolepis diminuta, a human parasite, but differ in size (43-56 x 31-50 μm in our samples vs. 60-79 x 72-86 μm in human samples; Thienpont et al. 1979). In Brazil, Hymenolepis cebidarum has been reported parasitizing Callicebus nigrifrons (Travassos 1965; Schmidt 1986) without mentioning the size or the morphology of the eggs. With the exception of Himenolepis nana, all other Hymenolepididae, such as H. diminuta, which could be consistent with the cestode we found, are characterised by indirect life cycles that include insects as intermediate hosts (Dunn 1963; Keymer 1981). This suggests that the relatively high prevalence found in our S. nigritus groups is due to the heavy reliance of these monkeys on arthropods as one of the major items in their diets. In fact, invertebrates in general, represent annually 40% to 57% of feeding time of this capuchin population at our study site (Brown & Zunino 1990; Agostini unpublished data). Moreover, since capuchins at our site groom each other frequently (Di Bitetti 1997), ingesting fleas containing the Hymenolepididae’s cysticercoid larvae in grooming context may enhance the chances of becoming infected with this cestode.
We found Ascaris sp. eggs in both Macuco and Spot groups, although at low prevalence. A record of this parasite was also reported for one individual of Sapajus macrocephalus in Tambopata National Reserve, Peru (Phillips et al. 2004). Given the rarity of Ascaris in New World monkeys, contrasting with the relative high prevalence recorded in humans (Michaud et al. 2003), it is likely that situations of close contact between humans and monkeys increase the probability of anthropozoonotic exchange of this parasite (Stuart et al. 1990; Michaud et al. 2003; but see Phillips et al. 2004).The other parasite taxa recovered in this study (i.e. Trichuris sp., the physalopterid, the subulurid, and the unknown trematode) had low overall prevalence and their presence was discontinuous across the four survey periods. As suggested by Parr et al. (2013), we should be cautious about including low-prevalence parasites, since they could have been accidentally acquired during the ingestion of some animal prey or animals’ faeces. Nevertheless, there are records of physalopterids, subulurids, and trematodes recovered from necropsies of Neotropical primates (Stoner & González-Di Pierro 2005; Nunn & Altizer 2006; Santos et al. 2010; Corrêa et al. 2016). Therefore, the presence of these species with low prevalence in Neotropical primates could indicate that they correspond to satellite parasite species.
Overall, the most common parasites found in our capuchin population have relatively low pathogenicity. Filariopsis sp. is known to form fibrous nodules containing worms and provoking small lesions on pulmonary tissues in capuchin monkeys (Rego & Schaeffer 1988). However, despite the high intensity of infection and significant pulmonary lesions found in lungs during necropsy, there has been no record of corresponding clinical signs of pulmonary disease in capuchin monkeys (Rego & Schaeffer 1988; Santa-Cruz et al. 1998; Parr et al. 2013). Cestodes belonging to Hymenolepididae are usually present in large numbers, however, infection with these parasites are rarely associated to clinical disease or enteric lesions. The principal clinical signs of infections with Hymenolepididae are diarrhoea and abdominal pain (Abee et al. 2012). Probably, the only parasite that may cause more severe clinical signs is Strongyloides. Infections with this parasite may provoke haemorrhagic or mucoid diarrhoea in nonhuman primates, as well as dermatitis, urticarial, anorexia, debilitation, vomiting, cough, and prostration among other signs. Occasionally, fatal cases of strongyloidiasis have been reported for nonhuman primates (reviewed by Abee et al. 2012).
