Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Mastozoología neotropical
versão impressa ISSN 0327-9383versão On-line ISSN 1666-0536
Mastozool. neotrop. vol.26 no.1 Mendoza jun. 2019
ARTÍCULO
Armadillos as natural pests control? Food habits of five armadillo species in Argentina
Jorge A. Gallo1, 2, Laura Fasola1 and Agustín M. Abba3
1 Dirección Regional Patagonia Norte, Administración de Parques Nacionales (APN), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), S. C. de Bariloche, Argentina [Correspondence: Jorge A. Gallo <jorge-gallo@hotmail.com>].
2 Programa de Estudios Aplicados a la Conservación, Parque Nacional Nahuel Huapi (CENAC-PNNH-CONICET), S. C. de Bariloche, Argentina.
3 Centro de Estudios Parasitológicos y de Vectores (CEPAVE), Facultad de Ciencias Naturales y Museo (UNLP), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), La Plata, Argentina.
ABSTRACT
Armadillos are among the most common mammals in agroecosystems in Argentina. Their insectivorous/omnivorous food habits raise the question about their putative role as pest controllers. The aim of this study is to describe the prey items of five armadillo species and evaluate their possible role as natural pest controllers. The stomach contents of 12 Dasypus hybridus, 10 Chaetophractus vellerosus, 14 Chaetophractus villosus, 4 Tolypeutes matacus and 9 Zaedyus pichiy were analyzed. We described the diet and identified prey items to family level, whenever possible, and computed the frequency of occurrence (FO) and relative abundance of prey items. With these values, the Item Categorization Index (ICI) was calculated to classify the items in order of importance (primary, secondary and tertiary items). Shannon diversity index was also computed. The armadillos studied here consumed arthropod species belonging to families of phytosanitary importance such as Acrididae, Scarabaeidae, Tenebrionidae, Lepidoptera, Formicidae, and Termitidae. Only two armadillos showed primary items in their diet; Z. pichiy (ants and tenebrionids) and T. matacus (termites and scarabid larvae). Important pest arthropods had FO values close to or greater than 50% in the diet of all armadillos. Armadillos consumed arthropods that are important pests for agriculture, forestry, and wood construction. Even though this study was mainly focused on armadillos’ diet, we suggest that the presence of these native species could benefit the productivity and health of agroecosystems by reducing the need for harmful agrochemicals.
RESUMEN
¿Son los armadillos controladores naturales de plagas? Hábitos alimentarios de cinco especies de armadillos en la Argentina.
Los armadillos son algunos de los mamíferos más comunes en los agroecosistemas de Argentina. Sus hábitos alimentarios omnívoro/insectívoro sugirieron la pregunta acerca de su rol como presuntos controladores de plagas. El objetivo de este trabajo es describir la dieta de cinco armadillos y evaluar su posible rol como controladores de plagas. Se analizaron los estómagos de 12 Dasypus hybridus, 10 Chaetophractus vellerosus, 14 Chaetophractus villosus, 4 Tolypeutes matacus y 9 Zaedyus pichiy. Se identificaron los ítems presa hasta el nivel de familia cuando fue posible. Se estimó la frecuencia de ocurrencia (FO) y la abundancia relativa. Con estos valores se calculó el Índice de Categorización de Ítems (ICI) para clasificar las presas siguiendo un orden e importancia (primarias, secundarias y terciarias). También se calculó el índice de diversidad de Shannon. Los armadillos consumieron especies de artrópodos pertenecientes a familias de importancia fitosanitaria como Acrididae, Scarabaeidae, Tenebrionidae, Lepidoptera, Formicidae y Termitidae. Solo dos armadillos mostraron ítems primarios en su dieta: Z. pichiy (hormigas y tenebrionidos) y T. matacus (termitas y larvas de escarábidos). Los artrópodos plaga encontrados en la dieta de los armadillos tuvieron una FO cercana o superior al 50%. Los armadillos consumieron especies de artrópodos que son plagas importantes para la agricultura, la industria forestal y la construcción maderera. Aunque este estudio estuvo enfocado principalmente a la dieta de armadillos, sugerimos que la presencia de estas especies nativas podría beneficiar la productividad y salud de los agroecosistemas, reduciendo la necesidad de de utlizar agroquímicos perjudiciales.
Key words: Armadillos; Agriculture; Argentina; Diet; Pest arthropods.
Palabras clave: Armadillos; Agricultura; Argentina; Artrópodos plaga; Dieta.
Recibido 23 abril 2018.
Aceptado 16 agosto 2018.
