Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Mastozoología neotropical
Print version ISSN 0327-9383On-line version ISSN 1666-0536
Mastozool. neotrop. vol.25 no.2 Mendoza Dec. 2018
ARTÍCULO
Functional subdivision of the vertebral column in four south american dolphins
María Constanza Marchesi1, Matías Sebastián Mora2, Enrique Alberto Crespo3, 4, Claudia C. Boy1, Rolando González-José5 and Rae Natalie P. Goodall1, 6†
1 Centro Austral de Investigaciones Científicas (CADIC-CONICET), Ushuaia, Tierra del Fuego, Argentina [Correspondencia: M. Constanza Marchesi <marchesimc@gmail.com>].
2 Instituto de Investigaciones Marinas y Costeras (IIMyC) – CONICET, Universidad Nacional de Mar del Plata, Mar del Plata, Buenos Aires, Argentina.
3 Centro para el Estudio de Sistemas Marinos (CESIMAR), Centro Nacional Patagónico (CENPAT-CONICET), Puerto Madryn, Chubut, Argentina
4 Universidad Nacional de la Patagonia San Juan Bosco (UNPSJB), Puerto Madryn, Chubut, Argentina.
5 Instituto Patagónico de Ciencias Sociales y Humanas, Centro Nacional Patagónico (CENPAT-CONICET), Puerto Madryn, Chubut, Argentina.
6 Museo Acatushún de Aves y Mamíferos Marinos Australes, Estancia Harberton, Canal Beagle, Tierra del Fuego, Argentina.
† Passed away on May 25th, 2015.
Abstract
Description and functional interpretation of morphological variation in the dolphin column can be facilitated by recognizing structural units. This information offers important clues to the proportion of the column involved in the oscillation and displacement of the flukes, and how swimming style can vary among species. Thus, the morphological characterization and functional subdivision of the vertebral column is of key importance to gain insights into the locomotor performance of cetacean species occurring in different environments. We employed traditional morphometrics to establish the functional subdivision of the vertebral column of Commerson’s dolphin (Cephalorhynchus commersonii), a coastal species, and the dusky dolphin (Lagenorhynchus obscurus), a shelf species. These species are closely related, and some of them are partially sympatric. We also compared the obtained results against information previously reported by Marchesi et al (2017) on Peale’s dolphin (Lagenorhynchus australis), a coastal species, and the hourglass dolphin (Lagenorhynchus cruciger), an oceanic species. These results bring further support to our hypothesis that coastal species have morphological traits associated with higher flexibility, whilst platform and oceanic species have features associated with higher stability in a greater proportion of their column.
Resumen
Subdivisión funcional de la columna vertebral en cuatro delfines sudamericanos.
La descripción e interpretación funcional de la variación morfológica en la columna de delfines pueden facilitarse mediante el reconocimiento de unidades estructurales a lo largo de toda la estructura de la columna. Esta información ofrece pistas importantes sobre la proporción de la columna involucrada en la oscilación y el desplazamiento de la aleta caudal, y cómo el estilo de natación puede variar entre las especies. Por lo tanto, la caracterización morfológica y la subdivisión funcional de la columna vertebral son de fundamental importancia para obtener información sobre el comportamiento locomotor de las especies de cetáceos que habitan ambientes diferentes. Se empleó morfometría tradicional para establecer la subdivisión funcional de la columna vertebral del delfín de la tonina overa (Cephalorhynchus commersonii), una especie costera, y el delfín oscuro (Lagenorhynchus obscurus), una especie de plataforma, realizando una primera caracterización morfológica de sus columnas vertebrales. Estas especies están estrechamente relacionadas y, en algunos casos, son parcialmente simpátricas. También comparamos los resultados obtenidos con información previamente reportada por Marchesi et al. (2017) para el delfín austral (Lagenorhynchus australis), una especie costera, y el delfín cruzado (Lagenorhynchus cruciger), una especie oceánica. Estos resultados apoyan nuestra hipótesis de que las especies costeras tienen rasgos morfológicos asociados con mayor flexibilidad, mientras que las especies oceánicas y de plataforma tienen características asociadas con una mayor estabilidad en una mayor proporción de su columna.
Key words: Commerson’s dolphin; Dusky dolphin; Flexibility; Morphometry; Vertebral column.
Palabras clave: Columna vertebral; Delfín oscuro; Flexibilidad; Morfometría; Tonina overa.
Recibido 27 noviembre 2017.
Aceptado 14 mayo 2018.
Editor asociado: R. Bastida
INTRODUCTION
There is an association between the flexibility and maneuverability of the cetacean body and swimming speed, food habits, and habitat-use patterns (Fish 2002; Woodward 2006). In this sense, fast swimming oceanic dolphins are characterized to have a relatively stable morphological configuration (e.g., Long et al. 1997; Pabst 2000; Fish 2002; Buchholtz & Schur 2004). Systems that stabilize the body during swimming can be either active or passive; the latter do not require energy and are basically determined by morphology, including vertebral shape (Long et al. 1997; Fish et al. 2003; Marchesi et al. 2017).
Vertebral morphology in dolphins dramatically varies across column regions (Long et al. 1997; Buchholtz & Schur 2004; Viglino et al. 2014; Marchesi et al. 2017). Such morphological variation along the spine reinforces or limits movements, and can be described through some morphological components such as the number of vertebrae, centrum shape and size, process structure and orientation, and accessory structures such as metapophyses (Buchholtz & Schur 2004). According to Buchholtz and colleagues (Buchholtz 2001; Buchholtz & Schur 2004; Buchholtz et al. 2005), the relative centrum length (RCL) can be an accurate descriptor of vertebral morphology that relates to the three variables (i.e., length, height, and width) of a centrum.
Description and functional interpretation of morphological variation is facilitated by recognizing structural units along the column (Buchholtz & Schur 2004). This information offers important clues about the proportion of the column involved in the oscillation and displacement of the flukes, and how the swimming style can vary among species (Buchholtz 2001; Buchholtz & Schur 2004). A morphological characterization and functional subdivision of the vertebral column is essential to gain insights into the locomotor performance of cetacean species that evolved in different environments.
