Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Mastozoología neotropical
Print version ISSN 0327-9383On-line version ISSN 1666-0536
Mastozool. neotrop. vol.26 no.1 Mendoza June 2019
ARTÍCULO
Richness, endemism and conservation of sigmodontine rodents in Argentina
Anahí Formoso1 and Pablo Teta2
1 Centro para el Estudio de Sistemas Marinos (CESIMAR-CENPAT-CONICET). Puerto Madryn, Chubut, Argentina.
2 División Mastozoología, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” Buenos Aires, Argentina. [Correspondence: Pablo Teta <antheca@yahoo.com.ar>]
ABSTRACT
Sigmodontine rodents, with 86 genera and ~430 living species, constitute one of the most successful radiations of Neotropical mammals. In this contribution, we studied the distributional ranges of 108 sigmodontine species in Argentina. Our objectives were (i) to establish geographical patterns of species richness and endemism, and (ii) to evaluate the regional conservation status of these taxa. We constructed a minimum convex polygon for each species, using information from literature and biological collections. Individual maps were superimposed on a map of Argentina divided into cells of 25 km on each side. For each cell, we calculated the species richness, which varied between 1 and 21 species, and its degree of endemism, which fluctuated between 0.001 and 3.28. There were 30 species of sigmodontine rodents distributed almost exclusively in Argentina, most of them restricted to forested areas (Southern Andean Yungas) or to arid and semiarid environments (High and Low Monte and Patagonian Steppe). Areas with high species richness and endemism scores corresponded grossly with the Southern Andean Yungas, the Humid Chaco plus the Paraná flooded savannas, the Alto Parana Atlantic forests plus the Araucaria moist forests, the High Monte and the ecotone between the Patagonian steppe and the Valdivian temperate forests. A reassessment of the conservation status of sigmodontine rodents distributed in Argentina retrieved 2 extinct species, 7 endangered, 7 vulnerable, 6 near threatened and 13 data deficient. These numbers suggest a much more serious situation than the expressed by previous evaluations, highlighting the urgent need to establish conservation measures for the protection of this group.
RESUMEN
Riqueza, endemismo y conservación de roedores sigmodontinos en Argentina.
Los roedores sigmodontinos, con 86 géneros y ~430 especies vivientes, constituyen una de las radiaciones más exitosas de mamíferos neotropicales. En esta contribución, estudiamos los rangos de distribución de 108 especies de sigmodontinos en Argentina. Nuestros objetivos fueron (i) establecer patrones geográficos de riqueza de especies y endemismo y (ii) evaluar el estado de conservación regional de estos taxones. Construimos un polígono convexo mínimo para cada especie, utilizando información de la literatura y colecciones biológicas. Los mapas individuales fueron superpuestos en un mapa de Argentina dividido en celdas de 25 km de lado. Para cada celda, calculamos la riqueza de especies, que varió entre 1 y 21, y su grado de endemismo, que fluctuó entre 0.001 y 3.28. Hubo 30 especies de roedores sigmodontinos distribuidos casi exclusivamente en Argentina, la mayoría de ellos restringidos a áreas boscosas (Yungas andinas del sur) o a ambientes áridos y semiáridos (Monte alto y bajo y Estepa Patagónica). Las áreas con mayor riqueza de especies y valores más altos de endemismo se correspondieron groseramente con las Yungas andinas del sur, el Chaco húmedo más las Sabanas inundadas de Paraná, el Bosque Atlántico del Alto Paraná más los Bosques húmedos de araucaria, el Monte alto y el ecotono entre la Estepa Patagónica y los Bosques templados valdivianos. Una reevaluación del estado de conservación de los roedores sigmodontinos distribuidos en Argentina recuperó 2 especies extintas, 7 en peligro, 7 vulnerables, 6 casi amenazadas y 13 con datos deficientes. Estas cifras sugieren una situación mucho más grave que la expresada en evaluaciones anteriores, destacando la necesidad urgente de establecer medidas de conservación para la protección de este grupo.
Key words: Cricetidae; Endangered species; Extinction; Muroidea; Rodentia.
Palabras clave: Cricetidae; Especie en peligro; Extinción; Muroidea; Rodentia.
Recibido 13 abril 2018.
Aceptado 24 diciembre 2018.
Editor asociado: G. D’Elia
INTRODUCTION
During the last 500 years, almost 100 species of mammals became extinct, rodent species being more than a half of them (Turvey 2009). Only in South and Central America, including both continental and insular areas, ~32 species of rodents disappeared in the last five centuries (Turvey 2009, Teta et al. 2014). If we consider rodent extinctions across the Holocene (i.e., the last 10 000 years), the global number of lost species ascends to 115 (Turvey 2009). This scenario contradicts the generalized perception that rodents lack major conservation problems (Lacher et al. 2017).
