Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Mastozoología neotropical
Print version ISSN 0327-9383On-line version ISSN 1666-0536
Mastozool. neotrop. vol.25 no.2 Mendoza Dec. 2018
ARTÍCULO
Bat assemblage of the Marumbi Peak State Park, Brazilian Atlantic Rainforest
João M. D. Miranda1, 2, João E. C. Brito3, Itiberê P. Bernardi1, 4 and Fernando C. Passos1, 5
1 Laboratório de Biodiversidade, Conservação e Ecologia de Animais Silvestres, Federal University of Paraná, Curitiba, Paraná, Brazil.
2 Biology Department, Midwest Paraná State University, Guarapuava, Paraná, Brazil. [Correspondence: João M. D. Miranda <guaribajoao@yahoo.com.br>]
3 Prominer Projetos Ltda., Brazil.
4 Laboratório de Ecologia e Conservação, Pontifical Catholic University of Parana, Curitiba, Paraná, Brazil.
5 Zoology Department, Federal University of Paraná. Curitiba, Paraná, Brasil.
ABSTRACT
The great biological diversity found in tropical forests has intrigued scientists for a long time. In this study, we used a bi-dimensional niche matrix to explain the coexistence of bat species in a Brazilian Atlantic Rainforest locality. Bats were caught with a set of mist nets and manually. The samples were taken between Jan/2009 and May/2010, totalizing 41 nights of effort (64800 m².h, only of standardized efforts). The bi-dimensional niche matrix was assembled using functional groups (using predominant feeding habits) and size classes created a posteriori. Seven size classes were defined on the basis of forearm lengths; these classes were shown to be different using a Kruskal-Wallis test (p<0.05). A total of nineteen bat species were recorded, of which sixteen were detected in systematical efforts. Sturnira lilium and Carollia perspicillata were the most abundant species. Five species were regarded as common, ten were intermediate, and four were rare in the studied assemblage. Most individuals sampled belonged to the frugivorous functional group. The aerial insectivore and frugivore functional groups were the richest functional groups, with seven species each. A niche matrix with 35 cells was created, of which 15 were occupied by bats; and only three of them occupied by two or more species. The analysis showed that a combination of feeding habits and size classes could account for resource sharing and coexistence of most of the nineteen bat species in the studied assemblage. The rest of coexistence can be explained by skull characteristics (gracile versus robust skulls for aerial insectivores) or some feeding specializations (for frugivore species).
RESUMO
Assembleia de morcegos do Parque Estadual Pico do Marumbi, Mata Atlântica Brasileira.
A grande biodiversidade encontrada nas florestas tropicais tem intrigado cientistas por um longo tempo. O objetivo do presente trabalho foi usar uma matriz de nicho bi-dimensional para explicar a coexistência das espécies de morcegos em uma localidade de Mata Atlântica brasileira. Os morcegos foram capturados com um conjunto de redes de neblina e também manualmente. As amostragens foram realizadas entre janeiro de 2009 e maio de 2010, totalizando 41 noites de esforço (64800 m².h apenas de esforço sistematizado). A matriz de nicho bi‑dimensional foi criada usando grupos funcionais (usando o hábito alimentar predominante) e classes de tamanho criadas a posteriori. Sete classes de tamanho foram definidas com base nos comprimentos do antebraço; essas classes mostraram-se diferentes usando o teste de Kruskal-Wallis (p<0,05). Um total de dezenove espécies foram registradas, das quais dezesseis foram detectadas apenas em esforços sistematizados. Sturnira lilium e Carollia perspicillata foram as espécies mais abundantes. Cinco espécies foram comuns, dez intermediárias e quatro raras na assembleia estudada. A maioria dos indivíduos capturados pertencia ao grupo funcional dos frugívoros. Os grupos funcionais dos insetívoros aéreos e dos furgívoros foram os grupos mais ricos, com sete espécies cada um. Foi criada uma matriz de nicho com 35 células, das quais 15 foram ocupadas por espécies de morcego, sendo apenas três delas ocupadas por duas ou mais espécies. A análise mostrou que a combinação de hábitos alimentares com classes de tamanho poderia explicar a partilha de recursos e a coexistência da maioria das dezenove espécies de morcegos na assembleia estudada. A coexistência restante pode ser explicada pelas características cranianas (crânios delicados versus robustos – para insetívoros aéreos) ou alguma especialização alimentar (para as espécies frugívoras).
Key words: Bat diversity; Biodiversity; Brazilian Atlantic Forest; Species coexistence.
Palavras-chave: Biodiversidade; Coexistência de espécies; Diversidade de morcegos; Mata Atlântica Brasileira.
Recibido 19 noviembre 2017.
Aceptado 15 agosto 2018.
Editor asociado: L. Aguirre
INTRODUCTION
Tropical rainforests harbor the highest species richness found in continental ecosystems of the world (Magurran 2011). "How can an environment conceal so many species?” is one of the most important questions in ecology (McArthur 1957; McNab 1971; Magurran 2011). Patterns in community structure, with rare and abundant species and their differential use of time, space, and food resources are some of the proposed mechanisms that drive species coexistence (McArthur 1957; Willig 1986).