Commerson’s dolphin (Cephalorhynchus commersonii) is a coastal species distributed in Patagonia from the Río Negro down to Cape Horn and Malvinas (Falkland) Islands (Best 2007). Stomach contents (Bastida et al. 1988) and stable isotope analysis (Riccialdelli et al. 2010) indicate that this species feeds in shallow waters of the continental shelf, capturing both pelagic and bentopelagic fish species, and occasionally frequenting deeper waters of the shelf where it feeds on pelagic prey such as squids. Although, the species mainly inhabits shallow waters, there have been occasional sightings up to 200 nautical miles (Pedraza 2008) from shore. Peale’s dolphin (Lagenorhynchus australis) is the most coastal species of the genus (Goodall et al. 1997a; b) and is found on both Pacific and Atlantic coastal areas of southern South America. In the southernmost areas, it is frequent in channels and fjords in association with the kelp beds of Macrocystis pyrifera, where it feeds on demersal and benthic prey (Goodall et al. 1997a; b; Viddi & Lescrauwaet 2005). In the northeastern part of the distribution it inhabits open coasts on the continental shelf, feeding on demersal and pelagic prey (Schiavini et al. 1997). Based on stable isotope analysis, Riccialdelli et al. (2010) suggested both benthic and pelagic prey for this species. Even though dedicated surveys have reported sightings in shelf waters, its abundance is negatively correlated with depth, supporting its preference for shallow waters (Dellabianca et al. 2016). The dusky dolphin (Lagenorhynchus obscurus) has a marked plasticity in its habit use, depending on location and season. In Argentina, it has been observed feeding diurnally on prey schools in shallow shelf waters (Würsig et al. 1997). Its abundance might be related to important pelagic prey concentration such as Argentine anchovy and juvenile hake (Schiavini et al. 1999). The hourglass dolphin (Lagenorhynchus cruciger) is a pelagic, cold water species with circumpolar distribution (Goodall et al. 1997c; Riccialdelli et al. 2010). Its basic biology and trophic ecology are poorly known due to its oceanic behavior and the low number of specimens found (Fernández et al. 2003). Based on a few analyzed animals, it has been suggested that they feed on prey that perform diurnal migrations in the water column, from deep to superficial water such as the squids Semirossia tenera, Loligo gahi and Illex argentinus, and the fish Merluccius hubbsi and Protomyctophum sp. (Goodall et al. 1997c; Fernández et al. 2003; Best 2007).
Since Le Duc et al. (1999), the dolphin subfamily Lissodelphininae has been considered to include three genera: Lissodelphis, Lagenorhynchus and Cephalorhynchus. Phylogenetic relationships within this group are widely discussed, with the genus Lagenorhynchus being reported as polyphyletic (Le Duc et al. 1999; Harling-Cognato & Honeycutt 2006; McGowen 2011; Banguera-Hinestroza et al. 2014). In fact, some authors have preferred to remove L. albirostris and L. acutus from the genus Lagenorhynchus for not finding them to be closely related to each other (Le Duc et al. 1999). Generic nomenclature is still a matter of discussion, with some authors proposing some or all the other Lagenorhynchus species to be placed in the genus Sagmatias (Le Duc et al. 1999; McGowen 2011). Despite this, the Committee on Taxonomy of the Society for Marine Mammalogy still includes all species within the same genus (Committee on Taxonomy 2017). This work focuses on four dolphin species belonging to the subfamily Lissodelphininae, with restricted distribution in the southern hemisphere. The close phylogenetic relationship of these four species has been supported by molecular, morphological and acoustic data (Le Duc et al. 1999; Tougaard & Kyhn 2010; McGowen 2011; Banguera-Hinestroza et al. 2014; Galatius & Goodall 2016). These species seem to have undergone a rapid adaptive radiation starting 5.3 million years ago in the South Atlantic Ocean mostly, mainly associated with differential adaptation to particular habitats and to dispersal processes (Banguera-Hinestroza et al. 2014; Galatius & Goodall 2016).
The aim of this work was to establish the functional subdivision of the vertebral column of Commerson’s dolphin (Cephalorhynchus commersonii) and the dusky dolphin (Lagenorhynchus obscurus). We compared our results with those reported by Marchesi et al. (2017) for Peale’s dolphin (L. australis) and the hourglass dolphin (L. cruciger). A quantitative comparison of the flexibility/stability of the column was made in order to establish regions of particular interest under the hypothesis that coastal species have features associated with greater flexibility, whereas oceanic fast swimming species have vertebral characteristics associated with enhanced stability. As a whole, our analyses provide a more complete picture of the inter-specific variation of a key phenotype involved in swimming performance and evolution.
MATERIALS AND METHODS
A total of 18 specimens, nine Cephalorhynchus commersonii and nine Lagenorhynchus obscurus specimens were measured in this study. For C.commersonii, seven specimens were males and only two females. For L. obscurus three of the specimens were males, two females and four were of unknown sex. Specimens are stored at the Goodall collection (RNP) from the Museo Acatushún de Aves y Mamíferos Marinos Australes, AMMA (Ushuaia, Tierra del Fuego, Argentina), and at the Centro Nacional Patagónico (CNPMAMM and LAMAMA; CENPAT, Puerto Madryn, Chubut, Argentina) (Table 1). Data for Lagenorhynchus australis and Lagenorhynchus cruciger were previously reported in Marchesi et al. (2017).
Table 1
Specimens of Commerson’s dolphin (Cephalorhynchus commersonii) and dusky dolphin (Lagenorhynchus obscurus) included in this study. ID: specimen collection number; VF: traditional vertebral formula; TC:total count; PM: Physical maturity; TCL: total centrum length in cm; F: female; M: male; -: indeterminate; +:incomplete skeleton. TCL estimated from regression equations are shown between parenthesis. RNP and MT:Museo Acatushún de Aves y Mamíferos Marinos Australes, Ushuaia, Argentina. CNPMAMM and LAMAMA: Centro Nacional Patagónico, Puerto Madryn, Argentina.

