Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Mastozoología neotropical
Print version ISSN 0327-9383On-line version ISSN 1666-0536
Mastozool. neotrop. vol.25 no.2 Mendoza Dec. 2018
ARTÍCULO
Environmental predictors of demography in the tuco-tuco of the dunes (Ctenomys flamarioni)
Felipe M. Garcias1, 6, José F. B. Stolz1, Gabriela P. Fernández2, Bruno B. Kubiak3, 7 ,Vinicius A. G. Bastazini4 and Thales R. O. Freitas1, 2, 3
1 Programa de Pós-Graduação em Ecologia. Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil.
2 Centro de Bioinvestigaciones (CeBio) Universidad Nacional del Noroeste de la provincia de Buenos Aires (UNNOBA)-CICBA/ Centro de Investigación y Transferencia CONICET-UNNOBA (CITNOBA) Pergamino, Buenos Aires, Argentina
3 Programa de Pós-Graduação em Biologia Animal, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil. [Correspondencia: Bruno B. Kubiak <busnelo@hotmail.com>]
4 Laboratório de Ecologia Quantitativa, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil.
5 Departamento de Genética, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil.
6 Instituto Pró-Pampa (IPPAMPA), Pelotas, Brazil.
7 Departamento de Ciências Biológicas da Universidade Regional Integrada do Alto Uruguai e das Missões – Campus de Frederico Westphalen, Fredeico Westphalen, Brazil.
ABSTRACT
We studied the population ecology of the South American subterranean rodent Ctenomys flamarioni at the Taim Ecological Station, Rio Grande do Sul, Brazil. A capture-mark-release program was conducted in two different time periods: (A) from February 2004 to November 2005 and (B) from December 2011 to March 2013; as a result, a total of131 individuals were marked. Some population parameters remained constant (average population density, survival rate), while others fluctuated (sex ratio, estimated population size, recruitment and mortality). The second sampling period had higher estimated population size and mortality and reduced recruitment compared to the first period. Sex ratio did not differ significantly from 1:1 in period A, but showed a significant departure from 1:1 in period B, with a greater number of females captured. Ctenomys flamarioni populations seem to be substantially influenced by local environmental factors; density was positively associated with temperature and negatively associated with precipitation. This is the first report of variation through time in reproduction and population rates for this species, which is under increasing risk of extinction.
RESUMO
Influência de variáveis ambientais sobre a demografia do tuco-tuco das dunas (Ctenomys flamarioni).
Nós estudamos a ecologia populacional do roedor subterrâneo sul-americano Ctenomys flamarioni na Estação Ecológica do Taim, Rio Grande do Sul, Brasil. Um programa de captura‑marcação-recaptura foi realizado em dois períodos diferentes: (A) de fevereiro de 2004 a novembro de 2005 e (B) de dezembro de 2011 a março de 2013. Como resultado, um total de 131 indivíduos foram marcados. Alguns parâmetros populacionais permaneceram constantes (densidade populacional média, taxa de sobrevivência), enquanto outros flutuaram (razão sexual, tamanho estimado da população, recrutamento e mortalidade). O segundo período de amostragem apresentou maior tamanho populacional estimado e mortalidade, por outro lado mostrou menor valor de recrutamento em relação ao primeiro período. A razão sexual não diferiu significativamente de 1:1 no período A, mas mostrou um desvio significativo de 1:1 no período B, com um maior número de fêmeas capturadas. As populações de Ctenomys flamarioni parecem ser substancialmente influenciadas por fatores ambientais locais; a densidade foi positivamente associada à temperatura e negativamente associada à precipitação. Este é o primeiro relato de variação ao longo do tempo nas taxas de reprodução e população para esta espécie, que está sob-risco crescente de extinção.
Key words: Conservation; Population biology; Population dynamics; Subterranean rodents.
Palavras-chaves: Conservação; Dinâmica populacional; Ecologia de populações; Roedores subterrâneos.
Recibido 2 febrero 2018.
Aceptado 26 junio 2018.
Editor asociado: A. Abba
INTRODUCTION
Population biology is considered one of the most important tenets of ecology (Berryman 2002), and data describing population characteristics are crucial for the design and implementation of conservation plans for threatened species (McCarthy & Thompson 2001; Brito & Figueiredo 2003). Furthermore, basic population metrics (e.g. abundance, density, vital rates) are required for effective decision-making with regard to species conservation and management (Royle et al. 2014). The relation between environmental factors and population dynamics is an important external element to be considered in population studies (Lundberg et al. 2000). Possible explanations about how environmental factors influence population dynamics involve food supply, predation, disease and parasites, or some interaction among these factors. Recognizing these factors and their relative influence on the population dynamics is critical for understanding the variation of population parameters through time (Boonstra et al. 1998).