Like other small mammals, rodents play a fundamental role in trophic chains acting as prey of other vertebrates (Jaksic 2002). Many rodent species occupy specialized ecological niches, contributing to energy and nutrient flow and providing important functions to the ecosystems, such as soil tilling and seed dispersal (Lacher et al. 2017). This situation contrasts with the lack of knowledge about this group, especially regarding the conservation status of their different members. A number of factors negatively influences over this situation, making it even more serious. On one hand, rodents are one of the most diverse and at the same time least known groups of Mammalia, with many species that are known only from the type specimen or series and sometimes from collections carried out more than 100 years ago (Amori et al. 2016). On the other hand, rodents are not charismatic species, attracting little attention from researchers and officials responsible for directing scientific resources and funding (Fleming & Bateman 2016).
Sigmodontine rodents, with 86 living genera and ca. 430 living species, constitute one of the most successful radiations of Neotropical mammals (Parada et al. 2015, Maestri et al. 2016). Species of this group are mostly distributed in South America and, to a lesser extent, in Middle and North America (D’Elía & Pardiñas 2015). Sigmodontine rodents occur from the humid rainforests of the Amazon to the extremely dry desert of Atacama (Maestri & Patterson 2016). Their diets are usually omnivorous, but there are species almost strictly herbivorous, insectivorous and fungivorous (Maestri et al. 2016). Despite their generalized morphology, sigmodontine rodents occupy from arboreal to cursorial, fossorial or semiaquatic niches (D’Elía & Pardiñas 2015).
At least 108 species of sigmodontine rodents have been recorded for Argentina, including representatives of nine tribes and some incertae sedis taxa (Patton et al. 2015, Teta et al. 2018). The tribes Akodontini and Oryzomyini were best represented in subtropical and temperate regions, both in forested and open environments. Abrotrichini and Phyllotini predominated towards high latitudes and Andean ranges. Phyllotini was also dominant in arid to semiarid open areas of western Argentina, where it reached its greatest diversity. Finally, Andinomyini and Euneomyini were distributed mostly in high mountain areas, Reithrodontini was widespread along open grassy areas and Thomasomyini and those taxa considered as incertae sedis occurred mostly in forested habitats (cf. Patton et al. 2015).
In this paper, two main issues were addressed. First, we evaluated geographical patterns of species richness and endemism of sigmodontine rodents in Argentina. Second, and based on the analysis of the available data, we proposed new conservation status for the species in this group at the regional level, demonstrating a more serious regional situation than previously reported.
MATERIALS AND METHODS
Argentina covers an area of almost 2.8 million km2, including both continental and insular portions. This country is the second largest in the Neotropics and the eight largest in the world (Real et al. 2003). Although the usage of political divisions to address biogeographical issues is often criticized because patterns and processes responsible for biological diversity do not recognize artificial boundaries (such as the frontiers among countries), we decided to choose this approach because the application of conservation measures is usually conducted at regional or national administrative levels (Real et al. 2003). In addition, it is widely accepted that political limits shape human activities and are not always completely arbitrary; in fact, political boundaries sometimes coincide with natural barriers (e.g., the Andean Cordillera to the west of Argentina), being suitable to detect some natural phenomena (Real et al. 2003). The scheme of ecoregions used in this work follows Olson et al. (2001).
Distributional data for 108 species of sigmodontine rodents inhabiting Argentina were taken from Patton et al. (2015) and updated when necessary (Jayat et al. 2016, Pardiñas et al. 2016a, Pardiñas et al. 2016b, Teta et al. 2017, Teta and D’Elía 2017). Taxonomy follows Teta et al. (2018). Point data for marginal records of each species were imported into Geographic Information System (GIS) and transformed to minimum convex polygons (= extent of the occurrence). Distributional ranges were calculated for each taxa, except for those with only 1 locality record (IUCN Species Survival Commission 2012). The polygons for those species exceeding the geographic limits of Argentina were clipped and the range size in km2 (area) for each species was calculated. Complete lists of localities were compiled only for those species with an extent of occurrence less than 20 000 km², as this value, combined with the number of localities, demography and threats, is used as a cut-off point by the UICN to separate between threatened and not-threatened categories.
For calculating species richness (S), a grid with square cells of 25 km2 was created and the number of species in each cell was counted. Data projections and calculations were carried out using the software ArcGIS Desktop 10.5.1 (ESRI 2017) and the projected coordinate system WGS84/UTM zone 19S (EPSG: 32719).