The specimens were classified as physically immature or mature based on the degree of fusion of the vertebral epiphyses, according to the criterion proposed by Perrin (1975) and modified by Goodall et al. (1988) and Lockyer et al. (1988). Based on these latter authors, specimens included in this study were classified as sub-adults and adults belonging to classes 2, 2b, 3, 3a and 3b (Table 1).
Three measurements were obtained on every post cervical vertebral centrum: length, width and height (CL, CW, CH respectively; Fig. 1), using a digital caliper to the nearest 0.1 mm. For the cervical vertebrae, only centrum length was measured. Positions corresponding to the change in orientation of the neural spines (Neural spine inclination=90º; see Fig. 1) were obtained from lateral photographs containing a scale bar and using the public domain NHI software Image J (Ferreira & Rasband 2012; Schneider et al. 2012). Variables employed to describe centrum shape (CL, CW and CH) were then plotted against the number of each vertebra and total centrum length (TCL) to describe variation along the column.
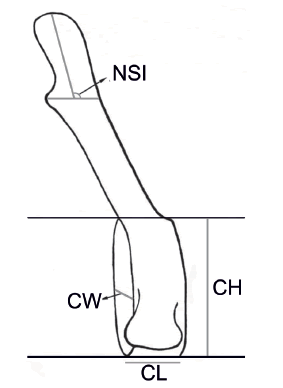
Fig. 1. Vertebral variables included in the study. CL: centrum length; CW: centrum width; CH: centrum height; NSI: neural spine inclination.
Anatomical regions in dolphins may differ from functional regions typically described in other mammals (Buchholtz & Schur 2004; Marchesi et al. 2016, 2017). Given this, the column was divided into series based on the functional criterion for cetaceans proposed by Buchholtz & Schur (2004). The torso comprises all vertebrae between the thoracic region (last rib bearing vertebra) and the first vertebra where CW<CH, the anterior limit of the tailstock (Fig. 2). The torso was further divided into three sub regions: anterior, mid-, and posterior torso; vertebrae where the neural spine changes its inclination are employed to define the anterior and posterior limits of the mid-torso (see Marchesi et al. 2017). Tailstock vertebrae are those where CW<CH ad fluke vertebrae are characterized by CW>CH.
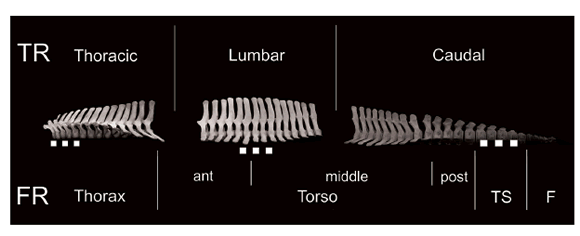
Fig. 2. Vertebral series for the analysis of postcranial skeleton for the Peale’s dolphin (Lagenorhynchus australis) according to the traditional regions (TR) and the functional regions (FR). The cervical region is not shown. TS: tailstock; F: fluke. Scale=10 cm. Modified from Marchesi et al. 2017.
Measurement on the vertebral centra (CL, CW, CH) were employed to calculate relative centrum length (RCL) as RCLi= [2CLi / (CWi + CHi)], following Buchholtz (2001). This is an accurate estimator of the relative size of the centrum faces. A value near 1 indicates a vertebral centrum with smaller faces and smaller contact area between adjacent vertebrae, allowing greater angular movements and higher flexibility among adjacent vertebrae (Long et al. 1997; Buchholtz 2001; Buchholtz & Schur 2004). On the other hand, lower values (RCL < 0.75) are associated with large, flat centrum faces, resulting in greater contact between adjacent vertebrae, long processes and, concomitantly, more stable columns (Buchholtz 2001; Buchholtz & Schur 2004; Woodward 2006).
For each specimen, total centrum length (TCL) was calculated as the sum of CL for the whole vertebral column (Table 1). TCL is an underestimation of the real length of the vertebral column, since intervertebral disc space is not accounted for its computation. In incomplete specimens, TCL was estimated for each species from a linear regression equation using the mean CL for a set of contiguous vertebrae following Buchholtz et al. (2005). The coefficient of determination (R2), its p-value and the percent prediction error (% PE), computed following Smith (1984), were analyzed for the mean CL of the cervical region (vertebrae 1-7) and for the CL of every individual vertebra up to the last vertebra of the torso. We selected the set of at least ten contiguous vertebrae with significant R2 coefficients higher than 0.8. Then, the regression equation for each species’ TCL was obtained for the mean CL of the resulting set of vertebrae.
The first characterization of the functional regions was done by plotting mean values of RCL versus mean values of TCL for the four species, following Buchholtz (2001) and Buchholtz et al. (2005). Values for L. australis and L. cruciger were obtained from Marchesi et al. (2017).
Each region occupies a fraction of the total length described as its relative percentage RPi=(CLri x 100) / TCL. Where, CLri, is the sum of centrum length in a particular functional region.
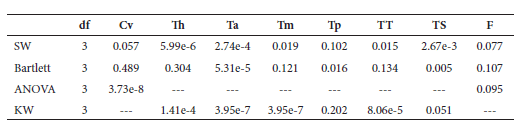
Normality and homoscedasticity of RP were analyzed using a Shapiro-Wilks test and a Barlett test, respectively (Zar 2010). Analyses of variance (ANOVA) followed by a Tuckey Test or a non-parametric Kruskal-Wallis test were employed to test for interspecific differences in RP (Table 2). Interspecific differences in vertebral counts for each region were evaluated by comparing the mode of the number of vertebrae for each region. Statistical analyses were performed by employing software R 3.3.3 (R Core Team 2016) and the package AGRICOLAE 1. 2-4 (De Mendiburu 2016) when needed.
Table 2
Degrees of freedom and pvalues for the statistical tests performed on the relative proportion values for the four species. SW: Shapiro-Wilks Normality Test; Bartlett: Bartlett Test for variance homocedasticity; KW:Kruskal-Wallis Test. Cv: cervical; Th: thorax; T (a, m, p): anterior, mid and posterior torso; TT: total torso; TS: tailstock; F: fluke.