Although several studies in the subterranean rodents of genus Ctenomys have been published in the last decades, few of them have evaluated changes in population parameters in a temporal scale (Pearson et al. 1968; Malizia 1998; Zenuto & Busch 1998; Cutrera et al. 2006; Marinho & Freitas 2006). The genus contains approximately 70 species (Gardner et al. 2014; Bidau 2015; Freitas 2016; one of these is Ctenomys flamarioni Travi 1981 (commonly known as "tuco-tuco-das-dunas”) that is endemic of coastal plain sand dunes in Rio Grande do Sul State, southern Brazil (Freitas 1995). The species is distributed along about 560 km of the nearly linear arrangement of dunes, from Arroio Texeira city in the north (Freitas 1994) to the Chui River in the south (Fernández-Stolz et al. 2007). Ctenomys flamarioni is classified as VU (Vulnerable) in the Brazilian List of Endangered Species (MMA 2014) and in the List of Endangered Fauna of Rio Grande do Sul (Fontana et al. 2003), and is listed as EN (Endangered) on the The International Union for Conservation of Nature’s Red List of Threatened Species (IUCN) (Catzeflis et al. 2008). Despite the efforts taken to improve their conservation status, the degree of threat to this species has been recently reevaluated and classified as EN at Rio Grande do Sul (FZB 2014).
The continued and increased threat of extinction to this species is probably due to the interaction of at least two factors: (i) strong urban pressure and habitat deterioration; and (ii) decreased population size caused by periodic and random oscillations in climate and habitat availability. An important challenge in conservation biology is extracting pertinent information from available data for endangered species (Buenau & Gerber 2004), from which conservation plans can be designed in order to reduce the degree of threat. Therefore, the principal aim of this study is to understand the population dynamics of Ctenomys flamarioni, using information from two distinct sampling periods to verify (1) the most important environmental factors acting over population parameters (e.g. survival, recruitment, population census size and density) and (2) variation of these parameters at different time periods. We provide clues on how to perform a remediation plan in order to protect this endangered subterranean rodent species.
MATERIAL AND METHODS
Study area
The study was performed at the Taim Ecological Station (ESEC-Taim) (Fig. 1), a protection and nature conservation area located in Rio Grande do Sul, Brazil, between the municipal limits of Rio Grande and Santa Vitória do Palmar. The total area is 10938.58 hectares (according to the establishment decree No. 92963 of 1986), and the site lies between latitudes 32°30¢00² and 32°55¢00² S and longitudes 52°25¢00² and 52°45¢00² W. The area comprises an extensive system of lagoons and wetlands, and contains high biological diversity (Schreiner 2012). The trapping area was delimited to ~6 ha (500 x 120 m) coastal plain sand dunes between the Atlantic Ocean to the east, and the Taim Swamp to the west; the southern boundary is defined by constant water flow from the swamp (1.5 m width). This area is highly affected by wind regimen originating from the NW or SW depending on temporal factors and ocean variations. Annual rainfall varies between 1000 and 1500 mm (Nimer 1977; Vieira & Rangel 1998). The wind regime forms moving dunes of approximately five meters in height, and the area is characterized by sparse vegetation including Blutaparon portulacoides, Panicum racemosum, Senecius crassiforus and Hydrocotyle bonariensis.
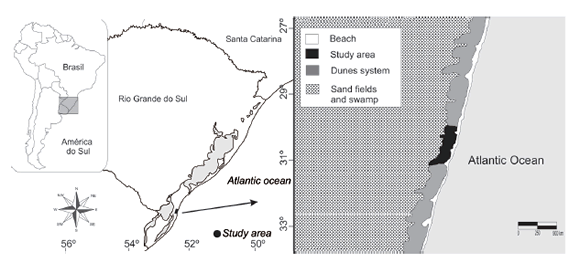
Fig. 1. (A) Geographical distribution of the "tuco-tuco-das-dunas” (Ctenomys flamarioni) along the seashore of Rio Grande do Sul, southern Brazil; (B) characteristics of the coastal dune system around the study area within the Taim Ecological Station (ESEC-Taim).
Trapping
We conducted a capture-mark-release program to obtain data on population structure and demography. The study included two sampling periods. The first (A) took place between February 2004 and November 2005, and the second (B) between December 2011 and March 2013. There were a total of eight sampling events in the first period and six in the second, with five consecutive days of trapping per sample event. We trapped animals using forty Oneida Victor nº "0” traps equipped with rubber for protection. Trapping took place from 08:00 to 16:00 h daily, for a total of eight hours per day in the field. The mean number of days between trapping events was 90 (the same for each sampling period). Traps were placed at the entrances of burrows, which were detected by the presence of freshly moved sand. Traps were checked every 15 minutes to avoid animal injury, and burrow identity was indicated with a numbered flag, which remained in place throughout the five-day sampling period. Field activities were performed only on periods with adequate conditions (e.g. little wind and no rain). The traps remained in each tunnel for at least four hours even if a tuco-tuco was captured, in case other individuals were using the same tunnel system. All burrows where animals were captured remained marked throughout the days of field activity. With this, it was possible to identify places of activity of individuals not captured, thus enabling to capture all (or almost all) the animals over the studied area. In addition, in all sample periods at least three researchers were present, guaranteeing similar capture effort and the collectors’ experience throughout the study.