Endemism was calculated both as categorical and continuous variables. First, we considered a taxon as endemic to Argentina if it occurs mostly (> 98% of its distributional range) or exclusively within the limits of the country. The rationale behind this procedure is that the conservation status of these species would depend almost exclusively on the policies adopted by the Argentinean legislative bodies at national or regional levels. Secondarily, we calculated endemism as a continuous variable, following the equation of Kryštufek and Griffiths (2002, see also Kryštufek et al. 2009): Ej = ∑1/Ai, for all i included in Sj, where Ai is the number of cells for every species with i = 1 to n (the maximum number of species). In each geographical cell j is the set of species Sj found within it. The sum of the weights of 1/Ai for every species found in set Sj produced an overall measure of endemism Ej. The contribution made by a species is constant for each cell (Nott & Pimm 1997, Kryštufek & Griffiths 2002). Species with range distributions above 106 km2 were excluded from the analysis, since their individual contribution to a cell would be < 10-3.
Areas with high levels of biodiversity were considered as “hotspots,” defining them as those cells with high species richness or high endemism scores, the cut-off point being the upper quartile (i.e. the top 25% squares; for a similar procedure, see Kryštufek & Griffiths 2002). Expressed in this way, this is the inverse of the definition of rarity proposed by Gaston (1994). Those cells with high species richness plus high endemism scores were considered as “top hotspots.” The statistical significance of “top hotspots” was tested using the Getis-Ord Gi* tool of ArcGIS (Getis & Ord 1992). This statistic allows the identification of “hotspots”, higher or lower in magnitude than one might expect to find by chance; in addition, this method compares the value for a given observation with locations in the neighborhood, thus providing a more explicit consideration of space (Nelson & Boots 2008).
Global conservation status were taken from the IUCN Red List (2011). New species assessments were based on the categories and guidelines defined by the IUCN for regional level (IUCN Species Survival Commission 2012). This methodology considers the number of known localities in which a certain species was documented and the extent of its distribution, plus additional information (if available) about demographic parameters (see IUCN Species Survival Commission 2012). For some species, preliminary categorizations were changed as long as there was a possibility of a “rescue effect” from populations outside the region (see IUCN Species Survival Commission 2012). With few exceptions, species based on historical records or documented for only one or two localities (e.g., Andalgalomys pearsoni, Necromys amoenus), with dubious taxonomic status (e.g., Graomys edithae), or both (e.g., Brucepattersonius guarani, B. misionensis, B. paradisus) were considered as Data Deficient.
RESULTS
Geographical range sizes for 108 sigmodontine species varied from 67.18 km2 to 1 439 529.18 km2, representing from 0.0024% to 51.77% of the total surface of Argentina (Table 1). Only 16 species have distributional ranges that covered more than 20% of the total area of the country, while the other 92 species occupied less than 19% each, with 52 species (this is 48.14% of total number of species) occurring in an area smaller than 1% (Table 1). Species with a range size less than 5501.58 km2 (= upper limit for the lower quartile of the species ranges annotated in Table 1) were considered rare (N = 27), according to the definition of Gaston (1994). Species richness varied between 1 and 21 species per grid cell (mean = 8.14). The highest values (i.e., S ≥ 14) mostly corresponded to five main areas: 1) Southern Andean Yungas, 2) Humid Chaco plus Paraná flooded savannas, 3) Alto Parana Atlantic forests plus Araucaria moist forests, 4) High Monte, and 5) the ecotone between the Patagonian steppe and the Valdivian temperate forests (Fig. 1).
Table 1
Sigmodontine rodents from Argentina: species list (arranged by range size), distributional range (occupied area in Argentina), global status according IUCN Red List (2011), regional status in Argentina according to Teta & Pardiñas (2012) and updated status (this work).


Fig. 1. Map of Argentina depicting species richness of sigmodontine rodents (left) and ecoregions according to Olson et al. 2001 (right).
There are 30 species almost exclusively distributed in Argentina, 13 belonging to Phyllotini, 13 to Akodontini, 2 to Abrotrichini and 2 to Oryzomyini. Most species in this category were distributed towards the western portion of the country (most cells ≥ 7 endemic species), both in forested areas (e.g., Southern Yungas) and open, arid to semiarid, areas (e.g., High and Low Monte, Patagonian steppe) (Fig. 2). The distributional range size of endemic species varied between 67.18 and 894 733.81 km2, with almost the half of them being distributed in less than 1000 km2 (Table 1).
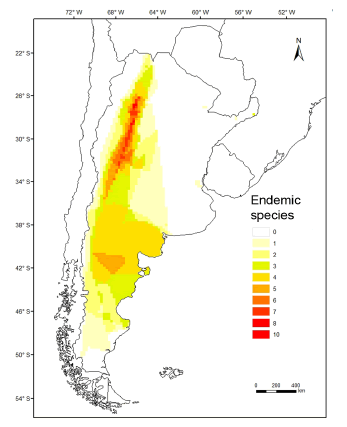
Fig. 2. Map of Argentina depicting the superimposed distributions of endemic species of sigmodontine rodents.