RESULTS
Variation along the column of centrum length, width and height (CL, CW and CH respectively) for Cephalorhynchus commersonii and Lagenorhynchus obscurus are summarized in Fig. 3. Values of the three variables tend to be similar in the middle of the thorax, between vertebra 12 and 16, being more similar in C.commersonii than in L. obscurus. In the anterior torso, CW and CH tended to increase, whereas CL tended to diminish progressively, although less markedly in C.commersonii. Excluding the fluke, the lowest mean CL values were observed in different areas for each species: the lowest values in C. commersonii were located in the limit between the mid- and posterior torso; whereas in L. obscurus, the lowest values occupied the whole mid-torso (Fig. 3). There was an area of maximum separation between CL and the other two variables between vertebrae 38 and 42 in C.commersonii and 32 and 46 in L. obscurus. In the posterior torso, there was a gradual increase in CL mean values, whereas CW and CH mean values remained constant for both species. Based on these results the definition of the tailstock, corresponding to high mean values of CL and vertebral centra that are taller than wide (CW<CH), was established between vertebrae 45 and 48 in C.commersonii and between vertebrae 54 and 60 in L. obscurus. For C.commersonii, CL values of the tailstock were notably inferior to maximum CL values of the thorax. On the contrary, in L. obscurus, these values were slightly higher than CL maximum values of the thorax. The fluke was comprised by vertebrae that are shorter and wider relative to their height (CL<CH<CW).

Fig. 3. Mean values of centrum length (CL), centrum width (CW) and centrum height (CH) versus the number of vertebrae for Cephalorhynchus commersonii and Lagenorhynchus obscurus. Vertical lines separate functional regions. Th: Thorax; Ta:anterior torso; Tm: mid-torso; Tp: posterior torso; TS: tailstock; F: fluke. N°V: number of vertebrae.
Changes in neural spine inclination (NSI) define the limits of the torso sub-regions. Even though we detected some intraspecific variation, the first change (from a posterior to an anterior inclination) was found mostly on the vertebra 27 in the case of C. commersonii and on the vertebra 25 for L. obscurus (Table3). Synclinal point, or second change in NSI (from an anterior to a posterior inclination) was located on the vertebra 43 for C. commersonii and on the vertebra 51 in L. obscurus (Table 3).
Table 3
Vertebra on which there is a shift of neural spine inclination in Cephalorhynchus commersonii and Lagenorhynchus obscurus. Anterior torso limit: shift from a posterior towards anterior inclination; Posterior torso limit: shift from an anterior towards a posterior inclination.

For both species, accuracy of the TCL estimation from CL varied according to the position within the skeleton (Table S1 and S2). For C. commersonii best fitted predictions were generated using vertebrae from the middle of the column, from the mid thoracic up to the posterior torso (vertebrae 16-42; Table S1). For L. obscurus, best fitted predictions were achieved using mid-torso vertebrae (vertebrae 33-42; Table S2). In all cases, vertebrae employed on the equation for estimation had a %PE smaller than 2.
Regression equations employed to estimate TCL of incomplete specimens for each species were selected based on the maximum explanatory value reached by the linear regression model (R2) and its significance (p):
C. commersonii:
y=33.48 x + 30.26, R2=0.93, n=5,
where: y=TCL in cm; x=mean CL for vertebrae 16 to 42 in cm.
L. obscurus:
y=46.85 x + 37.59, R2=0.94, n=5,
where: y=TCL in cm; x=mean CL for vertebrae 33 to 42 in cm.
The general pattern of variation considering the mean RCL values versus the mean TCL values was slightly different from the most coastal species relative to the most oceanic species (Fig.4). There were two areas of high RCL values: middle of the thorax and tailstock; and three areas of low RCL values: anterior portion of the column, torso and the base of the fluke. In sum, there were differences in the minimum and maximum values among species (mainly among those species with contrasting habitats), both in magnitude as in position along the column. For C.commersonii, maximum RCL mean values of the thorax were higher than in the other three species (C. commersonii=0.998, L. australis=0.896, L.obscurus=0.860, L.cruciger=0.808). The opposite was observed for the maximum mean values of the tailstock (although less evident than in the case of the maximum RCL mean values of the thorax), that were lower than in the other three species (C. commersonii=0.670, L. australis=0.777, L. obscurus=0.744, L.cruciger=0.708). In C.commersonii, decrease of RCL mean values from the maximum at the thorax towards minimum values at the torso was similar to that observed for L. australis, being less pronounced than in L. obscurus and L. cruciger. The RCL values of the torso were similar for C.commersonii and L. australis, but the minimum was placed in an anterior position for the latter species (relative position of minimum RCL: C. commersonii=68% TCL, L.australis=60% TCL). These minimums were higher than those observed for the other two species (RCL minimum mean values: C.commersonii= 0.515, L. australis=0.523, L.obscurus=0.428, L.cruciger=0.428). Regarding L. obscurus, maximum RCL mean values were intermediate between L. australis and L.cruciger both for the thorax and the tailstock. Minimum mean values of RCL were lower than those of L. australis and similar to those observed for L. cruciger, being placed in the same position of the skeleton as in the latter species (relative position of minimum RCL: L.obscurus=57% TCL, L. cruciger=56% TCL).

Fig. 4. Variation in the mean values of relative centrum length (RCL) versus the proportion of total centrum length (TCL) for the four species. Data for plotting Lagenorhynchus australis and Lagenorhynchus cruciger were obtained for Marchesi et al. 2017.