Tropical rainforests are currently among the most threatened ecosystems. The Brazilian Atlantic Rainforest was one of the largest rainforests in the world, though it is now restricted to 12.4% of its original area, with only 8.5% consisting of fragments larger than 100 ha (SOS Mata Atlântica 2018). Most of Brazilian Atlantic Rainforest fragments are incapable of sustaining viable populations of many species of animals and plants (Ranta et al. 1998; Meyer et al. 2014). Even in this disastrous situation, the Brazilian Atlantic Rainforest harbors around 20000 plant species and more than 2000 terrestrial vertebrate species (Mittermeier et al. 1999; SOS Mata Atlântica 2018). There are records of 298 mammalian species in the Brazilian Atlantic Rainforest (Paglia et al. 2012), of which nearly 40% are bats (Nogueira et al. 2014). Because of its great richness, high endemism, and a high degree of destruction, the Brazilian Atlantic Rainforest is considered one of the 25 hotspots for conservation actions of global biodiversity (Myers et al. 2000).
The loss and fragmentation of forests mainly affect large mammals, such as felids and primates (Chiarello et al. 2008), but the effects of these processes on bat fauna are still under discussion. Flight may allow bats to use a landscape formed by islands of forest. Also, some species are known to use altered environments, whereas others may be sensitive to environmental changes (Fenton 1997; Marinho-Filho & Sazima 1998).
Bats represent the richest order of mammals in many regions, occasionally with more than 100 coexisting species in a single area (Simmons & Voss 1998). They present a great variety of feeding habits, occupying several trophic levels and acting as plant pollinators, seed dispersers, insect population controllers and disease vectors (Kunz & Pierson 1994). These characteristics make bats good models for studying biodiversity and species coexistence (Patterson et al. 2003).
The study of species co-occurrence patterns and their association with environmental variables have been used to evaluate the structuring of communities (Pedro & Taddei 1997), as vertical stratification on bats (Bernard 2001; Carvalho et al. 2013), different habitat use (Barros et al. 2014) or differential selection of food (Andrade et al. 2013). However, community organization possibly results from complex interactions among biotic factors (such as competition, co-occurrence patterns, predation, mutualism, displacement patterns, food resource and shelter availability), historical factors (biogeographic and evolutionary processes) and abiotic factors (such as temperature, humidity, and precipitation) (Pedro & Taddei 1997; Stevens & Willig 2002; Patterson et al. 2003; Simmons & Conway 2003; Bloch et al. 2011; Stevens 2013). The coexistence via niche partitioning can be evaluated through the construction of a bi-dimensional niche matrix using functional groups and body sizes as dimensions (McNab 1971; Fleming et al. 1972). The definition of functional groups as a categorical variable has been discussed over the last few decades (McNab 1971; Fleming et al. 1972; Willig 1986; Tavares 2013). Even more critical, though, is the choice of which body-size related variable should be used in the matrix (for example weight versus forearm length), as well as the definition of thresholds that delimit size classes, since size is a continuous variable (Willig 1986).
The present work describes the organization of a bat assemblage and evaluates the hypothesis of resource partitioning among the species of a bat assemblage from the Brazilian Atlantic Rainforest. We expect assemblage organization to result from species competition avoidance through resource partitioning. In this case, each cell of the bi-dimensional niche matrix should allocate only one species.
MATERIAL AND METHODS
The present study was conducted in Marumbi Peak State Park—MPSP (i.e., Parque Estadual Pico do Marumbi), a conservation unit with 8745 ha of preserved and continuous Atlantic Rainforest (Fig.1). The MPSP is located in the Morretes, Piraquara, and Quatro Barras municipalities of Paraná State, Brazil (25º26¢21.3²S; 48º55¢07.3²W). Altitude in the MPSP ranges from 300 to 1539 m above sea level (Struminski 2001). The MPSP belongs to the Atlantic Ombrophilous Dense Forest domain and includes the sub formations of Sub-montane, Montane, and High-Montane (IBGE 2012). According to Köppen’s classification, the climate is Cfb, is characterized by cool summers, frequent frosts at the peaks (on winter), and no dry season, with cumulative annual rainfall of 3700 mm. Average temperatures are 22ºC and 18 ºC in the hottest and coldest months, respectively (Struminski 2001).
Fig. 1. Map of South America, depicting the Brazilian Atlantic Rainforest and Paraná State. Upper right: detail of Paraná State and location of the Marumbi Peak State Park location. Lower right: detail of the Marumbi Peak State Park.
Fieldwork included thirteen monthly samplings. First, a pilot survey was done during five days in January 2009; the resulting data were used only for the checklist of species and to define the bi-dimensional niche matrices. For quantitative analysis, in the last twelve samplings bats were captured monthly (during three nights in each month) with mist nets set in trails over streams (Figs. 2A, 2B), and with mist nets set in trails near mountain houses (Fig.2C). These samplings were done from June 2009 to May 2010 with standardized effort. For qualitative samplings, bats were captured with mist nets set at the opening of a potential shelter (abandoned railway tunnel) (Fig. 2D) and by bat search-and-capture in daytime shelters (such as tree hollows, cracks and holes in rocks, house shelters, double walls, abandoned railway tunnels, under bridges, and other natural or artificial cavities). All sampling efforts were conducted at montane subformation of Atlantic Ombrophilous Dense Forest, between 550 and 650 m above sea level.