Editor asociado: S. Zapata
INTRODUCTION
Armadillos are the most diverse xenarthran group with 9 genera and 20 extant species (Gardner 2005; Abba et al. 2015a). The food habits of the 9 living genera range from omnivorous-carnivorous to generalized and opportunistic insectivores (Redford 1985; McDonough & Loughry 2008).
The feeding activities of armadillos are associated to their morphological features. Their short thick limbs supplied with strong claws allow them to apply large forces when digging into the substrate, leaving simple structures within the soil known as “feeding holes” (“hozaduras” in Spanish) (Greegor 1980; Abba et al. 2005). Digging is the main activity not only to forage but also to build their nests (McDonough & Loughry 2008).
We studied the diet of five of the fifteen species of armadillos occurrying in Argentina: one of the subfamily Dasypodinae, Dasypus hybridus (southern long-nosed armadillo); three species of Euphractinae: Chaetophractus villosus (large hairy armadillo), Chaetophractus vellerosus (screaming hairy armadillo), Zaedyus pichiy (pichi); and one species of Tolypeutine Tolypeutes matacus (three-banded armadillo).
Dasypus hybridus is widely distributed in Argentina, occurrying in grassland areas with low disturbance and high vegetation cover from the northeast to the mid-east of the country. Although rare in agricultural lands, it is common in farmlands with extensive cattle ranching (Abba et al. 2007; Abba & Superina 2010). It is considered an opportunistic insectivorous with a strong tendency to myrmecophagy (Barlow 1965; Abba et al. 2011b).
Tolypeutes matacus is found from eastern Bolivia and south-western Brazil, southward through the Gran Chaco of Paraguay, to Argentina (San Luis province). This armadillo is also considered an opportunistic insectivorous and its diet varies seasonally. In winter it seems to prefer ants and termites, while in summer the most frequent food items are plants, mainly fruits. Some invertebrates, such as larvae of Coleoptera, occur throughout the year (Bolković et al. 1995; Cuellar 2008).
The two Chaetophractus species are known for its omnivorous-carnivorous habits (Redford 1985). Chaetophractus vellerosus ranges along the Chaco region of Bolivia, Paraguay and Argentina (Cuellar 2008; Abba et al. 2011a). A disjunct population occurs in eastern Buenos Aires province, Argentina. It occurs in xeric environments and grasslands, as well as in rangeland pastures and agricultural areas. In Buenos Aires province, it dwells on sandy calcareous soils and prefers grasslands with low vegetation height and high vegetation cover (Abba et al. 2011a). The big hairy armadillo, C. villosus, is present in the Gran Chaco of Bolivia, Paraguay, and Argentina as far south as Tierra del Fuego—Argentina and Chile (Gardner 2005; Poljak et al. 2007). It inhabits open areas and is frequent in agricultural lands of Buenos Aires province, even in degraded habitats (Abba et al. 2016). While there are many studies on the diet of the screaming hairy armadillo (Greegor 1980; Soibelzon et al. 2007; Abba et al. 2011a)Dasypodidae, only a few are available for the big hairy armadillo (see Casanave et al. 2003; Arriagada et al. 2017).
Zaedyus pichiy ranges from western Argentina (San Juan and La Rioja provinces) and eastern Chile south to the Strait of Magellan. This small armadillo has been previously described by Redford (1985)as a carnivore-omnivore, but Superina and Abba (2014) have shown that this armadillo feeds mainly on arthropods, small vertebrates, and plant materials, and can be regarded as an opportunistic omnivore. It occurs both in steppe and scrubland habitats, but seems to be more common in steppes (Abba et al. 2010). In Argentina, its diet is known only for Mendoza province (Superina 2007; Superina et al. 2009; Superina & Abba 2014), but reports for the Patagonian steppe, which comprises a major part of its distributional range, are lacking.
All five armadillos studied here are known to feed on insects and other arthropods, as reported in previous studies from Bolivia, Chile and Argentina (Greegor 1980; Bolković et al. 1995; Bruno & Cuéllar 2000; Superina 2007; Soibelzon et al. 2007; Abba & Cassini 2010; Abba et al. 2011b; Ciuccio 2014; Arriagada et al. 2017.
Because of the heavy damage they cause to crops, Scarabaeidae (white worms), Lepidoptera larvae (caterpillars), Orthoptera (locusts and crickets), Elateridaea (wireworms), leaf-cutter ants (Atta sp., Acromyrmex sp.) and carpenter ants (Camponotus sp.) are all considered important pests for agriculture in Argentina (Rizzo 1977; Bruno & Cuéllar 2000; Folgarait et al. 2002; Vitti et al. 2008). Cereals (wheat, soy, sunflower and corn) and pastures (Alvarado 1980; Vitti et al. 2008) are the crops most affected by these arthropods. In northern Argentina, other insects such isopterans (termites) are considered pests due to their impact on wooden structures and the forestry industry (Torales et al. 1995).