Captured animals were lightly anesthetized with Zoletil® for measurements and upon first capture, a passive integrated transponder tag (12x1.88mm AnimalTag®) was implanted. During recapture events, individuals were identified using a barcode reader and the transponder insertion site was checked for infection. In no case animals showed signs of health damages. Individual records included weight, sex, and reproductive status of the females (i.e., pregnant, lactating, or exhibiting signs of reproductive activity).
Animals were individually placed in a wooden box, and at the end of the day they were released into the same burrow in which they were captured. We distributed traps among different tunnel systems for each 5-day sample event to decrease the probability of capturing the same individuals, although in some cases it was unavoidable. This study was approved by the Brazilian government (IBAMA nº 02023.001955/03 license nº 144/2003, and ICMBIO nº 27938-1).
Demographic data
Analyses of demographic structure were carried out according to the qualitative data obtained in the capture events. The definition of age classes (I, II, III, IV and V) was established based on individual animal weights according to Stolz (2006). Class I individuals (≤ one year old) were considered juveniles, while individuals in classes II–V were classified as adults. Males were categorized into Class I: < 50 g; Class II: 50 g–180 g; Class III: 181 g–280 g; Class IV: 281 g–350 g; Class V: > 351 g; and females into Class I: individual cub (< 50 g); Class II: 50 g–180 g; Class III: 181 g–235 g; Class IV: 236 g–265 g; Class V: > 265 g.
Birth rates were evaluated taking into account the number of embryos that could be detected in the uterus of animals by abdominal palpation, and by the number of pups living with their mothers. We used a Student’s t-test to analyze differences between sampling periods. Simple linear regression was used to assess whether population sizes decreased over time for each sampling period. All analyses were conducted in the R program for statistical computing (R Development Core Team 2012).
Data analysis
We considered the two sampling periods as independent events, generating population estimates for both periods. Population size was calculated using Jolly-Seber stochastic estimates (Jolly 1965; Seber 1965). Survival and recruitment between seasons were also calculated using Jolly-Seber methods. Population density was calculated by dividing the population size by the total area (6 ha).
To determine the influence of environmental factors on population dynamics in both periods, we measured four variables: temperature (oC), precipitation (mm), humidity (%), and wind speed (km/h).
We used monthly measurements of each of these variables to estimate their averages for each season. The climatological data were obtained from the National Institute of Meteorology ("Instituto Nacional de Meterologia”) (INMET 2015), using as a reference data for the meteorological station located 150 km from the sample site (Santa Vitória do Palmar).
In order to evaluate the effects of each predictor and effect of sampling period over population density we used a model selection and multimodel inference approach, within an information-theoretic framework (Anderson 2008; Burnham & Anderson 2002). We measured model plausibility using second order Akaike Information Criterion (AICc), which is a measure of predictive accuracy of the model (Shmueli 2010) and AICc weight (w), which measures the relative likelihood of the model given the data, normalized across the set of candidate models to sum to one (Anderson 2008; Burnham & Anderson 2002). As predictors were not measured in the same scale, we standardized them to unit variance, in order to bring their means to zero and their variance to one (Legendre & Legendre 1998). As our main interest lied on the importance of the environmental variables, we first fitted three distinct models in order to decide whether or not sampling years should be included in the final models. The first model was a linear mixed model containing all environmental predictors and the sampling year as the random effect. The second one was a linear model containing the environmental predictors and sampling period as a factor, and the third model was linear and only environmental predictors were taken into account. As this last model showed the best fit to the data (AICc=53.6, AICc weight=0.56) we used it for further analysis. We estimated the average value for each model parameter using all possible nested models (Anderson 2008; Burnham & Anderson 2002). The relative importance value ranges from 0 to 1, where the larger the value of a predictor, the higher its relative importance compared to other predictors. Model selection and multimodel inference were computed using the packages bbmle (Bolker & R Development Core Team 2010) and glmulti (Calcagno & Mazancourt 2010) in the R environment (R Core Team 2012).
RESULTS
We captured 131 different individuals over 211 capture events in the two sampling periods. Period A resulted in 152 captures of 75 different individuals (38 males and 37 females). Period B resulted in 59 captures of 56 different individuals (18 males and 38 females). Sex ratio did not differ significantly from 1:1 (P=0.187; 37:38 female: male) in period A. Period B showed a significant departure from 1:1, with a greater number of females captured (38:18 female:male, P<0.05). Table 1 presents the means for seasonal survival rate, recruitment and mortality for sample period A and period B. Three of these parameters were significantly different between the two sampling periods (Fig. 2), period B showed higher estimates on population size and mortality values (t8=-2.208, P=0.029; t6=-2.563, P=0.021) and lower recruitment values (t6=3.318, P=0.014) compared to sampling period A.