We found similar prevalence of the most common parasites between our capuchin study groups, and temporal variation was evident only for Filariopsis, whose prevalence increased in the last winter study period compared to the previous sampling periods (both summer and winters). It is well known that seasonal changes can affect hosts and pathogens in ways that alter the rate at which infected hosts are produced (Altizer et al. 2006). The increase in prevalence recorded for Filariopsis could have been caused by particular climatic conditions, such as the high level of precipitation recorded in winter 2014 (937 mm; www.meteored.com.ar), compared to winter 2013 (656 mm) and winter 2012 (490 mm), that could have favoured the proliferation and transmission of this parasite among the study capuchins at Iguazú. Furthermore, as experimentally demonstrated by Agostini et al. (2017) in the same black capuchin study groups, an increase in food availability determines a decrease in the probability of infection, especially with the two most common parasites, Filariopsis and the Hymenolepididae, and a decrease in individuals’ overall parasite richness and probability of multiple infections. Thus, seasonal as well as inter-annual variations in food availability could be driving the observed changes in Filariopsis prevalence. However, other factors could have contributed to the observed rise in prevalence. We recorded changes in demography occurred in the two study groups during the last period, including immigration of new and potentially infected individuals into the groups that could have spread the parasite within the groups. Finally, during the 2014 winter period, we recorded events of frequent aggressions and social instability within both study groups. For the Macuco group, in particular, this instability later led to a male takeover. As it is known, situations of increased social stress can reduce immune-competence of individuals, making them more susceptible to become infected with parasites (Solomon 1969; Sheldon &Verhulst 1996).
In conclusion, our results provide an important baseline for future investigations of gastrointestinal parasites in Sapajus nigritus and related species. Further effort should be devoted to sampling other endoparasites, such as protozoa, which are the second most diverse group of parasites reported from wild primates (Nunn & Altizer 2006), and that can be at least partially recovered by non-invasive faecal sampling. In addition, future parasite surveys should include molecular analyses to facilitate more accurate species identification, thus providing a more precise evaluation of their zoonotic potential, their implications for primate conservation and management and for public health (Solórzano-García & Pérez-Ponce de León 2017).
ACKNOLEDGMENTS
We are grateful to Barbara Tiddi, Brandon Wheeler, Celia Baldovino and Paula Tujague for research collaboration and Patricia Casco, Isabella Bollini, Tabitha Allen, Melisa Unger, Maria Bobbio, Diana Sanmartín, Martin Fahy, Francisco Silva, Bartolo Silva, Fermino Silva, Esteban Pizzio, Angel Dolón Viejo, and Melina Brividoro for assistance in field data collection. We thank Mario Di Bitetti and three anonymous reviewers for discussion and comments on the manuscript, Nigel Parr for helping us in the identification of parasite taxa from pictures. We thank the National Park Administration (Administración de Parques Nacionales - APN) for research permits and the CIES (Centro de Investigaciones Ecológicas Subtropicales) for providing us with lodging facilities in Iguazú National Park. This research was supported by grant # 9219-12 of the National Geographic Society – Committee for Research and Exploration (CRE), and a Research Grant of the International Primatological Society to IA.