The loss of biodiversity, the dependence on non-renewable resources, and the heavy reliance on chemical fertilizers and pesticides characterize modern agroecosystems (Altieri 1999). The application of insecticides is the main control method for arthropod pests, but the social and ecological consequences of their use are raising concern. Although intended to kill pest arthropods, these chemical products can negatively impact other species, the soil and the entire agroecosystem (Devine et al. 2008). An unwanted consequence of the use of insecticides is the loss of biological control species, which can lead to the outbreak of other pests (Bohan et al. 2013). The World Health Organization (WHO) estimates that the exposure to insecticides causes about 20 000 deaths per year (Devine et al. 2008). Consumption of “organic” products promoted avoidance of products in which pesticides were applied. The use of native species as a biological control method is thus a strategy with important economic value for both farmers and society (Rusch et al. 2016).
All armadillos species studied in this work are present in agricultural lands (Abba & Superina 2010; Noss et al. 2014; Abba et al. 2016). In these degraded habitats numerous pest arthropods cause problems to human resources. It is estimated that 25-50% of the world’s crops are destroyed by herbivorous arthropods; a situation that is increasing with current climate change (Pimentel et al. 1991; Murrell 2017). Here, we describe the diet of five armadillo species, to familylevel whenever possible, provide new information on the species’ biology and assess their potential role as pest controllers based on the composition of their diets.
MATERIAL AND METHODS
Study area
Forty-nine stomach samples were collected for parasitological research (see Ezquiaga & Navone 2014; Ezquiaga et al. 2012, 2013, 2017) in three regions of Argentina: Pampas (n = 29), Chaco (n = 7) and Patagonian Steppe (n = 13); see Fig. 1. Eleven D. hybridus, 8 C. vellerosus, and 10 C. villosus were collected in the Pampas region of Buenos Aires. This region, which was originally covered by natural grassland, is today an extensive and productive flat plain, dominated by wheat-soybean relay cropping, maize, and sunflower plantations (FAO 2004; Paruelo et al. 2005; Abba et al. 2015b). In the Chaco region, 4 T. matacus, 2 C. vellerosus and 1 D. hybridus were collected. The Chaco was originally dominated by forests of quebracho (Schinopsis sp.) and algarrobo (Prosopis sp.). This region is today a heavily modified landscape with degraded subtropical forests and patches of natural grasslands, cereal, pasture crops and some cotton plantations (Riveros 2002; Paolasso et al. 2012). Forestry is an important activity in this region. Lastly, in the Patagonian steppe, 4 C. villosus and 9 Z. pichiy were collected. Vascular plants, grouped in shrubs, grasses, forbs with patches of extensive sheep farming, forestry and fruit plantations, characterize this region (Aguiar et al. 1996; Cingolani et al. 2008; Clausen et al. 2008).
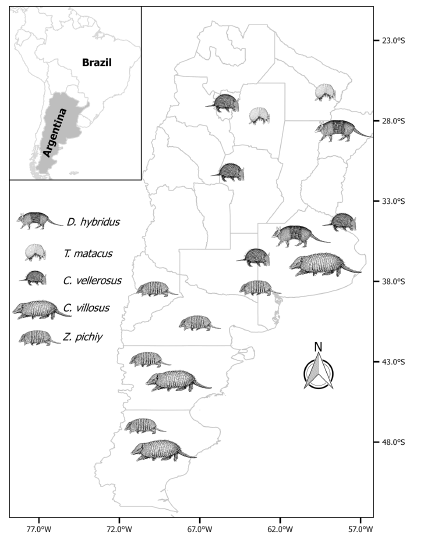
Fig. 1. Regions where armadillos samples were collected. Illustrations modified from Díaz and Barquez (2002) and Parera & Erize (2002).
Stomach content analysis
Stomachs were fixed in 10% formaldehyde. Stomach contents were sifted using a 0.1-cm2 sieve and placed in a petri dish for examination under a stereoscopic microscope. Particles < 0.1 cm2 in diameter were considered sediment. Prey items were identified to family level, whenever possible, by consulting specialists and specific bibliography (Alvarado 1980; Stehr 1987, 1991).