Table 1
Estimated population size of Ctenomys flamarioni, in ESEC Taim, during two different sampling periods, summer 2004 to spring 2005 (A) and spring 2011 to summer 2013 (B). Ni=estimated population size on time i. Фi,i+1= estimate survival between time i and i +1; Bi,i+1= estimated recruitment between time i and time i +1; Di,i+1= estimated mortality between time i and time i

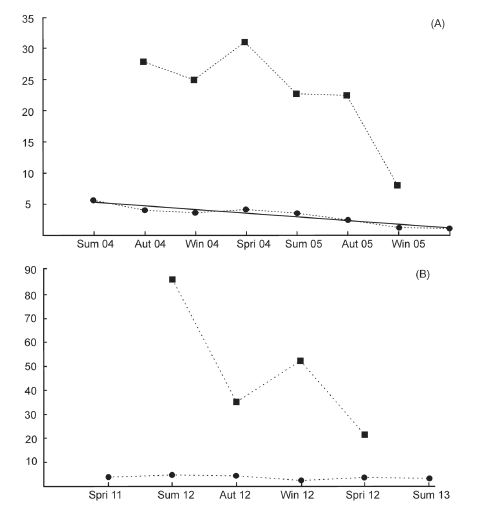
Fig. 2. Population size of Ctenomys flamarioni inhabiting sand dunes in southern Brazil. (A) during the period summer 2004 - spring 2005 and (B) spring 2011 - summer 2013. Squares represent estimated population size and circles represent population density. The solid line in (A) represents the line of simple linear regression.
Average population density did not differ significantly between sampling periods, (t4=-0.663, P=0.259), with 3.23ñ1.53 individuals/ha in period A and 3.96ñ0.88 individuals/ha in period B (Table 1). There was a reduction in the estimated population size and density over time in both periods, however a decrease in population density over time was only significant for period A (F1,6=50.147, P<0.001) (Fig. 2).
Temperature and precipitation were the best factors at predicting population densities of Ctenomys flamarioni for the entire sampling period (Fig. 3). Density was positively associated with temperature and negatively associated with precipitation (Table 2).

Fig. 3 Relative importance values for each predictor.
Table 2
Model-averaged parameter estimates of standardized environmental variables that might influence the population densities of Ctenomys flamarioni in ESEC Taim, based on all possible linear models.

Evaluation of population age structure indicated that the majority of males in both sampling periods were classified in Class III, considered to consist of sub-adults. Females sampled in period A were most often categorized as Class IV, while in period B they were more commonly categorized as Class II (Table3). We captured females with signs of reproductive activity throughout the year (Fig.4). However, the species presents a markedly seasonal reproductive cycle, with pups only found in spring and summer seasons. Nevertheless, except for the autumn and summer of 2011/2012, females in all other seasons showed evidence of pregnancy or parental care (lactating or with embryos). We captured a total of 11 pregnant females, seven of which had two embryos and four of which had only one embryo. Two of these females were sharing their burrows with pups (Fig. 4).
Table 3
Age structure of the population of Ctenomys flamarioni in ESEC Taim, during two different sampling periods: summer 2004 to spring 2005 (A) and spring 2011 to summer 2013 (B).


Fig. 4 Proportion of reproductive status in females of Ctenomys flamarioni in two different sampling periods (2004/2005 and 2011/2012) in a population in ESEC Taim.
DISCUSSION
The comparison of the population data for C.flamarioni at different time periods indicated that some population parameters stay constant (density), while others change through the time (estimated population size, mean seasonal survival rates, recruitment, and mortality). Thus, these population traits may differ spatially in different environments (Patton & Brylski 1987; Patton 1990; Cutrera et al. 2006) and temporally within the same environment. Some variables showed important differences between sampling periods (e.g. population size, survival rate, recruitment, and mortality). This may be explained by the low number of recaptures, within sampling periods, which may cause strong variation in these estimates (Otis 1978; Jolly 1965; Seber 1965). Density does not depend on the number of recaptures, thus, this population parameter did not significant differ between periods.
The estimated population density in this study was low in both sampling periods compared to other subterranean rodents, and even compared to other Ctenomys species (Pearson 1959; Nevo 1979; Rosi et al. 1992; Marinho & Freitas 2006). Our estimates of population density were less than the observed in C. australis, which lives in a similar habitat in Argentina (16.1ñ1.1 ind/ha, Zenuto & Busch 1998). Low density may be related to environmental characteristics (Mapelli & Kittlein 2009), such as plant biomass, which in the sand dunes habitat is smaller than that of adjacent habitats (Galiano et al. 2014; Kubiak et al. 2015). This may also influence the home range size, where animals occupying places with less plant biomass availability exhibit larger home range size (Kubiak et al. 2017) and may result in lower population densities.
Environmental variation is closely linked to population dynamics of this species, and fluctuations over time can cause population growth decrease to nearly zero (e.g., as seen in the population size and density estimates from period A). Our results suggest that population decrease may be related to precipitation rates, whereas higher temperatures tend to increase density. The increase in rainfall (mainly during the winter), and the subsequent increase if water volume in the lagoon system surrounding the tuco-tuco populations, flood the dunes and reduce the habitat available for the individuals. This way, increased precipitation influenced by the El Niño phenomenon may be another factor potentially contributing to the decrease in population density. This situation leads to a dynamics of local extinction and re-colonization as suggested by the population size estimated for each period. On the other hand, the increase in temperature seems to be related to high density rates, probably due to the fact that warmer temperatures lead to seasonal variation in reproductive rates. Despite the sexual activity observed throughout the year, pups were only found in warm seasons, suggesting there is only one birth period. Instead, temperature and precipitation should be related to the amount of biomass available in the habitat, directly influencing the home range size and population density of subterranean species (Šumbera et al. 2003; Romañach et al. 2005; Lövy et al. 2015); however more detailed studies that address these aspects are necessary to better understand the influence of these factors on the population density of C. flamarioni.