Endemism scores varied between 0.001 and 3.28 (mean = 0.02). Highest values for this variable mostly correspond to cells with high species richness (i.e, Southern Andean Yungas, Humid Chaco, Alto Parana Atlantic forests and High Monte) and those characterized by the presence of endemic species (e.g., Delta del Paraná, southeastern Mendoza, southwestern Buenos Aires) (Fig. 3). The Spearman correlation coefficient between species richness and endemism scores was 0.71 (p < 0.0001)

Fig. 3. Map of Argentina depicting endemism scores for sigmodontine rodents.
“Hotspots” were defined as those cells with species richness ≥ 10 or with an endemism score ≥ 0.018, retrieving 1257 cells (25.8% of the total surface of Argentina); of these, 730 (14.9% of the total surface of Argentina) are considered as “top hotspot”, since they outperformed in both parameters simultaneously (Fig. 4). Statistical significance of “top hotspots” is depicted on Supplement 1.

Fig. 4. Map of Argentina depicting “top hotspots” (= black cells) for sigmodontine rodents.
The reassessment of the conservation status of Argentinean sigmodontine rodents retrieved 2 Extinct species (1 Akodontini, 1 Oryzomyini), 7 Endangered (3 Akodontini, 4 Phyllotini), 7 Vulnerable (2 Akodontini, 1 Oryzomyini, 1 Phyllotini, 3 incertae sedis), 6 Near Threatened (4 Akodontini, 2 Phyllotini), 73 Least Concern and 13 Data Deficient (see Table 1; Fig. 5). Endangered species were mostly distributed in forested, arid to semiarid, and grassy environments (Fig. 6). Justification for each individual case is provided on Table 2.

Fig. 5.Histograms of species conservation status for sigmodontine rodents as defined by Teta and Pardiñas (2012; gray bars) and this work (black bars).

Fig. 6. Map of Argentina showing the superimposed distribution of threatened species of sigmodontine rodents.
Table 2
Justification of the threatened status of species of sigmodontine rodents from Argentina. An asterisk denotes a change in the conservation status based on the rescue effect from neighbor countries (see IUCN Species Survival Commission 2012). Use of term “location” follows the definition provided by the IUCN Species Survival Commission (2012).

DISCUSSION
The study and comprehension of geographical patterns of species richness and endemism is crucial for the conservation of biodiversity (e.g., Ceballos et al. 2005). Understanding how biodiversity is changing and responding to global change allows us to update, when necessary, the conservation status of species, in order to contribute preserving their populations (e.g., Lacher et al. 2017; this work). In this work, we analyzed the geographic distribution, species richness, endemism, and conservation status of 108 sigmodontine rodents that inhabit Argentina. The main results of our contribution are: (i) species richness, endemism scores and “hotspots” were moderately correlated and correspond to five main areas (see below); (ii) almost half of the studied species occupies, each, less than 1% of the country surface; (iii) the re-evaluation of conservation status at the regional level revealed a more critical situation than the one reported in a previous assessment (Teta & Pardiñas 2012).
High values of species richness and endemism scores coincided mostly in five main areas: 1) Southern Andean Yungas, 2) Humid Chaco plus Paraná flooded savannas, 3) Alto Parana Atlantic forests plus Araucaria moist forests, 4) High Monte, and 5) the ecotone between the Patagonian steppe and the Valdivian temperate forests. Most of those areas are adjacent to hilly or mountain chains (i.e., the Southern Andean Yungas, the High Monte and the ecotone between the Patagonian steppe and the Valdivian temperate forest are close to the Andes; the Alto Parana Atlantic forests and Araucaria moist forests are influenced by the relatively high elevations of the Serra do Mar and adjacent high plateaus of eastern Brazil), that offers high environmental heterogeneity (Amori et al. 2012). Mountain ranges, by establishing barriers to dispersal, creating vertical succession of habitats, and isolating populations, potentially lead speciation (mostly by allopatry) and contribute to generate high species richness and turnover (Parada et al. 2015, Maestri & Patterson 2016). In fact, species richness for sigmodontine rodents is strongly affected by elevation, which in South America is mostly dominated longitudinally by the Andes (Maestri & Patterson 2016). In accordance with our results, most of these same areas are recognized as diverse and distinctive centers of mammalian endemism, such as the Brazilian Atlantic forest or the Monte desert (cf. Mares 1992, Amori et al. 2012, Maestri & Patterson 2016).
Statistically significant “top hotspots” were broadly coincident with the Southern Andean Yungas and the Alto Parana Atlantic forests, highlighting the importance of forested environments as areas with high species richness and unique taxa (Amori et al. 2012). Further analyses, incorporating phylogenetic and functional diversity as additional variables, are much needed in order to determine “hotspots” with more accuracy (Mazel et al. 2018).