Most of the regions showed interspecific differences in the proportions of the skeleton they occupy (Fig. 5, Tables 2 and 4). For the cervical region, there were significant differences among species (Table 4). The proportion occupied by this region in C. commersonii was significantly greater when compared to the rest of the species (Fig. 5, Table 4). L. obscurus had the second largest cervical region. The smallest cervical region was found in L. australis and L.cruciger, for these two species cervical regions could not be considered significantly different based on the ANOVA results (Table 4). Regarding the thoracic region, Kruskal-Wallis test showed significant differences, defining three groups: C. commersonii, L. obscurus and L. cruciger, ordered in decreasing size of the region; whilst the thorax of L. australis differed from that of C. commersonii but not from that of its congeners (Fig. 5, Table 4). Both the anterior and the mid-torso showed significant differences among all the studied species (Fig. 5, Table 4). The anterior torso occupied a greater proportion of the skeleton in C. commersonii, followed by L. australis and L. obscurus, and finally by L. cruciger (Table4). An opposite pattern was observed for the mid-torso, with C. commersonii presentingthe smallest mid torso followed by L. australis and L. obscurus. L. cruciger had the greatest mid-torso relative to the four species (Fig. 5, Table 4). There were no differences in the proportion of the skeleton occupied by the posterior torso (Table 4). When the torso is considered as a whole (total torso) three groups can be distinguished (Table 4): C.commersonii, exhibiting the relatively smallest torso, L. australis and L.obscurus with a relatively intermediate-sized torso, and L. cruciger with the relatively biggest torso (Fig. 5, Table 4). The proportion of the skeleton occupied by the tailstock also differed among species, and three different groups were identified: one comprising C. commersonii with the lowest values, another one including L. australis and L. obscurus with the highest values, and the last one including L. cruciger, with values that do not differ from those of the other two groups (Fig. 5, Table 4). There were no differences in the proportion of the skeleton occupied by the fluke (Table 4).
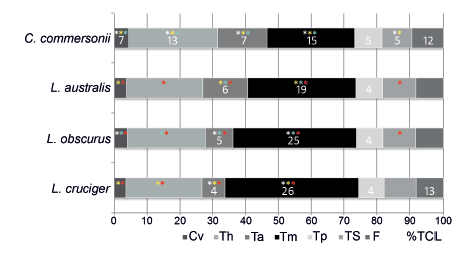
Fig. 5. Relative proportion of the vertebral column occupied by each functional region. Asterisks indicate significant differences (p<0.05; ANOVA and Tukey test for the cervical region and fluke; and Kruskal-Wallis test for the other regions): red, differences when compared to Cephalorhynchus commersonii; white, differences when compared to Lagenorhynchus australis; yellow, differences when compared to Lagenorhynchus obscurus; and light blue, differences when compared to Lagenorhynchus cruciger. Numbers refer to the number of vertebrae within each region, where they are absent is due to lack of difference from C. commersonii. Cv: cervical; Th: Thorax; Ta: anterior torso; Tm: mid-torso; Tp: posterior torso; TS: tailstock; F: fluke; %TCL: percentage of total centrum length.
Table 4
Regional distribution of the vertebral column for the Commerson’s dolphin (Cephalorhynchus commersonii), Peale’s dolphin (Lagenorhynchus australis), dusky dolphin (Lagenorhynchus obscurus) and hourglass dolphin (Lagenorhynchus cruciger). Cv: Cervical; Th: thorax; SD: Standard deviation; CLr: mean value of the sum of centrum length for a region; RP: mean value of the proportion of centrum length occupied by a region; V: mode of the number of vertebrae in the region; TCL:total centrum length. Letters indicate different groups resulting from Kruskall-Wallis (regular) and Tukey test (bold) after performing ANOVA.
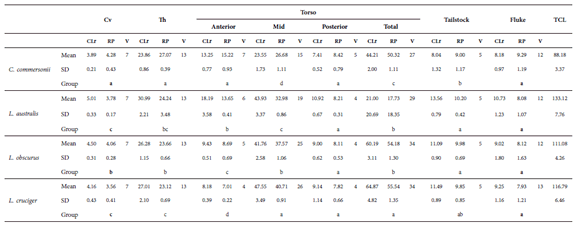
There were differences concerning the number of vertebrae of the anterior, mid- and posterior torso (Fig. 5, Table 4). In the anterior torso, C. commersonii had the greatest number of vertebrae with seven, followed by L. australis with six, L. obscurus with five and, last, L. cruciger with only four vertebrae. In the mid-torso, the observed pattern was the opposite: C. commersonii had the lowest number of vertebrae with a mode of 15, L. australis had 19, L. obscurus 25 and there were 26 vertebrae in L. cruciger. Based on these differences in the sub regions of the torso, the mode for the total number of vertebrae within the total torso differed among species, being smaller for C. commersonii, with only 27; followed by L. australis, with 29; and L. obscurus and L.cruciger had 34 vertebrae each (Fig. 5, Table 4).
DISCUSSION
The vertebral column of the studied cetacean species, Commerson’s dolphin (Cephalorhynchus commersonii) and the dusky dolphin (Lagenorhynchus obscurus), showed two areas of maximum potential flexibility (i.e. exhibiting high RCL values), namely the middle of the thorax and the tailstock (Fig.3). Our reference to the thorax as a potentially flexible area does not take into account the stabilization provided by the ribs and the sternum to this region and should therefore be interpreted with caution. Between those two areas, there is an area presenting shorter vertebrae with wide and high faces (Fig. 3), resulting in greater stability. Buchholtz & Schur (2004) and Marchesi et al. (2017) considered that morphological features result in a greater flexibility when CL is high relative to CW and CH. On the contrary, vertebrae that are short, wide and tall (low CL, high CW and CH) result in disk-shaped morphology, allowing greater contact between adjacent vertebrae and providing a stable section in the skeleton (Long et al. 1997; Buchholtz 2001; Buchholtz & Schur 2004). When the three features have low values, and CW and CH diverge, centra tend to have an oval morphology, with the width of the vertebrae as the main axis (the centra are nearly rectangular). This substantial difference in vertebral morphology determines the most important differences in the degree of flexibility of certain regions of the axial skeleton (Buchholtz & Schur 2004). As was reported by Marchesi et al. (2017) for Peale’s dolphin (Lagenorhynchus australis)and the hourglass dolphin (Lagenorhynchus cruciger), the vertebral columns of C. commersonii and L. obscurus have an anterior stable zone followed by a flexible one in the middle of the thorax. This first potentially flexible zone has the same relative position in both species and it is followed by an area of potentially higher stability with vertebral centra of disk-shaped morphology. Particularly, C. commersonii specimens have fewer disk-shaped vertebrae, and transition to such morphology occurs in a more posterior position when it is compared to L. obscurus specimens. Moreover, the position of the second zone of flexibility varies between species, being placed more anteriorly in C.commersonii. In this way, disk-shaped morphology of the centra is extended through at least seven more vertebrae (and also in relative percentage of the skeleton, see below) in L.obscurus. In both species, this second zone of flexibility is located where vertebrae are taller than wide, corresponding to the tailstock. Following this region, vertebrae are shorter and the height/width ratio is smaller, resulting in oval vertebrae that form the skeleton of the fluke.