Population size fluctuations in Ctenomys flamarioni have previously been revealed in genetic and phylogeographic studies utilizing microsatellite loci and mitochondrial DNA, which include evidence of loss of genetic variation and, in many cases, population bottlenecks (Fernández-Stolz 2007; Fernández-Stolz et al. 2007). The actual instability of the coastal habitats across Rio Grande do Sul state could partially explain this pattern. However, the evolutionary history of the species could also play a key role over the loss of genetic variation and the populational decline, because of its hypothesized origin from a small number of founder individuals (Massarini & Freitas 2005), followed by demographic expansion through the vacant coastal dunes (Fernández-Stolz 2007). Subsequent reductions in population size could then have been caused by periodic oscillations in climate, and habitat availability determined by the Quaternary glacio-eustatic fluctuations in sea level, through at least four successive transgression-regression cycles (Villwock et al. 1986). The most recent interglacial sea-level event formed the most recent lagoon system, about 5000 years ago (ya) (Tomazelli Dillenburg & Villwock 2000; Esteves et al. 2002). There is strong evidence (e.g. radiocarbon dating of marine fossils) of a marine ingression into Mirim Lagoon (Buchmann, Barbosa & Villwock 1998), supporting the hypothesis of important reductions in suitable areas for occupation, and a related reduction in population size. Urban development in many coastal locations is also a probable modern factor that contributes to long- and short-term coastal erosion in Rio Grande do Sul (Esteves et al. 2002; Medeanic, Dillenburg & Toldo 2001), influencing population dynamics and genetic patterns in these populations (Fernández-Stolz 2007; Fernández-Stolz et al. 2007).
Despite the sexual activity observed throughout the year, pups were only found in spring and summer, indicating that there is only one birth period. This may lead to a lower rate of population increase. This becomes a major problem for species conservation in the area because the highest mortality rates are in young individuals. Sex ratio can vary within Ctenomys, with some species biased toward females (e.g., C. talarum [Pearson 1959; Malizia & Busch 1991; Zenuto & Busch 1998]), and others having a more or less balanced sex ratio (e.g., C. mendocinus, [Rosi et al. 1996); C. talarum (Malizia & Busch 1997; Malizia 1998); C. minutus (Marinho & Freitas 2006]). Sex ratio was balanced in the first sampling period but skewed toward females in the second one. Previous studies with C. flamarioni have also found balanced sex ratios (Bretschneider 1987; Fernández 2002; Fernández-Stolz et al. 2007). However, when only adults were considered, there were fewer males than females (Fernández-Stolz et al. 2007). This suggests that birth rate, but not mortality rate, is balanced among the sexes.
We recognize that the method used to estimate birth rates has limitations, since embryo detection in the mother’s uterus is linked to the size of the embryo, and that small pups may be less likely to be captured using Victor traps. However, we come to believe that the number of embryos estimated for the pregnant females is realistic, since we expect embryos siblings to present similar size and then the same probability of being detected. That is, whenever it was possible to use this methodology, all existing embryos were detected. Probably other methodologies are more adequate to verify the number of pups that are sharing the tunnel system with the mother, such as the nest search and consequently the destruction of the tunnel system, however this type of methodology is impracticable in a population study and can be applied in future studies. In spite of this methodologic constraint, the results presented here show certain congruence with the results of Zenuto & Busch (1998) for C. australis, a phylogenetically close species that occupies similar habitats.
According to some authors, mortality rates within the genus Ctenomys are connected to body size and age (Pearson 1968; Malizia 1998). There is evidence of a high mortality from predation in young subjects, while in adults patterns of mortality are less clear (Lacey et al. 2000). The observed population age structure of C. flamarioni in this study matched our expectations, since increased mortality seems to occur during the first year when individuals are smaller and more susceptible to predation. Mature individuals have greater body mass and are less susceptible to predator attacks, thus adults are more common in the population (Lacey et al. 2000). Further, as mentioned litter size could be underestimated due to limitations in our ability to identify embryos by touching, and estimates for numbers of young individuals could be inaccurate due to limitations of the trap system to catch animals with low body mass and rates of movement.
In sum, this study provides important information for C. flamarioni conservation. Our results indicate that population dynamics are strongly influenced by environmental factors, with these relationships potentially amplified by the peculiar dynamics caused by the wind and hydric regimes of the region, which influence the conformation and structure of the coastal dunes. These factors cause a high degree of population parameters variation (estimated population size, mean seasonal survival rates, recruitment, and mortality), with apparent immigration and re-colonization from neighboring populations, in response to the availability of resources. This is the first work to generate information about reproduction and population rates over the time for this species, which has been threatened by increasing risk of extinction. The habitat specificity of this species coupled with the natural instability of the coastal habitat and human activities (Fernández-Stolz 2007) makes C. flamarioni one of the most endangered tuco-tuco species of the Rio Grande do Sul Coastal Plain (Fernandes et al. 2007). Considering that this study was conducted in a strictly protected area, the values obtained here can serve as a basis for comparisons with degraded areas, and to describe potential management activities for species conservation.