Understanding diversity implies to consider not only species richness or endemism but also rarity (Halffter 1994). Rare species are those that occur in a small distributional range or with low abundances through large areas (Gaston 1994, Halffter 1994). Species with small geographic ranges are expected to be more vulnerable to habitat deterioration and other localized anthropogenic disturbances. Our results showed that almost half of the sigmodontine rodents distributed in Argentina (N = 52) occupies, each, less than 1% of the total surface of the country; among these, 27 (including 18 endemic) could be considered rare following the definition of Gaston (rarity being defined as the inverse of range size, i.e. the converse of widespread). This situation is not surprising, because prevalence of rarity is a common phenomenon among other taxonomic groups in different geographical regions (Gaston 1994). In this study, species with restricted distributions occur mostly in the Southern Andean Yungas, the Alto Parana Atlantic forests plus Araucaria moist forests, and the Monte desert. Most species in the Southern Andean Yungas and the Monte desert are, according to current knowledge, truly restricted (Table 1); while most of those of the Alto Parana Atlantic forests plus Araucaria moist forests have reduced distributions in Argentina (due to the small range occupied by these forests in this country), although most of them are relatively widespread along the forested portions of eastern Paraguay and southeastern Brazil (cf. Patton et al. 2015).
The reassessment of the conservation status of Argentinean sigmodontine rodents showed a situation more critical and severe than currently envisioned. Our findings differ from the evaluation carried out by Teta & Pardiñas (2012) who reported 1 Extinct species (1 Akodontini), 2 Endangered (2 incertae sedis), 4 Vulnerable (1 Akodontini, 1 Euneomyini, 2 Phyllotini) and 1 Near Threatened (1 Akodontini). Despite some methodological issues resulting in such different figures, our results suggest a more worrying regional situation than that formerly reported. Regarding this point, it is important to note that all changes recorded since Teta & Pardiñas (2012) are non-genuine (i.e., those resulting—for example—from improved knowledge, taxonomic changes, or correction of earlier errors; see IUCN Species Survival Commission 2012), because of new available information or mistakes in the interpretation of some guidelines (e.g., Teta & Pardiñas [2012] do not consider the “rescue effect” from populations in neighbor countries or misunderstood the concept of locality according to the IUCN). We found that threatened species inhabit mostly forested environments, but also open, arid, semiarid to temperate, steppes and grasslands. Among those species that we considered as rare, there are 2 listed as Extinct, 7 Endangered and 3 Vulnerable (Table 1). Extinct species include the swamp rat Gyldenstolpia fronto (last seen in Brazil in the decade of 1990; the only known Argentinian specimen was secured in 1886 [Pardiñas et al. 2008, Bezerra 2011]) and the marsh rat Holochilus lagigliai (last seen in the decade of 1950 [Pardiñas et al. 2013]). Amori et al. (2012), from a continental perspective, reported no more than one threatened species per region across Argentina. Here, we documented a much different situation, with up to 3-4 threatened species in some parts of the High Monte and the Alto Parana Atlantic forests plus the Araucaria moist forests. These results highlight the need of regional assessments, especially when this type of information constitutes the base for future research or is used to taking regional conservation measures (see IUCN Species Survival Commission 2012).
Most threatened species shared some features that influence its conservation status, such as small distributional ranges, low abundances or a reduced number of locality records (Table 2). In most cases, these species occur in habitats where both their extension and quality are declining, such as the Alto Parana Atlantic forests, the Araucaria moist forests (e.g., Castoria angustidens, Delomys dorsalis, Juliomys pictipes) or the grasslands of Humid Pampas in central-eastern Argentina (e.g., Deltamys kempi, Necromys obscurus). The situation of the leaf-eared mice of the genus Phyllotis is outstanding, since at least 4 species of this taxon are considered under some threatened category. Species of Phyllotis are mostly restricted to rocky outcrops and high grassy areas, having, in some cases, extremely restricted ranges (Steppan & Ramírez 2015) in threatened environments (e.g., Southern Andean Yungas).
Our work has two potential sources of bias, one primarily related to taxonomic issues and the other linked to artifacts in species mapping (Kryštufek & Griffiths 2002, Isaac et al. 2004). For example, Andalgalomys olrogi is here categorized as endangered; however, this rodent was included by some researchers (e.g., Diaz et al. 2006) into the synonymy of A. roigi, a species categorized as of least concern. For the genus Brucepattersonius, there is at least three species with very restricted distributional areas (i.e., Brucepattersonius guarani, B. misionensis, B. paradisus), which were categorized as Data Deficient due to their dubious taxonomic status and potential conspecificity (cf. Lanzone et al. 2018). Another example is the case of Phyllotis tucumanus from northwestern Argentina, whose taxonomic splitting would increase the endemism scores in an area already recognized as a “hotspot” and also reduce the average range sizes for the different species in this genus (see Jayat et al. 2016). Contrarily, the opposite effect would occur if P. alisosiensisis is included into the synonymy of P. anitae, as is suggested by the available morphological and molecular evidence (see Jayat et al. 2016). Regarding the species distribution datasets, our approach ignores that real ranges of species are better represented as mosaics than as continuous polygons in a map, which do not always represent the real geographic distribution of the species. Fortunately, it is well documented that broad biogeographic patterns are not sensitive to grid square size (see Schall & Pianka 1978, Di Marco et al. 2013).