High values of RCL represent articular faces relatively small, with small contact area. This geometry allows greater angular movements and greater flexibility (Long et al. 1997; Buchholtz 2001; Buchholtz & Schur 2004); in turn, greater flexibility is associated with greater maneuverability and coastal habits (Fish 2002, Woodward 2006). Variation in the pattern of RCL along the column (RCL versus TCL; Fig. 4) is similar for all the species addressed here, evidencing two potentially flexible areas (middle of the thorax and tailstock) and three stable areas (anterior portion of the column, torso and base of the fluke). Differences in these morphological patterns mainly separate C. commersonii from the other dolphin species. In this species, based only on vertebral morphology, both the thorax and the anterior torso are potentially more flexible than in the other three species. In contrast, the tailstock seems to be less flexible. This might be related with counteracting the high flexibility of the anterior part of the body or with the capacity of this species to move to platform waters, where it has been reported to feed (Pedraza 2008; Riccialdelli et al. 2010, 2013; E.A. Crespo pers. obs.). For this species, it has been reported that feeding behavior and group size depends on habitat type. Group size may reach one hundred individuals when the dolphins are feeding on schooling pelagic fish like anchovies or juvenile hake; this could favor stability over flexibility. When they are feeding in tidal areas in front of rias, in kelp forests and in waters influenced by river discharge, they are more typically alone or in small groups and feeding from benthic prey (Loizaga de Castro et al. 2013), thus, requiring certain degree of flexibility while searching for food. This plasticity in habitat type, behavior and prey preference is reflected in the regional morphology of the vertebral column. In the case of L. obscurus, RCL values of the mid-torso are very similar to those of L. cruciger but values of the flexible areas are intermediate between L. australis and L. cruciger, in accordance to the capacity of the species to move and feed both in shallow and deep platform waters (Würsig & Würsig 1980; Degrati et al. 2012). Conventional knowledge indicates that dusky dolphins show a schooling behavior pursuing anchovies or other pelagic fish as the main prey (Würsig & Würsig 1980). In fact, during the warm season, dusky dolphins mostly forage using a feeding-traveling sequence (Würsig & Würsig 1980). However, in the cold season, a greater proportion of diving activity appeared and surface feeding decreased.In Golfo San José, Argentina, Würsig & Würsig (1980) suggested that dusky dolphins were feeding below and not at the surface during winter, possibly on different prey, more individually, and in small groups near the shore. Results from Degrati et al. (2012) are in concordance with this suggestion. The latter authors reported that, during winter, dusky dolphins were observed in coordinated diving apparently in a feeding activity, contrasting with the surface feeding observed during summer. Squid would be the target, as the second more important prey in their diet (Koen Alonso et al. 1998), while diving could be the strategy to catch them. Depending on catching strategy when diving, stability may not be an important requirement but lack of underwater behavior data prevents us from drawing conclusions.
On the other hand, relative proportion of the skeleton represented by a particular region of the column has biomechanical implications as it provides information regarding the percentage of the skeleton that may be considered flexible or stable. Our results (Fig. 5, Table4) suggest that coastal species (C.commersonii and L.australis) tend to have a relatively large thorax and anterior torso containing more vertebrae, but relatively smaller mid-torso and with fewer vertebrae than platform (L.obscurus) or oceanic (L.cruciger) species. The relatively large cervical region of C.commersonii could be translated into a greater flexibility of the anterior part of the body that might be beneficial for allowing greater movement potential for the head during maneuvers. At the same time, the relatively short tailstock opens the question whether it could be adding to the high flexibility of this region when compared with the other three species. For L. obscurus, the proportion occupied by the anterior torso is smaller than in L. australis but larger than in L. cruciger. This, in conjunction with a smaller proportion for the cervical region and a bigger thorax when comparing with its congeners, might be indicating a greater flexibility of the anterior portion of the column. This helps to counteract the high stability (great development and high number of vertebrae of the mid-torso) when this species moves and feeds in shallow waters.
To sum up, recognizing structural units along the vertebral column is fundamental when making functional inferences relative to swimming performance and habitat characteristics. Our results allow us to functionally subdivide the vertebral column of C. commersonii and L.obscurus, comparing these morphological patterns with those observed in other cetacean species. By comparing them with results from Marchesi et al. (2017) we established that they are in accordance with the hypothesis that coastal species have morphological traits associated with higher flexibility, whereas platform and oceanic species have features associated with higher stability in a greater proportion of their column. In addition, this type of approach helps us to discover species with intermediate morpho-functional capacities, which would present greater plasticity in exploiting contrasting environmental situations. Moreover, it allows us to make an a priori interpretation about the possible associations of species with their most frequented habitats. This is a very good example of how studies in functional morphology can help us to reveal characteristics of the species in relation to their habitats and behaviors, providing base knowledge for future conservation efforts. The difficulty of having information on the functional subdivision of the skeleton in museum specimens makes this work essential before comparing in detail vertebral morphology and biomechanical implications.
ACKNOWLEDGMENTS
We dedicate this paper to the memory of Dr. Natalie R. Prosser Goodall: a very important woman for the scientific community, a true mentor for MCM and a friend of us all. Natalie, you will always be remembered and your work will be carried on by all the people you touched during your life. The authors are grateful to the Committee for Research and Exploration (CRE) of the National Geographic Society for continuing grants to support the field work carried out in Tierra del Fuego. We thank Total Austral S.A., the Fundación RNP Goodall, and several other companies for support to the Museo Acatushún de Aves y Mamíferos Marinos Australes (AMMA). MCM would like to thank the Society for Marine Mammalogy and the Cetacean Society International for their grants to support the data collection for this work. To N. García at the Laboratorio de Mamíferos Marinos (CENPAT, Puerto Madryn, Argentina) for helping with the preparation of specimens. This research project is a part of MCM’s PhD thesis for which the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) from Argentina granted her a postgraduate fellowship. We are very thankful to Dr. Emily Buchholtz for her review on an early version of the manuscript; her comments have improved this paper enormously.