Statistical analysis
A relative abundance scale (A) ranging between Null (~ 0%), Very Rare (~ 5%), Rare (~ 10-20%), Common (~ 20%-40%), Abundant (~ 40%-60%) and Very abundant (~60% -100%) was used to quantify the abundance of different prey items. Frequency of Occurrence (FO; Gallina-Tessaro 2011) was estimated as the percentage of stomachs in which each item appears. Diet diversity was expressed using the Shannon-Weaver index (H’, Magurran 1988). In addition, we computed the Item Categorization Index (ICI, Grosman 1995) as ICI = and ranked food items as Primary (ICI>10), Secondary (ICI = 5-10), Tertiary (ICI=1-5) or Accidental (ICI<1). This index integrates all previous metrics, (Grosman 1995; Mancini & Grosman 1998; Grosman et al. 2001, 2002). We conducted a Chi-square independence test in R (R Core Team 2016) to test for differences in FOs for each prey item between the five armadillos. We excluded T. matacus because of low sample size (n = 4).
RESULTS
Thirty-six different food items were found in the diet of the 5 armadillo species (Table 1). These included 19 insect families from 8 orders; 1 family of Isopoda, 2 Arachnid orders, 1 Annelid order, 1 class of Myriapod, 3 families of Amphibians, 1 Lepidosauria, plant material, bird eggshell fragments and carrion. There were differences in FOs for the different prey items among species of armadillos (Fig. 2, χ2 =18.04, p = 0.034).
Table 1
Values of the Item Categorization Index (in parentheses) for food items found in this study. Letters indicate previous studies where diets of armadillos were described. a: Abba et al. 2010; b: Ciuccio 2014; c: Greegor 1980; d: Soibelzon et al. 2007; e: Abba et al. 2011; f: Arriagada et al. 2017; g: Bolkovic et al. 1995; h: Bruno & Cuellar 2000; i: Superina 2007.


Fig. 2. Principal food items of five armadillo species of Argentina. (L): Larvae; (A): Adult. Illustrations modified from Díaz & Barquez (2002) and Parera & Erize (2002).
Dasypus hybridus showed the most diverse diet, with 27 food items and H’ = 4.0, including only arthropods and plant material. The most frequent items were ants (Hymenoptera: Formicidae) with 91.7% of occurrences, acridids (Orthoptera: Acrididae) and scarabid larvae (Coleoptera: Scarabaeidae), both with 50% of occurrences (Fig. 2). ICI values indicate that these are tertiary food items (Table 1).
Chaetophractus vellerosus consumed 16 different food items (H’ = 3.04). Plant material (fruits, seeds, roots and leaves fragments) and tenebrionids (Coleoptera: Tenebrionidae) were the food items most consumed by this armadillo (both with FOs at 70%). Scarabid larvae, lepidopteran larvae and vertebrate carrion (muscular tissue and mammal fur) were also common items in the diet of C. vellerosus (Fig. 2). For this armadillo, ICI values indicate that these are tertiary food items (Table 1).
Chaetophractus villosus consumed mainly vertebrates (91.7%), plant material (54.5%) and coleopterans including scarabid larvae and carabid adults (both with FOs ~ 45%, Fig. 2). Its diet comprised 16 different prey items (H’ = 0.297), and included vertebrate remains of 3 different amphibian families (Leptodactylidae, Bufonidae, and Odontophrynidae) as well as bird eggshell fragments—C. villosus was the only armadillo studied here that included these last items. ICI values for plant material and carrion were the highest for this armadillo (Table 1).
Tolypeutes matacus included 8 different food items, H’ = 1.4. All stomachs had scarabid adults and termites (Isoptera: Termitidae) both with FOs at ~ 50% (Fig. 2). According to ICI values, none of the food items were occasional or accidental, scarabid larvae were a primary item, termites were secondary and the rest were tertiary (Table 1).
Finally, Zaedyus pichiy included 14 different food with H’ = 3.3. As shown in Table 1, FOs for scarabid larvae, tenebrionids, acridids, and ants were greater than 50% (Fig. 2), and all considered as secondary with regard to their ICI values. Lepidosauria was exclusively found in the diet of this armadillo.
DISCUSSION
Numerous insectivorous and omnivorous vertebrates such as seagulls, owls, bats and shrews are considered as pest controllers (see Buckner 1966; Ghys & Favero 2004; Biondi et al. 2005; Douglas 2008; Carevic 2011; Guevara & Sainoz Aguirre 2012) and armadillos are not the exception. Although Soibelzon et al. (2007) suggested that armadillos could be considered as pest controllers, studies on their diet preferences to evaluate this prospect are lacking. Our analyses filled a gap regarding the diet composition of five species of armadillos in different regions of Argentina. The regular consumption of pest arthropods by armadillos could change human perception about their ecological role in agroecosystems.