LITERATURE CITED
1. Abee, C., K. Mansfield, S. Tardif, & T. Morris. 2012. Nonhuman primates in biomedical research: diseases (2nd edition). American College of Laboratory Animal Medicine Series; USA. [ Links ]
2. Agostini, I., E. Vanderhoeven, M. S. Di Bitetti, & P. M. Beldomenico. 2017. Experimental testing of reciprocal effects of nutrition and parasitism in wild black capuchin monkeys. Scientific Reports 7: 12778. [ Links ]
3. Altizer, S., A. Dobson, P. Hosseini, P. Hudson, M.Pascual, & P. Rohani. 2006. Seasonality and the dynamics of infectious diseases. Ecology Letters 9:467-484. [ Links ]
4. Brown, A. D., & G. E. Zunino. 1990. Dietary variability in Cebus apella in extreme habitats: evidence for adaptability. Folia Primatologica 54:187-195. [ Links ]
5. Bush, A. O., K. D. Lafferty, J. M. Lotz, & A. W. Shostak. 1997. Parasitology meets ecology on its own terms: Margolis et al. Revisited. The Journal of Parasitology 83:575. [ Links ]
6. Chinchilla, M., O. M. Guerrero, G. A. Gutiérrez-Espeleta, R. Sánchez, & I. V. Campos. 2007. Parásitos en monos carablanca Cebus capucinus (Primates: Cebidae) de Costa Rica. Parasitologia Latinoamericana 62:170-175. [ Links ]
7. Chinchilla, M., B. Urbani, I. Valerio, & J. C. Vanegas. 2010. Parasitosis intestinal en monos capuchinos cariblancos Cebus capucinus (Primates: Cebidae) de un área protegida en la provincia de Limón, noreste de Costa Rica. Revista de Biología Tropical 58:1335-1346. [ Links ]
8. Corrêa, P., C. Bueno, R. Soares, F. M. Vieira, & L. C. Muniz-Pereira. 2016. Checklist of helminth parasites of wild primates from Brazil. Revista Mexicana de Biodiversidad 87:908-918. [ Links ]
9. Cox, D. D., & A. C. Todd. 1962. Survey of gastrointestinal parasitism in Wisconsin dairy cattle. Journal of the American Veterinary Medical Association 141:706-709. [ Links ]
10. Crespo, J. A. 1982. Ecología de la comunidad de mamíferos del Parque Nacional Iguazú, Misiones. Revista MACN, Ecología 3:45-162. [ Links ]
11. Di Bitetti, M. S. 1997. Evidence for an important social role of allogrooming in a Platyrrhine Primate. Animal Behaviour 54:199-211. [ Links ]
12. Di Bitetti, M. S. 2001. Food-associated calls in the tufted capuchin monkey Cebus apella. Tesis de Doctorado. Universidad Stony Brook, New York, EE.UU. [ Links ]
13. Dunn, F. L. 1963. Acanthocephalans and cestodes of South American monkeys and marmosets. The Journal of Parasitology 49:717-722. [ Links ]
14. Dunn, O. J. 1964. Multiple comparisons using rank sums. Technometrics 6:241-252. [ Links ]
15. Ezquiaga, M., M. Superina, & G. T. Navone. 2009. Parásitos intestinales de Zaedyus pichiy (Xenarthra: Dasypodidae) de Mendoza, Argentina. Mastozoología Neotropical 16:309-319. [ Links ]
16. Fragaszy, D. M., E. Visalberghi, & L. M. Fedigan. 2004. The Complete Capuchin: The Biology of the Genus Cebus. Cambridge University Press, Cambridge. [ Links ]
17. Freeman, G. H., & J. H. Halton. 1951. Note on an exact treatment of contingency, goodness of fit and other problems of significance. Biometrika 38:141-149. [ Links ]
18. Janson, C. H. 1996. Toward an experiemental socioecology of primates. Examples from Argentine brown capuchin monkeys (Cebus apella nigritus). Adaptive Radiations of Neotropical Primates (C. H. Janson, M. A. Norconk, A. L. Rosenberger & P. A. Garber, eds.). Plenum Press, New York. [ Links ]
19. Kaur, T., & J. Singh. 2009. Primate-parasitic zoonoses and anthropozoonoses: a literature review. Primate Parasite Ecology: the Dynamics and Study of Host-Parasite Relationships (M. A. Huffman & C. A. Chapman, eds.). Cambridge University Press, Cambridge. [ Links ]