Fig. 2. Four different forest profiles where the mist nets were set during samplings at Marumbi Peak State Park, Paraná State, Brazil. (A) Mist net set at a trail in the forest. (B) Mist net set over a stream. (C) Mist net set at a trail near mountain houses. (D) Mist net set at the opening of an abandoned railway tunnel.
We used ten mist nets in each sampling (eight nets with 7x3 m and two with 12x3 m; 20 mm of mesh size), and nets remained open for six hours after sunset. The height of the opened nets ranged between 0.2 and 0.5 m from the ground and reached 3.5 m of height. The nets were installed alone or combined (in line or in "L”), the vertical combinations reached up to 6 m of height (see Fig.2D). The sampling effort with the mist nets added up to 66816 m2.h (sensu Straube & Bianconi 2002), being 2016 m2.h in the pilot sampling (Jan/2009) and 64800 m2.h in the monthly standarized samplings (Jun/2009-Aug/2010).
Captured bats were placed in cotton bags and taken to the field base for identification, forearm measurements and weighing. The forearm length was measured with a Mitutoyo™ analogical caliper (ñ0.05mm), and bats were weighed with a Pesola™ dynamometer of 100 and 200 g (ñ1 and ñ2g, respectively). The captured specimens were identified according to identification keys available in the literature (Barquez et al. 1999; Gardner 2008; Barquez & Diaz 2009). Up to five first specimens of each species sampled were collected, prepared as vouchers and deposited in the Scientific Collection of Mammals of the Federal University of Paraná State (Appendix). The remaining bats were marked with numbered metallic rings and released at the same place they were captured. This work was carried out with following permits SISBIO 25516-1 and IAP 290-11.
Species richness was estimated using the nonparametric estimator Chao2 (with 1000 bootstraps of the original data). A rarefaction curve was built with the twelve systematic fieldwork samples (Mao Tau procedure). Sampling effort was evaluated through complementary analysis given by the percentage of recorded species relative to the average number of species estimated (Chao2).
Species relative abundance (RA) was calculated as the percentage that each species represented relative to the sum of all captures. A constancy (CO) class was determined for each species based on its relative frequency (RF) at the 12 standardized samples: common, when RF ≥ 51%; intermediate, when 26% ≤ RF<50% or rare, when RF<25% (adapted from Bernardi & Passos 2012).
Each registered species was categorized into a functional group (according to Fleming et al. 1972; Willig 1986; Tavares 2013), based on predominant feeding habit and foraging method: (1) aerial insectivores; (2) gleaning animalivores; (3) frugivores; (4)nectarivores/palynivores; and (5) hematophagous.
A bi-dimensional niche matrix was built to evaluate functional groups relative to body size (based in forearm lengths). We chose forearm length over body mass as a body-size measure because it is more stable metric that reduces the effects of seasonal variation and reproductive status (as in Willig 1986). Differences in forearm length within functional groups were evaluated using Kruskal-Wallis non-parametric analysis of variance (following by Mann-Whitney post hoc tests). The species in same functional group with no size differences were placed in same cell of the bi-dimensional matrix. Sexual dimorphism in forearm size was considered, assuming that the intraspecific variation was smaller than the interspecific variation.
From the construction of the bi-dimensional niche matrix, species allocated in the same cell had their coexistence analyzed using two approaches: (1)constancy of the species in the community and (2) skull shape of insectivorous bats (robust versus gracile skulls—sensu Freeman 1979; 1981; 1998). With regards to species constancy, we expected limited overlap in cell occupancy by species, especially those that were more frequent. Among insectivorous bats, we distinguished between species with robust skulls from those with gracile skulls. Insectivorous bats with robust skulls (e.g. Eptesicus spp., Histiotus spp. and Molossus spp.) tend to have a broader feeding niche, feeding both on hard-bodied (e.g., Coleoptera) and soft-bodied insects (e.g., Lepidoptera, Hymenoptera, Diptera). Bats with gracile skulls (e.g., Myotis spp. and Tadarida spp.), on the other hand, tend to have narrower feeding niches, feeding mostly on soft-bodied insects (Freeman 1981; Swartz et al. 2003; Emiliano et al. 2017). All analyses were performed using the PAST™ software, and a significance level of 0.05 was considered (Hammer 2012).
RESULTS
A total of 247 specimens belonging to 19 species were captured. In the standardized effort (12 monthly samplings) 207 captures were made (excluding ten recaptures of five species) and 16 bat species were recorded (Table1). Three other species were only recorded in shelter searches and non-systematic samplings (Trachops cirrhosus, Diphylla ecaudata, and Molossus rufus). The rarefaction curve tended to stabilize between the sixth and eighth monthly samples (Fig.3). Bat richness of MPSP was estimated (by Chao2) in 15.7ñ1.48 species, indicating that the effort made reached 93.1% of the maximum estimated richness.
Table 1
Bat species and families recorded in the Marumbi Peak State Park, Paraná State, Brazil, and traits used in this study. (*) Species sampled only during preliminary, non-systematic surveys. Skull building: G=gracile skull; R=robust skull (sensu Fremann 1981).


Fig. 3. Rarefaction curves (red line) with 95% confidence intervals (blue lines) for the bat assemblage of the Marumbi Peak State Park, Paraná State, Brazil.