ACKNOWLEDGMENTS
The study was funded by Fundação O Boticário de Proteção à Natureza (no 0634_20042), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). We want to thank the people of Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) TAIM Ana Carolina Canary, Seu Laudelino and Everson. Special thanks to Loreci Silva, Fabiano Fernandes, Sérgio Althof, Rodrigo Fornel, Breno Matte, Gisele Rebelato and Daniel Galiano for help in the field.
LITERATURE CITED
1. Anderson, D. R. 2008. Model based inference in the life sciences: a primer on evidence. Springer, New York. [ Links ]
2. Berryman, A. A. 2002. Population: a central concept for ecology. Oikos 97:439-442. [ Links ]
3. Bidau, C. J. 2015. Familiy Ctenomyidae Lesson, 1842. Mammals of South America (J. L. Patton, U. F. J. Pardiñas & G. D’Elía, eds.). The university of Chicago Press, Chicago and London.
4. Bolker, B., & R Development Core Team. 2010. bbmle: Tools for general maximum likelihood estimation. R package version 0.9, 5. [ Links ]
5. Boonstra, R., C. J. Krebs, & N. C. Stensth. 1998. Population cycles in small mammals: the problem of explaining the low phase. Ecology 79:1479-1488. [ Links ]
6. Bretschneider, D. 1987. Alguns aspectos da biologia e ecologia de Ctenomys flamarioni Travi, 1981 (Rodentia: Ctenomyidae). Dissertation, University Federal of Rio Grande do Sul. [ Links ]
7. Brito, D., & M. S. L. Figueiredo. 2003. Minimum viable population and conservation status of the Atlantic forest spiny rat Trinomys eliasi. Biological Conservation 112:153-158. [ Links ]
8. Buenau, K. E., & L. R. Gerber. 2004. Developing recovery and monitoring strategies for the endemic Mount Graham red squirrels (Tamiascirus hudsonicus grahamensis) in Arizona. Animal Conservation 7:177-22. [ Links ]
9. Buchmann, F. S., V. P. Barbosa, & J. A. Willwock. 1998. Sedimentología e paleoecología durante o maximo transgressivo holocenico na Lagoa Mirim, RS, Brasil. Revista Acta Geologica Leopoldinesia 21:21-26. [ Links ]
10. Burnham, K. P., & D. R. Anderson. 2002. Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York. [ Links ]
11. Calcagno, V., & C. de Mazancourt. 2010. glmulti: an R package for easy automated model selection with (generalized) linear models. Journal of Statistical Software 34:1-29. [ Links ]
12. Catzeflis, F., J. Patton, A. Percequillo, C. Bonvicino, & M. Weksler. 2008. Ctenomys flamarioni. The IUCN Red List of Threatened Species 2008:e.T136464A4295063. [ Links ]
13. Cutrera, A. P., C. D. Antinuchi, M. S. Mora, & A. I. Vassallo. 2006. Home-range size and activity patterns of the South American subterranean rodent Ctenomys talarum. Journal of Mammalogy 91:1425-1434. [ Links ]
14. Esteves L. S., E. E Toldo Jr, S. R. Dillenburg, & L.J. Tomazelli. 2002. Long- and short-term coastal erosion in Southern Brazil. Journal of Coastal Research 36:273-282. [ Links ]
15. Fernandes, F. A., G. P. Fernández-Stolz, C. M. Lopes, & T. R. O. Freitas. 2007. The conservation status of the tuco-tucos, genus Ctenomys (Rodentia: Ctenomyidae) in southern Brazil. Brazilian Journal of Biology 67:839-847. [ Links ]
16. Fernández, G. P. 2002. Análise da estrutura populacional e da variabilidade genética em três populações de Ctenomys flamarioni (Rodentia-Ctenomyidae) através de loci de microssatélites. Dissertation, University Federal of Rio Grande do Sul. [ Links ]
17. Fernández-Stolz, G. P. 2007. Estudos evolutivos, filogeográficos e de conservação em uma espécie ameaçada de extinção, Ctenomys flamarioni (Rodentia-Ctenomyidae), através de loci de microssatélites e DNA mitocondrial. Dissertation, University Federal of Rio Grande do Sul. [ Links ]
18. Fernández-Stolz, G. P., J. F. B. Stolz, & T. R. O. Freitas. 2007. Bottlenecks and dispersal in the tuco-tuco-das-dunas, Ctenomys flamarioni (Rodentia:Ctenomyidae) in southern Brazil. Journal of Mammalogy 88:935-945. [ Links ]
19. Fontana, C. S., G. A. Bencke, & R. E. Reis. 2003. Livro vermelho da fauna ameaçada de extinção no Rio Grande do Sul. Edipucrs, Porto Alegre. [ Links ]
20. Freitas, T. R. O. 1994. Geographical variation of heterochromatin in Ctenomys flamarioni (Rodentia: Octodontidae) and its cytogenetic relationship with other species of the genus. Cytogenetics and Genome Research 67:193-198. [ Links ]
21. Freitas, T. R. O. 1995. Geographic distribution and conservation of four species of the genus Ctenomys in Southern Brasil. Studies on Neotropical Fauna and Environment 30:53-59. [ Links ]
22. Freitas, T. R. O. 2016. Family Ctenomyidae (Tuco-tucos). Lagomorphs and Rodents I. (D. E. Wilson, T. E. Lacher & R. A. Mittermeier, eds.). Lynx Edicions Publications, Barcelona. [ Links ]
23. FZB. 2014. Lista de espécies da fauna gaúcha ameaçadas de extinção. <http://www.fzb.rs.gov.br/conteudo/4444/?RS_tem_280_esp%C3%A9cies_de_animais_amea%C3%A7adas_de_extin%C3%A7%C3%A3o> [ Links ].