The fragmentation of habitats and the loss of biodiversity are in constant advance and it seems to become even worse in coming decades. Human activities are the main cause of habitat loss, fragmentation and species extinction, but climate change is projected to be equally or even more important in the near future (Hoffman et al. 2010, Pereira et al. 2010, Dawson et al. 2011). Global biodiversity is currently under a huge pressure of change and threat (Ceballos et al. 2005). Recognizing and understanding this problem is crucial for carrying out plans for managing and protecting the species that more need it (Ceballos et al. 2005). For example, protected areas such as National Parks are scattered through Argentina, covering ca. 13% of its total surface. This protection is unevenly distributed, being mostly coincident with areas of natural attractions or impressive landscapes (e.g., Alto Parana Atlantic forests, Valdivian temperate forests). From our data, it is clear that additional protected areas are strongly needed in some distinctive ecoregions, such as the High Monte that supports numerous endemic species. In many countries, and more especially in developing ones, funds designated to conservation are limited, even when the need for constant updates on the knowledge of species is crucial for their conservation (Ceballos et al. 2005). The situation of the order Rodentia is paradigmatic, because historical records suggest that the species in this group are among the most vulnerable mammals to extinction due to human activities (Turvey 2009). Simultaneously, this group includes some of the least known mammal species of the world. Funding of specific conservation projects for rodents is a first step to maintaining the exceptional diversity of this order on our planet.
ACKNOWLEDGEMENTS
This work has benefited from the numerous discussions held over the past years with colleagues and friends, including G. Cassini, S. Cirignoli, E. Cuéllar, G. D’Elía, D. Flores, N. Fracassi, C. Galliari, G. Gil, P. Jayat, C. Lanzone, S. Lucero, R. Ojeda, P. Ortíz, U. F. J. Pardiñas, J. Pereira, D. Podestá and D. Udrizar. Similarly, two anonymous reviewers and the asociated editor (G. D’Elía) provided valuable input on an earlier version of this contribution. We also want to acknowledge the help provided by P. Dell’Aciprete, I. Minoli and V. Alonso Roldán with some methodological issues.
LITERATURE CITED
1. Amori, G., F. Chiozza, B. D. Patterson, C. Rondinini, J. Schipper, & L. Luiselli. 2012. Species richness and distribution of Neotropical rodents, with conservation implications. Mammalia 77:1-19. https://doi.org/10.1515/mammalia-2012-0050 [ Links ]
2. Amori, G., G. Alari Esposito, & L. Luiselli. 2016. Known from a handful of specimens: analyzing the worldwide patterns of occurrence and conservation of rodents and shrews recorded only from the type locality. Journal of Threatened Taxa 8:8556-8563. https://doi.org/10.11609/jott.2405.8.3.8556-8563 [ Links ]
3. Bezerra, A. M. R. 2011. Collection records of Gyldesntolpia planaltensis (Avile-Pires, 1972) (Rodentia, Cricetidae) suggest the local extinction of the species. Mastozoología Neotropical 18:119-123. [ Links ]
4. Ceballos, G., P. R. Ehrlich, J. Soberón, I. Salazar, & J. P. Fay. 2005. Global mammal conservation: what must we manage? Science 309:603-607. https://doi.org/10.1126/science.1114015 [ Links ]
5. Dawson, T. P., S. T. Jackson, J. L. House, C. Prentice, & G. M. Mace. 2011.Beyond predictions: biodiversity conservation in a changing climate. Science 332:53-58. https://doi.org/10.1126/science.1200303 [ Links ]
6. D’ Elía G. & U. F. J. Pardiñas. 2015. Subfamily Sigmodontinae Wagner, 1843. Mammals of South America, Volume 2 Rodents (J. L. Patton, U. F. J. Pardiñas & G. D’Elía, eds.). The University of Chicago Press, Chicago and London.