The most abundant species were Sturnira lilium, Carollia perspicillata, Myotis nigricans, and Molossus molossus, which represented 18.8%, 17.4%, 11.6%, and 10.1% of the sum of captures, respectively (Table 1). As for constancy, five species were considered common, seven species were intermediate, and four species were rare (Table 1). The frugivores was the functional group with the highest number of sampled individuals, accounting for 52.1% of the total abundance. It was followed by the aerial insectivores (27.9%), the nectarivores (16.6%), and the gleaning animalivores (0.4%). The hematophagous species (D. ecaudata) was only captured in non-standardized efforts. The aerial insectivore and frugivore functional groups were the richest functional groups, with seven species each.
The analysis of forearm length classes relative to functional groups revealed four size classes for aerial insectivores (H=59.340; p<0.001) (Fig. 4), five classes for frugivores (H=88.1; p<0.001) (Fig. 4), and two classes for nectarivores/palynivores (H=30.7; p<0.001) (Fig.4). The two gleaning animalivore species were allocated in two classes because of their great size differences (Fig. 4). The hematophagous bat included a single class. A bi-dimensional niche matrix with 35 cells (seven size classes and five functional groups) was made, fourteen of these cells were occupied; of these, only three cells were occupied by two or more species (Table 2).
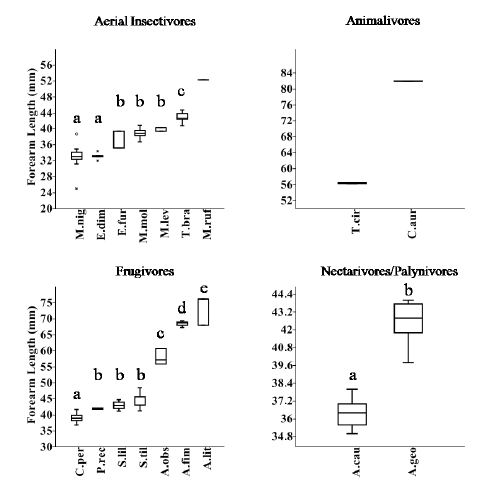
Fig. 4. Box Plot showing species Forearm Length (mm) (median, one and two quartiles deviation), and size classes at each Functional Group. Different letters above each box plot indicate significant differences between species following Mann-Whitney post hoc tests (p<0.05). M.nig=Myotis nigricans; E.dim=Eptesicus diminutus; E.fur=Eptesicus furinalis; M.mol=Molossus molossus; M.lev=Myotis levis; T.bra=Tadarida brasiliensis; M.ruf=Molossus rufus; T.cir=Trachops cirrhosus; C.aur=Chrotopterus auritus; C.per=Carollia perspicillata; P.rec=Platyrrhynus recifinus; S.lil=Sturnira lilium; S.til=Sturnira tildae; A.obs=Artibeus obscurus; A.fim=Artibeus fimbriatus; A.lit=Artibeus lituratus; A.cau=Anoura caudifer; A.geo=Anoura geoffroyi.
Table 2
Bi-dimensional niche matrix considering the aerial insectivores, gleaning animalivores, frugivores, nectarivores/palynivores, and hematophagous bat functional groups, with their respective size classes (forearm length in millimeters), defined a posteriori using the Kruskal-Wallis / Mann-Whitney post hoc tests between species in each functional group (as in Willig 1986).

DISCUSSION
The rarefaction curves and richness estimator (Chao2) showed that the sampling effort was sufficient to sample the bat assemblage of MPSP. Contrary to Bergallo et al. (2003), which proposed that well-sampled areas would be those with 700 to 1000 captures, the present work reached sampling sufficiency with only 207 captures. Perhaps this difference in capture number may be related to the latitudinal gradient. At the lower latitudes of Atlantic Rainforest there is a greater richness and a higher rate of captures, whereas at higher latitudes lower richness and lower capture rates are expected (Willig & Selcer 1989; Stevens 2013). Moreover, another study (not directed to bat sampling) conducted in MPSP recorded only one additional species that was not observed in the present work: Pygoderma bilabiatum (Kaehler et al. 2005). This species seems to do vertical migration (Esberard et al. 2011), and was captured (by Kaehler et al. 2005) at 1100 m above sea level in high montane forest, an environment not sampled in present work.
The 19 recorded species make MPSP a relatively rich bat area considering the southern Brazilian Atlantic Rainforest (Bernard et al. 2010; Stevens 2013). They represent 16.9% of the bat species recorded in this biome (Paglia et al. 2012; Varzinczak et al. 2015; Carvalho et al. 2017) and 25.3% of species recorded for the Southern Region of Brazil (Bianconi et al. 2009; Scultori et al. 2009; Passos et al. 2010; Suckow et al. 2010; Carvalho et al. 2014). Trachops cirrhosus stands out, as the MPSP represents the southernmost limit of its known distribution (Passos et al. 2010). Additional relevant records include Platyrrhinus recifinus, which had been recorded at only three localities in Southern Brazil (Passos et al. 2010; Carvalho & Fabián 2011).