24. Galiano, D., B. B. Kubiak, G. E. Overbeck, & T. R. O Freitas. 2014. Effects of rodents on plant cover, soil hardness, and soil nutrient content: a case study on tuco-tucos (Ctenomys minutus). Acta Theriologica (Warsz) 59:583-587. [ Links ]
25. Gardner, S. L., J. Salazar-Bravo, & J. A. Cook. 2014. New Species of Ctenomys Blainville 1826 (Rodentia: Ctenomyidae) from the Lowlands and Central Valleys of Bolivia. Special Publications - The Museum, Texas Tech University. 62: 1-34 [ Links ]
26. IUCN. 2015. Red List of Threatened Species, version 2015.3. <http://www.iucnredlist.org/details/699/0. Accessed 16 November 2015> [ Links ].
27. INMET. 2015. Available online at: http://www.inmet.gov.br. Accessed 23 December 2015. [ Links ]
28. Jolly, G. M. 1965. Explicit estimates from capture-recapture data with both death and immigration-stochastic model. Biometrica 52:225-247. [ Links ]
29. Kubiak, B. B., D. Galiano, & T. R.O. Freitas. 2015. Sharing the space: Distribution, habitat segregation and delimitation of a new sympatric area of subterranean rodents. PLoS One 10:1-10. [ Links ]
30. Kubiak, B. B., D. Galiano, & T. R. O. Freitas. 2017. Can the environment influence species home-range size? A case study on Ctenomys minutus (Rodentia, Ctenomyidae). Journal of Zoology 302:171-177. [ Links ]
31. Lacey, E. A., J. L. Patton, & G. N. Cameron. 2000. Life Underground. The University of Chicago Press. Chicago. [ Links ]
32. Legendre, P., & L. Legendre. 1998. Numerical Ecology. 2nd edition. Elsevier, Amsterdam. [ Links ]
33. Lövy, M., J. Šklíba, E. Hrouzkova, V. Dvorakova, E. Nevo, & R. Šumbera. 2015. Habitat and burrow system characteristics of the blind mole rat Spalax galili in an area of supposed sympatric speciation. PLoS ONE 10 e0133157.
34. Lungberg, P., E. Ranta, J. Ripa, & V. Kaitala. 2000. Population variability in space and time. Reviews. Trends in Ecology & Evolution 15:460-464. [ Links ]
35. Malizia, A. I. 1998. Population dynamics of fossorial rodent Ctenomys talarum (Rodentia: Octodontidae). Journal of Zoology 244:545-551. [ Links ]
36. Malizia, A. I., & C. Busch. 1991. Reproductive parameters and growth in the fossorial rodent Ctenomys talarum (Rodentia: Octodontidae). Mammalia 55:293-305. [ Links ]
37. Malizia, A. I., & C. Busch. 1997. Breeding biology of the rodent Ctenomys talarum (Rodentia: Octodontidae). Journal of Zoology 242:463-471. [ Links ]
38. Mapelli, F. J., & M. J. Kittlein. 2009. Influence of patch and landscape characteristics on the distribution of the subterranean rodent Ctenomys porteousi. Landscape Ecology 24:723-733. [ Links ]
39. Marinho, J. R., & T. R. O. Freitas. 2006. Population structure of Ctenomys minutes (Rodentia, Ctenomyidae) on the coastal plain of Rio Grande do Sul, Brazil. Acta theriologica 51:53-59. [ Links ]
40. Massarini, A. I., & T. R. O. Freitas. 2005. Morphological and cytogenetics comparison in species of the mendocinus-group (genus Ctenomys) with emphasis in C. australis and C. flamarioni (Rodentia-Ctenomyidae). Caryologia 58:21-27. [ Links ]
41. McMarthy, M. A., & C. Thompson. 2001. Expected minimum population size as a measure of threat. Animal Conservation forum 4:351-355. [ Links ]
42. Medeanic, S., S. R. Dillenburg, & E. E. Toldo Jr. 2001. Novos dados palinológicos da transgressão marinha pós-glacial em sedimentos da Laguna dos Patos, RS, Brasil. Revista Universidade de Guarulhos 6:64-76. [ Links ]
43. MMA. 2014. Lista das Espécies da Fauna Brasileira Ameaçadas de Extinção. <http://www.icmbio.gov.br/portal/biodiversidade/fauna-brasileira/lista-de-especies.html> [ Links ].