LITERATURE CITED
1. Banguera-Hinestroza, E., A. Hayano, E. Crespo, & A. R. Hoelzel. 2014. Delphinid systematics and biogeography with a focus on the current genus Lagenorhynchus: Multiple pathways for antitropical and trans-oceanic radiation. Molecular phylogenetics and Evolution 80:217-230. [ Links ]
2. Bastida, R., V. Litchtschein, & R. N. P Goodall. 1988. Food habits of Cephalorhynchus commersonii off Tierra del Fuego. Reports of the International Whaling Commission, Special Issue 9 (R. L. Brownell & G. P. Donovan, eds). International Whaling Commission, Cambridge, UK. [ Links ]
3. Best, P. B. 2007. Whales and dolphins of the Southern African Subregion. Cambridge University Press, United Kingdom. [ Links ]
4. Buchholtz, E. A. 2001. Vertebral osteology and swimming style in living and fossil whales (Order: Cetacea). Journal of Zoololgy 253:175-190. [ Links ]
5. Buchholtz, E. A., & S. A. Schur. 2004. Evolution of vertebral osteology in Delphinidae (Cetacea). Zoological Journal of the Linnean Society 140:383-401. [ Links ]
6. Buchholtz, E. A., E. M. Wolkovich, & R. J. Cleary. 2005. Vertebral osteology and complexity in Lagenorhynchus acutus (Delphinidae) with comparison to other delphinoid genera. Marine Mammal Science 21:411-428. [ Links ]
7. Committee on Taxonomy. 2017. List of marine mammal species and subspecies. Society for Marine Mammalogy, <http://www.marinemammalscience.org> [ Links ].
8. De Mendiburu, F. 2016. AGRICOLAE: Statistical Procedures for Agricultural Research. R package version 1.2-4. <https://CRAN.R-project.org/package=agricolae> [ Links ].
9. Degrati, M., S. L. Dans, G. V. Garaffo, & E. A. Crespo. 2012. Diving for food: a switch of foraging strategy of dusky dolphins in Argentina. Journal of Ethology 30:361-367. [ Links ]
10. Dellabianca, N. et al. 2016. Spatial models of abundance and habitat preferences of Commerson’s and Peale’s Dolphin in Southern Patagonian Waters. PLoS ONE 11(10): e0163441.
11. Fernández M., B. Berón-Vera, N. A. García, A. A. Raga, & E. A. Crespo. 2003. Food and parasites from two hourglass dolphins, Lagenorhynchus cruciger (Quoy and Gairmard 1824), from Patagonian waters. Marine Mammal Science 19:832-836. [ Links ]
12. Ferreira, T., & W. Rasband. 2012. ImageJ User Guide [online] 198 pp. [ Links ]
13. Fish, F. E. 2002. Balancing requirements for stability and maneuverability in cetaceans. Integrative and Comparative Biology 42:85-93. [ Links ]
14. Fish, F. E., J. Peacock, & J. Rohr. 2003. Stabilization mechanism in swimming odontocete cetaceans by phased movements. Marine Mammal Science 19:515-528. [ Links ]
15. Galatius, A., & R. N. P. Goodall. 2016. Skull shapes of the Lissodelphininae: radiation, adaptation and asymmetry. Journal of Morphology 277:776-785. [ Links ]
16. Goodall, R. N. P. et al. 1988. Studies of Commerson's dolphins, Cephalorhynchus commersonii, off Tierra del Fuego, 1976-1984, with a review of information of the species in South Atlantic. Reports of the International Whaling Commission, Special Issue 9 (R. L. Brownell & G. P. Donovan, eds). International Whaling Commission, Cambridge, UK. [ Links ]
17. Goodall, R. N. P, A. N. Baker, P. B. Best, M. Mayer, & N. Miyazaki. 1997c. On the biology of the hourglass dolphin, Lagenorhynchus cruciger (Quoy and Gaimard, 1824). Reports of the International Whaling Commission 47:985-999. [ Links ]
18. Goodall, R. N. P, J. C. de Haro, F. Fraga, M. A. Iñiguez, & K. S. Norris. 1997b. Sightings and behaviour of the Peale’s dolphins, Lagenohrynchus australis, with notes on dusky dolphins, L. obscurus, off southernmost South America. Reports of the International Whaling Commission, 47:757-775.
19. Goodall, R. N. P. et al. 1997a. Review and update on the biology of Peale´s dolphin, Lagenorhynchus australis. Reports of the International Whaling Commission 47:777-796. [ Links ]
20. Harlin-Cognato, A. D., & R. L. Honeycutt. 2006. Multi-locus phylogeny of dolphins in the subfamily Lissodelphininae: character synergy improves phylogenetic resolution. BMC Evolutionary Biology 6:87. [ Links ]
21. ImageJ. Image Processing and Analysis in Java. Available in <http://rsb.info.nih.gov> [ Links ].
22. Koen Alonso, M., E. A. Crespo, N. A. Garcia, S. N. Pedraza, & M. Coscarella. 1998. Diet of dusky dolphins (Lagenorhynchus Obscurus), in waters of Patagonia, Argentina. Fishery Bulletin 96:366-374. [ Links ]
23. Le Duc, R. G., W. F. Perrin, & A. E. Dizon. 1999. Phylogenetic relationships among the Delphinid cetaceans based on full citochrome b sequences. Marine Mammal Science 15:618-648. [ Links ]
24. Lockyer, C., R. N. P. Goodall, & R. A. Galeazzi. 1988. Age and body length characteristics of Cephalorhynchus commersonii from incidentally-caught specimens off Tierra del Fuego. Reports of the International Whaling Commission, Special Issue 9 (R. L. Brownell & G. P. Donovan, eds). International Whaling Commission, Cambridge, UK. [ Links ]
25. Loizaga de Castro, R., S. L. Dans, M. A. Coscarella & E. A. Crespo. 2013. Living in an estuary: Commerson's dolphins’ habitat use and behavioural pattern at the Santa Cruz River, Patagonia, Argentina. 2013. Latin American Journal of Aquatic Science 41:985-991.