The bat assemblage organization pattern found in MPSP, with a few dominant species (C. perspicillata and S. lilium in this work) and many other species with low abundance is widespread throughout the Neotropics (e.g. Cunha et al. 2011 in the savanna; Silva et al. 2015; Feijó & Rocha 2017 in the Caatinga; Bernardi & Passos 2012; Miranda & Zago 2015 in the Atlantic Rainforest; Barnett et al. 2006; Miranda et al. 2015 in Amazonia) and fits the Brocken Stick type of distribution model (Magurran 2011). Frugivore bats dominate the bat assemblage in MPSP, which seems to be a pattern in Neotropical rainforests (e.g., Pereira et al. 2010; Luz et al. 2011; Carvalho et al. 2013; Miranda et al. 2015).
The bat assemblage of MPSP includes seven species of aerial insectivorous bats. This coexistence can occur through tree enabling mechanisms: (1) species differ in size classes (Hutchinson 1959), as in the tree size classes found in this work; (2) species differ in skull building (robust versus gracile) (Freeman 1979, 1998; Swartz et al. 2003) and likely feed on different insects (see Freeman 1981; Aguiar & Antonini 2008; Bracamonte 2013; Emiliano et al. 2017), as Eptesicus versus Myotis or Eptesicus and Molossus versus Myotis in the two smaller size classes; and (3) species belong to the same size class and present the same skull building but are not common species. The last case is illustrated by the fact that only one (M. nigricans) of seven insectivore species was considered to be common in the bat assemblage of MPSP.
Allocation of frugivore bats to distinct size-class was likely the primary factor that contributed to their coexistence, since the seven frugivore species comprised five different size classes. The coexistence between P. recifinus, S. lilium, and S. tildae can be explained by constancy differences in the assemblage; among these species, only S. lilium is common. Finally, whereas Sturnira spp. has an association with Solanum spp. (Passos et al. 2003; Andrade et al. 2013), P. recifinus seems to feed on Ficus spp. and Cecropia spp. (Tavares et al. 2007; Tavares & Velazco 2010).
The two coexisting, gleaning animalivore species are different in size and feeding habits. Although both prey on insects, T. cirrhosus frequently preys on anurans amphibians as well (Bonato & Facure 2000), whereas C. auritus preys on small mammals such as rodents, marsupials, and other bats (Medelín 1988; Bonato et al. 2004; Brito et al. 2010). Differences in size can explain the coexistence of the two nectarivore species. Moreover, it is possible that both species have some different feeding habits, as found by Muchhala & Jarrin-V. (2002).
The bi-dimensional niche matrix, with size classes created a posteriori (as suggested by Willig 1986) proved to be a good approach to understand the organization of the bat assemblage and to explain the coexistence of most of the 19 species occurring on MPSP. There are still questions worth investigating concerning the coexistence of bats in rainforests, for instance, if insectivorous bats with robust skulls and gracile skulls indeed use different food resources (as proposed by Freeman 1981). Likewise, further coexistence mechanisms are still to be explored, such as the different use of space and time.
ACKNOWLEDGEMENTS
We thank CAPES for JECB and IPB scholarships, CNPq for FCP productivity grant, IAP for sampling authorizations, and the staff of Marumbi Peak State Park for the support during field work. We also thank Juliett Maritza González Carvajal for help with map drawing.
LITERATURE CITED
1. Aguiar, M. S. L., & Y. Antonini. 2008. Diet of two sympatric insectivores bats (Chiroptera: Vespertilionidae) in the Cerrado of Central Brazil. Revista Brasileira de Zoologia 25:28-31. [ Links ]
2. Andrade, T. Y., W. Thies, P. K. Rogeri, E. K. V. Kalko, & M. A. R. Mello.2013. Hierarchical fruit selection by Neotropical leaf-nosed bats (Chiroptera: Phyllostomidae). Journal of Mammalogy 94:1094-1101. [ Links ]
3. Barnett, A. A. et al. 2006. Bats of Jaú National Park, central Amazonia, Brazil. Acta Chiropterologica 8:103-128. [ Links ]
4. Barquez, R. M., & M. M. Díaz. 2009. Los murciélagos de Argentina: clave de identificación. Editorial Magna, Tucumán. [ Links ]
5. Barquez, R. M., M. A. Mares, & J. K. Braun.1999. The Bats of Argentina. Special Publications of the Museum Texas Tech University 42:1-275. [ Links ]
6. Barros, M. A. S., D. M. A. Pessoa, & A. M. Rui. 2014. Habitat use and seasonal activity of insectivorous bats (Mammalia: Chiroptera) in the grasslands of Southern Brazil. Zoologia 31:153-161. [ Links ]
7. Bergallo, H. G., C. E. L. Esbérard, M. A. Mello, V. Lins, G. G. S. Melo, & M. Baptista. 2003. Bat sampling in Atlantic Forest: how much should the minimum effort be? Biotropica 35:278-288. [ Links ]
8. Bernard, E. 2001. Vertical stratification of bat communities in primary forests of central Amazon, Brazil. Journal of Tropical Ecology 17:115-126. [ Links ]
9. Bernard, E, L. M. S. Aguiar, & R. B. Machado. 2010. Discovering the Brazilian bat fauna: a task for two centuries? Mammal Review 2010:1-17. [ Links ]
10. Bernardi, I. P., & F. C. Passos. 2012. Estrutura de comunidade de morcegos em relictos de Floresta Estacional Decidual do Sul do Brasil. Mastozoología Neotropical 19:9-20. [ Links ]
11. Bianconi, G. V., R. Gregorin, & D. C. Carneiro. 2009. Range extension of the Peale’s Free-tailed bat Nyctinomops aurispinosus (Molossidae) in Brazil. Biota Neotropica 9:267-270.