44. Nevo, E. 1979. Adaptive convergence and divergence of subterraneam mammals. Annual review of ecology and systematics 10:269-308. [ Links ]
45. Nimer, E. 1977. Clima. Geografia do Brasil, Região Sul (IBGE). SERGRAF-IBGE, Rio de Janeiro. [ Links ]
46. Otis, D. L., K. P. Burnham, G. C. White, & D.R. Anderson. 1978. Statistical inference from capture data on closed animal populations. Wildlife Monographs 3-135. [ Links ]
47. Patton, J. L. 1990. Geomyid evolution: the historical, selective, and random basis for divergence patterns within and among species. Evolution of subterranean mammals at the organismal and molecular levels (E.Nevo & O. A. Reig, eds.). Wiley-Liss, New York. [ Links ]
48. Patton, J. L., & P. V. Brylski. 1987. Pocket gophers in alfalfa fields: causes and consequences of habitat-related body size variation. The American Naturalist 4:493-503. [ Links ]
49. Pearson, O. P. 1959. Biology of subterranean rodents, Ctenomys in Peru. Memorias del Museo de Historia Natural Javier Prado 9:1-56. [ Links ]
50. Pearson, O. P. et al. 1968. Estructura social, distribución espacial y composición por edades de una población de tuco-tucos (Ctenomys talarum). Investigaciones Zoológicas Chilenas 18:47-80. [ Links ]
51. R Development Core Team. 2012. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. <http://www.R-project.org> [ Links ].
52. Romañach, S. S., O. J. Reichman, & E. W. Seabloom. 2005. Seasonal influences on burrowing activity of a subterranean rodent, Thomomys bottae. Journal of Zoology 266:319-325. [ Links ]
53. Rosi, M. I., M. I. Cona, S. Puig, F. Videla, & V. G. Roig. 1996. Size and structure of burrow systems of the fossorial rodent Ctenomys mendocinus in the piedmont of Mendoza Province, Argentina. Z. Saugetierkd 61: 352-364. [ Links ]
54. Rosi, M. I., S. Puig, F. Videla, L. Madoery, & V. G. Roig. 1992. Estudio ecológico del roedor subterráneo Ctenomys mendocinus en la precordillera de Mendoza, Argentina: ciclo reproductivo y estructura etaria. Revista Chilena de Historia Natural 65:221-233. [ Links ]
55. Royle, J. A., R. B. Chandler, R. Sollman, & B. Gardner. 2014. Spatial capture-recapture. Academic Press, Waltham, Mass. [ Links ]
56. Schereiner, G. M. 2012. Proposta de cenários para a delimitação da zona de amortecimento de impactos na Estação Ecológica do Taim. Dissertation, University Federal of Rio Grande do Sul. [ Links ]
57. Seber, J. A. F. 1965. A note on the multiple-recapture census. Biometrika 52:249-259. [ Links ]
58. Shmueli, G. 2010. To explain or to predict? Statistical Science 289-310. [ Links ]
59. Stolz, J. F. B. 2006. Dinâmica populacional e relações espaciais do tuco-tuco-das-dunas (Ctenomys flamarioni-Rodentia-Ctenomyidae) na Estação Ecológica do Taim-RS/Brasil. Dissertation, University Federal of Rio Grande do Sul. [ Links ]
60. Šumbera, R., H. Burda, W.,N. Chitaukali, & J. Kubová. 2003. Silvery mole-rats (Heliophobius argenteocinereus, Bathyergidae) change their burrow architecture seasonally. Naturwissenschaften 90:370-373.
61. Travi, V. H. 1981. Nota prévia sobre nova espécie do gênero Ctenomys, Blaiville, 1986 (Rodentia: Octodontidae). Iheringia 60:123-124. [ Links ]
62. Tomazelli, L. J., S. R. Dillenburg, & J. A. Willwock. Late Quartenary geological history of Rio Grande do Sul coastal plain, southern Brazil. Revista Brasileira de Geociências 30:474-476. [ Links ]
63. Vieira, E., & S. S. Rangel. 1998. Planície costeira do Rio Grande do Sul: geografia física, vegetação e dinâmica sócio-demográfica. Sagra, Porto Alegre. [ Links ]
64. Villwock, J. A., L. . Tomazelli, E. L. Loss, E. A. Dehnhardt, N. O. Horn, F. A. Bachi, & B. A. Dehnhardt. 1986. Geology of Rio Grande do Sul coastal province. Quartenary of South America and Antarctic Peninsula (J. Rabbassam, eds.). A. A. Balkeman, Rotterdam. [ Links ]
65. Zenuto, R., & C. Busch. 1998. Population biology of the subterranean rodent Ctenomys australis (tuco-tuco) in a coastal dune field in Argentina. Journal of Mammalian Biology 63:357-367. [ Links ]