20. Keymer, A. 1981. Population dynamics of Hymenolepis diminuta in the intermediate host. Journal of Animal Ecology 50:941-950. [ Links ]
21. Kowalewski, M. M., J. S. Salzer, J. C. Deutsch, M.Raño, M. S. Kuhlenschmidt, & T. R. Gillespie. 2011. Black and gold howler monkeys (Alouatta caraya) as sentinels of ecosystem health: patterns of zoonotic protozoa infection relative to degree of human-primate contact. American Journal of Primatology 73:75-83. [ Links ]
22. Lynch Alfaro, J. W., J. D. Jr Silva, & A. B. Rylands. 2012. How different are robust and gracile capuchin monkeys? An argument for the use of Sapajus and Cebus. American Journal of Primatology 74:273-286. [ Links ]
23. Michaud, C., M. Tantalean, C. Ique, E. Montoya, & A. Gozalo. 2003. A survey for helminth parasites in feral New World non-human primate populations and its comparison with parasitological data from man in the region. Journal of Medical Primatology 32:341-345. [ Links ]
24. Mollericona, J. L., J. Martínez, R. Limachi, P. Carvajal, & E. Alandia-Robles. 2013. Primer reporte de parásitos intestinales en Callicebus modestus del departamento de Beni, Bolivia. Neotropical Primates 20:18-24. [ Links ]
25. Navone, G. T. 1988. Estudios parasitologicos en edentados argentinos IV. Cestodes, pertenecientes a la familia Anoplocephalidae Cholodkoski, 1902, parásitos de Dasypodidos. Neotropica 34:51-61. [ Links ]
26. Notarnicola, J., C. M. Pinto, & G. T. Navone. 2008. Host occurrence and geographical distribution of Dipetalonema spp. (Nematoda: Onchocercidae) in Neotropical monkeys and the first record of Dipetalonema gracile in Ecuador. Comparative Parasitology 75:61-68. [ Links ]
27. Nunn, C. L., & S. Altizer. 2006. Infectious diseases in primates: behavior, ecology and evolution. Oxford University Press, Oxford. [ Links ]
28. Parr, N. A., L. M. Fedigan, & S. J. Kutz. 2013. A coprological survey of parasites in white-faced capuchins (Cebus capucinus) from Sector Santa Rosa, ACG, Costa Rica. Folia Primatologica 84:102-114. [ Links ]
29. Phillips, K. A., M. E. Haas, B. W. Grafton, & M.Yrivarren. 2004. Survey of the gastrointestinal parasites of the primate community at Tambopata National Reserve, Peru. Journal of Zoology 264:149-151. [ Links ]
30. Pinto, N., J. Lasky, R. Bueno, T. H. Keitt, & M. Galetti. 2009. Primate densities in the Atlantic Forest of Southeast Brazil: the role of habitat quality and anthropogenic disturbance. South American Primates: Comparative Perspectives in the Study of Behavior, Ecology, and Conservation (P. A. Garber, A. Estrada, J. C. Bicca-Marques, E. W. Heymann & K. B. Strier, eds.). Springer Science+Business Media, New York. [ Links ]
31. R Development Core Team. 2016. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org [ Links ]
32. Rego, A. A., & G. Schaeffer. 1988. Filariopsis barretoi (Travassos, 1921) (Nematoda: Metastrongyloidea) lung parasite of primates from South America - taxonomy, synonyms and pathology. Memórias do Instituto Oswaldo Cruz 83:183-188. [ Links ]
33. Ribeiro, M. C., A. C. Martensen, J. P. Metzger, & M.Fortin. 2011. The Brazilian Atlantic Forest: a shrinking biodiversity hotspot. Biodiversity hotspots (F. E. Zachos & J. C. Habel, eds.). Springer-Verlag, Berlin, Heidelberg. [ Links ]
34. Robinson, J. G., & C. H. Janson. 1987. Capuchins, squirrel monkeys and atelines: sociological convergence with Old World Monkeys. Primate societies (B. B. Smuts, D. L. Cheney, R. M. Seyfarth, R. W. Wrangham & T. T. Struhsaker, eds.). University of Chicago Press, Chicago. [ Links ]
35. Santa Cruz, A., J. Borda, M. de Rott, & L. Gómez. 1998. Pulmonary Filariopsis arator in capuchin monkeys (Cebus apella). Laboratory Primate Newsletter 37:15-17. [ Links ]