12. Bloch, C. P., R. D. Stevens, & M. R. Willig. 2011. Body size and resource competition in New World bats: a test of spatial scaling laws. Ecography 34:460-468. [ Links ]
13. Bonato, V., & K. G. Facure. 2000. Bat predation by the fringe lipped bat Trachops cirrhosus (Chiroptera: Phyllostomidae). Mammalia 64:241-243. [ Links ]
14. Bonato, V., K. G. Facure, & W. Uieda. 2004. Food habits of bats of subfamily Vampirinae in Brazil. Journal of Mammalogy 85:708-713. [ Links ]
15. Bracamonte, J. C. 2013. Hábitos alimenticios de un ensamble de murciélagos insectívoros aéreos de un bosque montano en las Yungas Argentinas. Chiroptera Neotropical 19:1157-1162. [ Links ]
16. Brito, J. E. C., J. M. D. Miranda, I. P. Bernardi, & F.C. Passos. 2010. Predação de Tadarida brasiliensis por Chrotopterus auritus no sul do Brasil. Chiroptera Neotropical 16:46-47. [ Links ]
17. Carvalho, F., & M. E. Fabián. 2011. Mammalia, Chiroptera, Phyllostomidae, Platyrrhinus recifinus (O.Thomas, 1901): first confirmed record in the state of Santa Catarina, southern Brazil. Check List 7:139-141. [ Links ]
18. Carvalho, F., M. E. Fabián, & J. Menegheti. 2013. Vertical structure of an assemblage of bats (Mammalia: Chiroptera) in a fragment of Atlantic Forest in Southern Brazil. Zoologia 30:491-498. [ Links ]
19. Carvalho, F., V. Mottin, J. M. D. Miranda, & F. C. Passos. 2014. First Record of Vampyrodes caraccioli (Thomas, 1889) (Chiroptera: Phyllostomidae) for the state of Paraná, and range extension to southern region of Brazil. Check List 10:1189-1194. [ Links ]
20. Carvalho, F., D. A. S. Bôlla, F. M. Patel, J. M. D. Miranda, S. L. Althoff, & J. J. Zocche. 2017. Ampliação de distribuição de Eumops patagonicus (Chiroptera: Molossidae) e primeiro registro em ambiente de restinga na costa leste do Brasil. Mastozoología Neotropical 24:443-450. [ Links ]
21. Chiarello, A. G., L. M. S. Aguiar, R. Cerqueira, F.R. Melo, F. H. G. Rodrigues, & V. M. F. Silva. 2008. Mamíferos: Livro vermelho da fauna brasileira ameaçada de extinção (A. B. M. Machado, G.M. Drummond & A. P. Paglia, eds.). Fundação Biodiversitas/Ministério do Meio Ambiente, Belo Horizonte. [ Links ]
22. Cunha, N. L., E. Fischer, & C. F. Santos. 2011. Bat assemblage in savanna remnants of Sonora, central-western Brazil. Biota Neotropica 11:197-201. [ Links ]
23. Emiliano, S. B., L. A. Pereira, S. Pressinatte Júnior, & J. M. D. Miranda. 2017. Dieta de morcegos insetívoros (Mammlia: Chiroptera) em fragmentos de Floresta de Araucárias, Sul do Brasil. Revista Brasileira de Zoociências 18:187-196. [ Links ]
24. Esberard, C. E. L. et al. 2011. Evidence of vertical migration in the Ipanema bat Pygoderma bilabiatum (Chiroptera: Phyllostomidae: Stenodermatinae). Zoologia 28:717-724. [ Links ]
25. Feijó, A., & P. A. Rocha. 2017. Morcegos da Estação Ecológica Aiuaba, Ceará, Nordeste do Brasil: uma Unidade de Proteção Integral na Caatinga. Mastozoología Neotropical 24:333-346. [ Links ]
26. Fenton, M. B. 1997. Science and the conservation of bats. Journal of Mammalogy 78:1-14. [ Links ]
27. Fleming, T. H., E. T. Hooper, & D. E. Wilson. 1972. Three Central American bat communities: structure, reproductive cycles, and movement patterns. Ecology 53:556-569. [ Links ]
28. Freeman, P. W. 1979. Specialized insectivory: beetle-eating and moth-eating molossid bats. Journal of Mammalogy 60:467-479. [ Links ]
29. Freeman, P. W. 1981. Correspondence of food habits and morphology in insectivorous bats. Journal of Mammalogy 62:166-173. [ Links ]
30. Freeman, P. W. 1998. Form function, and evolution in skulls and teeth of bats. Bat biology and conservation (T. H. Kunz & P. A. Racey, eds.). Smithsonian Institution Press, Washington, DC. [ Links ]
31. Gardner, A. L. 2008. Mammals of South America: marsupials, xenarthrans, shrews, and bats. Vol. 1., The University of Chicago Press, Chicago and London . [ Links ]
32. Hammer, Ø. 2012. PAST: Paleontological Statistics. Version 2.14. Reference manual. University of Oslo Press, Oslo. [ Links ]
33. Hutchinson, G. E. 1959. Why are there so many kinds of animals? American Naturalist 93:145-159. [ Links ]
34. IBGE. 2012. Manual técnico da vegetação brasileira. Série Manuais Técnicos em Geociências: Número 1. Instituto Brasileiro de Geografia e Estatística, Rio de Janeiro. [ Links ]
35. Kaehler, M., I. G. Varassin, & R. Goldenberg. 2005. Polinização em uma comunidade de bromélias em Floresta Atlântica Alto-Montana no Estado do Paraná, Brasil. Revista Brasileira de Botânica 28:219-228. [ Links ]
36. Kunz, T. H., & E. D. Pierson.1994. Bats of the world: an introduction. Walker’s bats of the world. (R. M. Nowak, ed.). Johns Hopkins University Press, Baltimore and London.