26. Long, J. H. Jr., D. A. Pabst, W. R. Shepherd, & W. McLellan. 1997. Locomotor design of dolphin vertebral columns: Bending mechanics and morphology of Delphinus delphis. Journal of Experimental Biology 200:65-81. [ Links ]
27. Marchesi, M. C., L. E. Pimper, M. S. Mora, & R. N. P. Goodall. 2016. The vertebral column of the hourglass dolphin (Lagenorhynchus cruciger Quoy and Gaimard, 1824): With notes on its functional properties in relation to its habitat. Aquatic Mammals 42:306-316. [ Links ]
28. Marchesi, M. C., M. S. Mora, L. E. Pimper, E. A. Crespo, & R. N. P. Goodall. 2017. Can habitat characteristics shape vertebral morphology in dolphins? An example of two phylogenetically related species from southern South America. Marine Mammal Science 33:1126-1148. [ Links ]
29. McGowen, M. R. 2011. Toward the resolution of an explosive radiation—a multilocus phylogeny of oceanic dolphins (Delphinidae). Molecular Phylogenetics and Evolution 60:345-357.
30. Pabst, D. A. 2000. To bend a dolphin: convergence of force transmission designs in cetaceans and scombrid fishes. American Zoologist 40:146-155. [ Links ]
31. Pedraza, S. N. 2008. Ecología poblacional de la tonina overa Cephalorhynchus commersonii (Lacépéde, 1804) en el litoral patagónico. Tesis Doctoral, Universidad de Buenos Aires, Buenos Aires, Argentina. [ Links ]
32. Perrin, W. F. 1975. Variation of spotted and spinner porpoises (genus Stenella) in the eastern tropical Pacific and Hawaii. Bulletin Scripts of the Institute of Oceanography 21:1-205. [ Links ]
33. R Core Team. 2016. R: A language and environment for statistical computing. R Foundation Statistical Computing, Vienna, Austria. <https://www.R-project.org/> [ Links ].
34. Riccialdelli, L., S. D. Newsome, M. L. Fogel, & R. N. P. Goodall. 2010. Isotopic assessment of prey and habitat preferences of a cetacean community in the southwestern South Atlantic Ocean. Marine Ecology Progress Series 418:235-248. [ Links ]
35. Riccialdelli, L., S. D. Newsome, N. A. Dellabianca, R. Bastida, M. L. Fogel & R. N. P. Goodall. 2013. Ontogenetic diet shift in Commerson’s dolphin (Cephalorhynchus commersonii commersonii) off Tierra del Fuego. Polar Biology 36:617-627. DOI: 10.1007/s00300-013-1289-5.
36. Schiavini, A. C. M., R. N. P Goodall, A. K. Lescrauwaet, & M. Koen Alonso. 1997. Food habits of Peale’s dolphin, Lagenorhynchus australis; Review and New Information. Reports of the International Whaling Commission 47:827-834.
37. Schiavini, A. C. M., S. N. Pedraza, E. A. Crespo, R. González, & S. L. Dans. 1999. Abundance of dusky dolphins (Lagenorhynchus obscurus) off north and central Patagonia, Argentina, in spring and comparison with incidental catch in fisheries. Marine Mammal Science 15:828-840. [ Links ]
38. Schneider, C., W. Rasband, & K. Eliceiri. 2012. NIH to ImageJ: 25 years of image analysis. Nature Methods 9:671-675. [ Links ]
39. Smith, R. J. 1984. Allometric scaling in comparative biology: problems of concept and method. American Journal of Physiology 246:R152-R160. [ Links ]
40. Tougaard, J., & L. A. Kyhn. 2010. Echolocation sounds of hourglass dolphins (Lagenorhynchus cruciger) are similar to the narrow band high-frequency echolocation sounds of the dolphin genus Cephalorhynchus. Marine Mammal Science 26:239-245. [ Links ]
41. Viddi, F. A., & A. K. Lescrauwaet. 2005. Insights on habitat selection and behavioral patterns of Peale’s dolphins (Lagenorhynchus australis) in the Strait of Magellan, southern Chile. Aquatic Mammals 31:176-183.
42. Viglino, M., D. A. Flores, M. D. Ercoli, & A. Álvarez. 2014. Patterns of morphological variation of the vertebral column in dolphins. Journal of Zoology 294:267-277. [ Links ]
43. Woodward, B. 2006. Locomotory strategies, dive dynamics, and functional morphology of the myticetes: using morphometrics, osteology, and DTAG data to compare swim performance in four species of balleen whales. Doctoral Thesis. University of Maine, EEUU. [ Links ]
44. Würsig, B., F. Cipriano, E. Slooten, R. Constantine, K.Barr, & S. Yin. 1997. Dusky dolphins (Lagenorhynchus obscurus) off New Zealand: status of present knowledge. Reports of the International Whaling Commission 47:715-722. [ Links ]
45. Würsig, B., & M. Würsig. 1980. Behavior and ecology of dusky dolphins, Lagenorhynchus obscurus, in the South Atlantic. U.S. Fishery Bulletin 77:871-890. [ Links ]
46. Zar, J. R. 2010. Biostatistical Analysis, 5th Edition. Prentice Hall, New Jersey. [ Links ]
Supplement 1
https://www.sarem.org.ar/wp-content/uploads/2018/10/SAREM_MastNeotrop_25-2_Marchesi-sup1.pdf
Table S1. Regression equations for predicting total centrum length (TCL) from centrum length (CL) of individual vertebrae, except for fluke vertebrae, and for the mean CL of the selected set of vertebrae (see text) for Cephalorhynchus commersonii.
Table S2. Regression equations for predicting total centrum length (TCL) from centrum length (CL) of individual vertebrae, except for fluke vertebrae, and for the mean CL of the selected set of vertebrae (see text) for L. obscurus.