36. Santa Cruz, A., J. Borda, L. Gómez, & M. de Rott. 2000. Endoparasitosis in captive Cebus apella. Laboratory Primate Newsletter 39:10-13. [ Links ]
37. Santos, S. I., C. R. Ruiz-Miranda, & C. D. P. Santos. 2010. Helminths found in marmosets (Callithrix penicillata and Callithrix jacchus) introduced to the region of occurrence of golden lion tamarins (Leontopithecus rosalia) in Brazil. Veterinary Parasitology 171:123-129. [ Links ]
38. Schmidt, G. D. 1986. Handbook of tapeworm identification. CRC Press, Florida. [ Links ]
39. Sheldon, B. C. & S. Verhulst. 1996. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends in Ecology and Evolution 11:317-321. [ Links ]
40. Siegel, S., & N. J. Castellan. 1988. Nonparametric statistics for the behavioral sciences (2nd ed.). McGraw-Hill, New York. [ Links ]
41. Siemers, B. M. 2000. Seasonal variation in food resource and forest strata use by brown capuchin monkeys (Cebus apella) in a disturbed forest fragment. Folia Primatologica 71:181-184. [ Links ]
42. Solomon, G. B. 1969. Host hormones and parasitic infection. International Review of Tropical Medicine 3:101-158. [ Links ]
43. Solórzano-García, B., & G. Pérez-Ponce de León. 2017. Helminth parasites of howler and spider monkeys in Mexico: insights into molecular diagnostic methods and their importance for zoonotic diseases and host conservation. International Journal for Parasitology: Parasites and Wildlife 6:76-84. [ Links ]
44. Stoner, K. E. 1994. Population density of the mantled howler monkey (Alouatta palliata) at La Selva Biological Reserve, Costa Rica: a new technique to analyze census data. Biotropica 26:332-340. [ Links ]
45. Stoner, K. E., & A. M. González-Di Pierro. 2005. Intestinal parasitic infections in Alouatta pigra in tropical rainforest in Lacandona, Chiapas, Mexico: implications for behavioral ecology and conservation. New perspectives in the study of Mesoamerican primates: distribution, ecology, behavior, and conservation (A. Estrada, P. A. Garber, M. S. M. Pavelka & L. Luecke, eds.). Springer, New York. [ Links ]
46. Stuart, M. D., L. L. Greenspan, K. E. Glander, & M.R. Clarke. 1990. A coprological survey of parasites of wild mantled howling monkeys, Alouatta palliata palliata. Journal of Wildlife Diseases 26:547-549. [ Links ]
47. Stuart, M., V. Pendergast, S. Rumfelt, S. Pierberg, L. Greenspan, K. Glander, et al. 1998. Parasites of wild howlers (Alouatta spp.). International Journal of Primatology 19:493-512. [ Links ]
48. Sweet, D. L. 2017. A collection of nonparametric hypothesis tests. R package, Version 1.0.1. [ Links ]
49. Terborgh, J. 1983. Five New World Primates: a study in comparative ecology. Princeton University Press. [ Links ]
50. Thienpont, D., F. Rochette, & O. F. J. Vanparijs. 1979. Diagnóstico de las helmintiasis por medio del examen coprológico. Janssen Research Foundation, Beerse, Belgium. [ Links ]
51. Travassos, L. 1965. Contribuicão para o inventário crítico da zoologia no Brasil. Fauna helmintológica: consideracões preliminares - cestódeos. Publicações Avulsas do Museu Nacional, Rio de Janeiro. [ Links ]
52. Trejo-Macías, G., A. Estrada, & M. Á. Mosqueda Cabre-ra. 2007. Survey of helminth parasites in populations of Alouatta palliata mexicana and A. pigra in continuous and in fragmented habitat in southern Mexico. International Journal of Primatology 28:931-945. [ Links ]
53. Vitazkova, S. K., & S. E. Wade. 2006. Parasites of free-ranging black howler monkeys (Alouatta pigra) from Belize and Mexico. American Journal of Primatology 68:1089-1097. [ Links ]
54. Yamaguti, S. 1959. The cestodes of vertebrates. Systema helminthium. Vol. II. Interscience Publishers, New York. [ Links ]