37. Luz, J. L., L. M. Costa, E. C. Lourenço, & C. E. L. Esbérard. 2011. Morcegos (Mammalia, Chiroptera) da Reserva Rio das Pedras, Rio de Janeiro, Sudeste do Brasil. Biota Neotropica 11:95-101. [ Links ]
38. Magurran, A. E. 2011. Medindo a diversidade biológica. Editora UFPR, Curitiba. [ Links ]
39. Marinho-Filho, J., & I. Sazima.1998. Brazilian bats and conservation biology; a first survey. Bat biology and Conservation (T. H. Kunz & P. A. Racey, eds.). Smithsonian Institution Press, Washington and London. [ Links ]
40. Mcarthur, R. H. 1957. On the relative abundance of bird species. Proceedings of the National Academy of Sciences USA 43:293-295. [ Links ]
41. Mcnab, B. K.1971. The structure of tropical bat faunas. Ecology 52:352-358. [ Links ]
42. Medelín, R. 1988. Prey of Chrotopterus auritus, with notes on feeding behaviour. Journal of Mammalogy 69:841-844. [ Links ]
43. Meyer, A. S., M. R. Pie, & F. C. Passos. 2014. Assessing the exposure of lion tamarins (Leontopithecus spp.) to future climate change. American Journal of Primatology 76:551-562. [ Links ]
44. Miranda, J. M. D., & L. Zago. 2015. Assembleia de morcegos em remanescente de Floresta Ombrófila Mista no planalto de Guarapuava, Paraná, Brasil. Mastozoologia Neotropical 22:55-62. [ Links ]
45. Miranda, J. M. D., L. Zago, F. Carvalho, M. B. G. Rubio, & I. P. Bernardi. 2015. Morcegos (Mammalia: Chiroptera) da região do Médio Rio Teles Pires, Sul da Amazônia, Brasil. Acta Amazonica 45:89-100. [ Links ]
46. Mittermeier, R. A., N. Myers, & C. G. Mittermeier. 1999. Hotspots: Earth’s biologically richest and most endangered terrestrial ecoregions. CEMEX/Conservation International, Washington, DC.
47. Muchhala, N., & P. Jarrín-V. 2002. Flower visitation by bats in cloud forests of Western Ecuador. Biotropica 34:387-395. [ Links ]
48. Myers, N., R. A. Mittermeier, C. G. Mittermeier, G.A. B. Fonseca, & J. Kent. 2000. Biodiversity hotspots for conservation priorities. Nature 403:853-858. [ Links ]
49. Nogueira, M. R., I. P. Lima, R. Moratelli, V. C. Tavares, R. Gregorin, & A . L. Peracchi. 2014. Checklist of Brazilian bats, with comments on original records. Check List 10:808-821. [ Links ]
50. Paglia, A. P. et al. 2012. Annoted checklist of Brazilian mammals. 2nd Edition. Occasional Papers in Conservation Biology 6:1-82. [ Links ]
51. Passos, F. C., W. R. Silva, W. A. Pedro, & M. R. Bonin. 2003. Frugivoria em morcegos (Mammalia, Chiroptera) no Parque Estadual Intervales, sudeste do Brasil. Revista Brasileira de Zoologia 20:511-517. [ Links ]
52. Passos, F. C., J. M. D. Miranda, I. P. Bernardi, N. Y. Kaku-Oliveira, & L. C. Munster. 2010. Morcegos da Região Sul do Brasil: análise comparativa da riqueza de espécies, novos registros e atualizações nomenclaturais (Mammalia, Chiroptera). Iheringia (Série Zoologia) 100:25-34. [ Links ]
53. Patterson, B. D., M. R. Willig, & R. D. Stevens. 2003. Trophic strategies, niche partitioning, and patterns of ecological organization. Bat Ecology (T. H. Kunz & M. B. Fenton, ed.). University Chicago Press, Chicago and London. [ Links ]
54. Pedro, W. A., & V. A. Taddei. 1997. Taxonomic assemblage of bats from Panga Reserve, southeastern Brazil: abundance patterns and trophic relations in the Phyllostomidae (Chiroptera). Boletim do Museu de Biologia Mello Leitão (Nova Série) 6:3-21. [ Links ]
55. Pereira, M. J. R., J. T. Marques, & J. M. Palmerim. 2010. Vertical stratification of bat assemblages in flooded and unflooded Amazonian forests. Current Zoology 56:469-478. [ Links ]
56. Ranta, P., T. Blom, J. Niemela, E. Joensuu, & M.Siitonen.1998. The fragmented Atlantic Forest of Brazil: Size, shape and distribution of forest fragments. Biodiversity and Conservation 7:385-403. [ Links ]
57. Scultori, C., D. Dias, & A. L. Peracchi. 2009. Mammalia, Chiroptera, Phyllostomidae, Lampronycteris brachyotis (Dobson, 1879): first record in the state of Paraná, southern Brazil. Check List 5:872-875. [ Links ]
58. Silva, S. S. P. et al. 2015. Bats (Mammalia: Chiroptera) from the Caatinga Scrublands of the Crateus Region, Noertheastern Brazil, with new records for the State of Ceará. Mastozoología Neotropical 22:335-348. [ Links ]
59. Simmons, N. B., & T. M. Conway. 2003. Evolution of ecological diversity in bats. Bat Ecology (T. H. Kunz & M. B. Fenton, eds.). University of Chicago Press, Chicago and London. [ Links ]
60. Simmons, N. B., & R. S. Voss. 1998. The mammals of Paracou, French Guiana: a Neotropical lowland rainforest fauna part 1. Bats. Bulletin of American Museum of Natural History 237:1-279. [ Links ]