7. Díaz, M. M., P. Teta, U. F.J . Pardiñas, & R. Barquez. 2006. Phyllotini Vorontzov, 1959. Mamíferos de Argentina: sistemática y distribución (R. Barquez, M. Díaz & R. Ojeda, eds.). Sociedad Argentina para el Estudio de los Mamíferos, Mendoza. [ Links ]
8. Di Marco, M., C. Rondinini, L. Boitani, & K. A. Murray. 2013. Comparing multiple species distribution proxies and different quantifications of the human footprint map, implications for conservation. Biological Conservation 165:203-211. https://doi.org/10.1016/j.biocon.2013.05.030 [ Links ]
9. ESRI. 2017. Environmental Systems Research Institute. ArcGIS Desktop version 10.5.1. Redlands, CA. [ Links ]
10. Fleming, P. A., & P. W. Bateman. 2016. The good, the bad, and the ugly: which Australian terrestrial mammal species attract most research? Mammal Review 46:241-254. https://doi.org/10.1111/mam.12066 [ Links ]
11. Gaston, K. J. 1994. Rarity. Population and community biology series, vol. 13. Springer, Dordrecht. [ Links ]
12. Getis, A. & J. Ord. 1992. The analysis of spatial association by use of distance statistics. Geographical Analysis 24:189-206. https://doi.org/10.1111/j.1538-4632.1992.tb00261.x [ Links ]
13. Halffter, G. 1994. Qué es la biodiversidad? Butlletí de la Institució Catalana d’Historia Natural 62:5-14.
14. Hoffmann M. et al. 2010. The impact of conservation on the status of the World’s Vertebrates. Science 330:1503-1509.
15. Isaac, N. J. B., J. Mallet, & G. M. Mace. 2004. Taxonomic inflation: its influence on macroecology and conservation. Trends in Ecology and Evolution 19:464-469. https://doi.org/10.1016/j.tree.2004.06.004 [ Links ]
16. IUCN 2011. International Union for the Conservation of the Nature, Red List of Threatened Species. Version 2011.2. [ Links ]
17. IUCN Species Survival Commission, 2012. Guidelines for Application of IUCN Red List Criteria at Regional and National Levels: Version 4.0. International Union for the Conservation of the Nature. [ Links ]
18. Jaksic, F. M., A. Iriarte, & J. E. Jiménez. 2002. The raptors of Torres del Paine National Park, Chile: biodiversity and conservation. Revista Chilena de Historia Natural 75:449-461. https://doi.org/10.4067/s0716-078x2002000200014 [ Links ]
19. Jayat, P. J., P. E. Ortiz, R. F. González, & G. D’Elia. 2016. Taxonomy of the Phyllotis osilae species group in Argentina; the status of the “Rata de los nogales” (Phyllotis nogalaris Thomas, 1921; Rodentia: Cricetidae). Zootaxa 4083:397-417. https://doi.org/10.11646/zootaxa.4083.3.5
20. Kryštufek, B., & H. I. Griffiths. 2002. Species richness and rarity in European rodents. Ecography 25:120-128. https://doi.org/10.1034/j.1600-0587.2002.250114.x
21. Kryštufek, B., V. Vohralik, & J. Obuch. 2009. Endemism, vulnerability and conservation issues for small terrestrial mammals from the Balkans and Anatolia. Foloia Zoologica 58:291-302.
22. Lacher, T. E., W. J. Murphy, J. Rogan, A. T. Smith, & N. S. Upham. 2017. Evolution, phylogeny, ecology, and conservation of the Clade Glires: Lagomorpha and Rodentia. Handbook of mammals of the world, Volume 6: lagomorphs and rodents (D. E. Wilson, J. T. E. Lacher & R. A. Mittermeier, eds.). Lynx Ediciones, Barcelona. [ Links ]
23. Lanzone, C., C. A. Labaroni, A. Formoso, L. M. Buschiazzo, F. Da Rosa, & P. Teta. 2018. Diversidad, sistemática y conservación de roedores en el extremo sudoccidental del Bosque Atlántico Interior. Revista del Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” 20:151-164. https://doi.org/10.22179/revmacn.20.566
24. Maestri, R. & B. D. Patterson. 2016. Patterns of species richness and turnover for the South American rodent fauna. PlosOne 11: e0151895. https://doi.org/10.1371/journal.pone.0151895 [ Links ]
25. Maestri, R., L. R. Monteiro, R. Fornel, N. S. Upham, B. D. Patterson, & T. R. O. de Freitas. 2016. The ecology of a continental evolutionary radiation: Is the radiation of sigmodontine rodents adaptive? Evolution 71:610-632. https://doi.org/10.1111/evo.13155 [ Links ]
26. Mares, M. A. 1992. Neotropical mammals and the myth of Amazonian biodiversity. Science 255:976-979. https://doi.org/10.1126/science.255.5047.976 [ Links ]
27. Mazel, F. et al. 2018. Prioritizing phylogenetic diversity captures functional diversity unreliably. Nature Communications 9 (2888):1-9. [ Links ]
28. Nelson, T. A., & B. Boots. 2008. Detecting spatial hot spots in landscape ecology. Ecography 31:556-566. https://doi.org/10.1111/j.0906-7590.2008.05548.x [ Links ]
29. Nott, M. P., & S. L. Pimm. 1997. The evaluation of biodiversity as a target for conservation. The ecological basis of conservation. Heterogeneity, ecosystems, and biodiversity (S. T. A. Pickettetal, eds.). Chapmanand Hall. https://doi.org/10.1007/978-1-4615-6003-6_13 [ Links ]
30. Olson, D. M. et al. 2001. Terrestrial ecoregions of the world: a new map of life on Earth. BioScience 51:933-938. [ Links ]
31. Parada, A., G. D’Elía, & R. E. Palma. 2015. The influence of ecological and geographical context in the radiation of Neotropical sigmodontine rodents. BMC Evolutionary Biology 15:172. https://doi.org/10.1186/s12862-015-0440-z
32. Pardiñas, U. F. J., G. D’Elía, & P. Teta. 2008. Una introducción a los mayores sigmodontinos vivientes: revisión de Kunsia y descripción de un nuevo género. Arquivos do Museu Nacional, Rio de Janeiro, 66:509-594.