61. SOS MATA ATLÂNTICA. 2018. SOS Mata Atlântica. <https://www.sosma.org.br/nossa-causa/a-mata-atlantica/> [ Links ].
62. Stevens, R. D. 2013. Gradients of bat diversity in Atlantic Forest of South America; Environmental, seasonality, sampling effort and spatial autocorrelation. Biotropica 45:764-770. [ Links ]
63. Stevens, R. D., & M. R. Willig. 2002. Geographical ecology at the community level: perspectives in the diversity of New World bats. Ecology 83:545-560. [ Links ]
64. Straube, F. C., & G. V. Bianconi. 2002. Sobre a grandeza e a unidade utilizada para estimar esforço de captura com utilização de redes de neblina. Chiroptera Neotropical 8:150-152. [ Links ]
65. Struminski, E. 2001. Parque Estadual Pico do Marumbi. Editora da Universidade Federal do Paraná, Curitiba. [ Links ]
66. Suckow, U. M. S., G. V. Bianconi, L. C. Parolin, & I. P. Lima. 2010. First occurrences of the greater bonneted Eumops perotis (Molossidae) in the State of Paraná and synthesis of known records for Brazil. Biota Neotropica 10:453-456. [ Links ]
67. Swartz, S. M., P. W. Freeman, & E. F. Stokwell. 2003. Ecomorphology of bats: comparative and experimental approaches relating structural design to ecology. Bat Ecology (T. H. Kunz & M. B. Fenton, eds.). The University of Chicago Press. Chicago and London. [ Links ]
68. Tavares, V. C. 2013. Phyllostomid bat wings from Atlantic Forest bat ensambles: an ecomorphological study. Chiroptera Neotropical 19:57-70. [ Links ]
69. Tavares, V. C., & P. M. Velazco. 2010. Platyrrinus recifinus. Mammalian Species 42:119-123. [ Links ]
70. Tavares, V. C., F. A. Perini, & J. A. Lombardi. 2007. The bat communities (Chiroptera) of the Parque Estadual do Rio Doce, a large remnant of Atlantic Forest in southeastern Brazil. Lundiana 8:35-47. [ Links ]
71. Varzinczak, L., I. P. Bernardi, & F. C. Passos. 2015. Is the knowledge of bat distribution in the Atlantic Rainforest sufficient? Comments about new findings and a case study in the Paraná State coastal area, Brazil. Mammalia 79:1‒7. [ Links ]
72. Willig, M. R 1986. Bat structure in South America: a tenacious chimera. Revista Chilena de Historia Natural 59:151-168. [ Links ]
73. Willig, M. R., & K. W. Secler. 1989. Bat species density gradients in the New World: a statistical assessment. Journal of Biogeography 16:189-195. [ Links ]
Voucher specimens collected in this work and preserved in Scientific Mammal Collection of the Federal University of Paraná. Acronym DZUP:
Anoura caudifer (820, 1233, 1234, 1241, 1245); Anoura geoffroyi (819, 1244, 1246, 1247, 1248); Artibeus fimbriatus (1235, 1236, 1249, 1260, 1316); Artibeus lituratus (1661); Artibeus obscurus (1293, 1317, 1325); Carollia perspicillata (801,802, 803, 804, 805); Chrotopterus auritus (1302); Diphylla ecaudata (1319, 1320, 1321, 1322); Eptesicus diminutus (1299,1300, 1301); Eptesicus furinalis (1239, 1257, 1297); Molossus molossus (807, 808, 809, 810, 1254); Molossus rufus (800); Myotis levis (1230, 1231, 1318); Myotis nigricans (813, 814, 815, 816, 817); Platyrrhinus recifinus (1232, 1253, 1326); Sturnira lilium (821, 822, 1227, 1276, 1277); Sturnira tildae (1240, 1250, 1282, 1283); Tadarida brasiliensis (1273,1274, 1275, 1305, 1306); Trachops cirrhosus (811, 812).