33. Pardiñas, U. F. J., P. Teta, D. Voglino, & F. J. Fernández. 2013. Enlarging rodent diversity in west-central Argentina: a new species of the genus Holochilus. Journal of Mammalogy 91:231-240. https://doi.org/10.1644/12-mamm-a-216 [ Links ]
34. Pardiñas, U. F. J., P. Teta, J. Salazar-Bravo, P. Myers, & C. A. Galliari. 2016a. A new species of arboreal rat, genus Oecomys (Rodentia, Cricetidae) from Chaco. Journal of Mammalogy 97:1177-1196. https://doi.org/10.1093/jmammal/gyw070 [ Links ]
35. Pardiñas, U. F. J, L. Geise, K. Ventura, & G. Lessa. 2016b. A new genus for Habrothrix angustidens and Akodon serrensis (Rodentia, Cricetidae): again paleontology meets neontology in the legacy of Lund. Mastozoología Neotropical 23:93-115. [ Links ]
36. Patton, J. L., U. F. J. Pardiñas, & G. D’Elía. 2015. Mammals of South America, Volume 2: Rodents. The University of Chicago Press, Chicago and London.
37. Pereira, H. M. et al. 2010. Scenarios for Global Biodiversity in the 21st Century. Science 330:1496-1501. [ Links ]
38. Real, R. et al. 2003. Relative importance of environment, human activity and spatial situation in determining the distribution of terrestrial mammal diversity in Argentina. Journal of Biogeography 30:939-947. https://doi.org/10.1046/j.1365-2699.2003.00871.x [ Links ]
39. Schall, J. J., & E. R. Pianka. 1978. Geographical trends in numbers of species. Science 201:679-686. https://doi.org/10.1126/science.201.4357.679 [ Links ]
40. Steppan, S. J., & O. Ramírez. 2015. Genus Phyllotis Waterhouse, 1837. Mammals of South America, Volume 2, Rodents (J. L. Patton, U. F. J. Pardiñas & G. D’Elía, eds). The University of Chicago Press Chicago and London.
41. Teta, P. et al. 2018. Lista revisada de los mamíferos de Argentina. Mastozoología Neotropical 25:163-198. https://doi.org/10.31687/saremmn.18.25.1.0.15 [ Links ]
42. Teta, P., & G. D’Elía. 2017. Taxonomic notes on the long-clawed mole mice of the genus Geoxus (Cricetidae), with the description of a new species from an oceanic island of southern Chile. Hystrix, The Italian Journal of Mammalogy, 26. https://doi.org/10.4404/hystrix-27.2-11996
43. Teta, P., C. Cañon, B. D. Patterson, & U. F. J. Pardiñas. 2017. Phylogeny of the tribe Abrotrichini (Cricetidae, Sigmodontinae): integrating morphological and molecular evidence into a new classification. Cladistics 33:153-182. https://doi.org/10.1111/cla.12164 [ Links ]
44. Teta, P., A. E. Formoso, M. N. Tammone, D. De Tommaso, F. J. Fernández, J. Torres, & U. F. J. Pardiñas. 2014. Micromammals, climate change and human impact: How much changed the communities of southern South America in the last 500 years? Therya 5:7-38. [ Links ]
45. Teta, P., & U. F. J. Pardiñas. 2012. Familia Cricetidae. Libro Rojo de Mamíferos Amenazados de Argentina (R. Ojeda, V. Chillo & G. B. Diaz Isenrath, eds.). Buenos Aires, Sociedad Argentina para el Estudio de los Mamíferos. [ Links ]
46. Turvey, S. T. 2009. Holocene extinctions. Oxford University Press, Oxford. [ Links ]
Supplement 1
https://www.sarem.org.ar/wp-content/uploads/2019/08/SAREM_MastNeotrop_26-1_Formoso-sup1.doc
Fig. S1. Maps of Argentina depicting the statistical significance of “top hotspots” for sigmodontine rodents (left, species richness; right, endemism scores).