Dasypus hybridus is classified as an opportunistic insectivore (Redford 1985) and our results agree with this classification. Insects are the most common prey in the diet of this armadillo and judging from the ICI values obtained there is no specialization for any item. Furthermore, the high FO for ants (91%) was previously reported by Abba et al. (2011b), who suggested that this species tends to myrmecophagy.
The other opportunistic insectivore is Tolypeutes matacus (Redford 1985, Bolkovic et al. 1995). The Shannon-Weaver index showed low dietary diversity. We found that T. matacus stomachs collected during winter had only insects, in agreement with previous studies that found also insects as the preferred prey during winter, while plants were suggested as the main source of energy in summer (see Bolković et al. 1995). Considering the ICI values, the primary item in T. matacus diet is scarabid larvae (with 100% occurrence, as high as to 350 larvae found in an individual’s stomach). However, to rule out opportunistic feeding more information is needed. Termites followed in importance with FO at 50% and with a secondary ICI categorization.
The two omnivorous-carnivorous Chaetophractus species(Redford 1985) studied here showed similarities in the type of prey items consumed. H’ values indicates that C. vellerosus has the most diverse diet. All frequent food items had tertiary ICI categorization, suggesting an opportunistic foraging habit. The case of C. villosus is similar. Even though this armadillo has been described as an opportunist, very high ICI values for carrion and plant material, suggest that these items are by far more important than tertiary ones. Previous studies on the diet of C. villosus considered findings of vertebrate as carrion (see Arriagada et al. 2017). However, we here found entire amphibians and bird eggshells in the stomach contents, confirming that this armadillo actively preys upon vertebrates. This behavior has been reported before for other species of armadillos: Euphractus sexcinctus (Bezerra 2001; Foster et al. 2017) and D. novemcinctus, (Nesbitt et al. 1977; Staller et al. 2005; Rader et al. 2007).
The most recent study on the diet of Zaedyus pichiy in Argentina (Superina et al. 2009) described this armadillo as an opportunistic omnivore, consumer of invertebrates, plant material and carrion. We considered that plant material was consumed accidentally as it only occurred in one of the analyzed stomachs from northern Patagonia. This contrasts with reports from Mendoza province (Superinaet al. 2009), where plant materials were found in all of the samples. Our results showed that Z. pichiy relies on invertebrates and carrion in their southern distribution, and on account of this it has to be considered as an opportunistic-carnivore, at least in the Patagonian steppe. Only Z. pichiy and both Chaetophractus species included carrion and vertebrate in their diets. ICI value for ants, mainly of the genera Solenopsis and Camponotus, was the highest and exceeded that recorded in D. hybridus, known for its tendency to myrmecophagy (Abba et al. 2011b)
According to the Integrated Pest Management Program (FAO-UN) and the Argentine National Pest Surveillance and Monitoring System, the arthropod groups of phytosanitary importance consumed by armadillos are Acrididae, Scarabaeidae, Tenebrionidae, Lepidoptera, Formicidae, and Termitidae. All these families had a high frequency of occurrence and ICI values in the diet of armadillos, and thus can be disregarded as accidental or occasional prey. All produce heavy damage on crops, pastures and other agricultural activities. Tenebrionid larvae and adults are phytophagous, feeding on roots, seeds, and foliage (Boito et al. 2009). Acridide adults and nymphs are herbivorous feeding on foliage of crops and forage (De Wysiecki & Sánchez 1992; Luiselli et al. 2002). Lepidopteran larvae are herbivorous, feeding on foliage of corn and soybean, two of the main crops in Argentina (Sagadin & Gorla 2002; Riquelme Virgala et al. 2006). Scarabid white grubs are also considered to be an important pest. These hypogeal larvae feed on roots of forage, annual crops and fruit trees (Rodríguez et al. 2004). Insecticides are the principal control method for these insects, but movement of the larvae inside the soil reduces their effectiveness (Villani et al. 1990). In Chaco province, northeastern Argentina, termite infestations can be a problem in the forestry industry as well as in human dwellings made of wood (Torales et al. 1995). Leafcutter ants are considered one of the most important pests of agriculture and forestry in all subtropical South America (Hölldobler & Wilson 1990). The harmful effects of ants genera Atta and Acromyrmex led governments to declare them as pests in 1917 (Daguerre 1945). These leaf cutter ants produce negative impacts by modifying plant communities and soil structure. They also assist in the dispersion of agricultural weeds and exotic plant species (Farji-Brener 1992). Other ants of economic importance in the Argentinean agroecosystem are the carpenter ants of the genus Camponotus. Although they do not represent a direct hazard to crops, as they are neither herbivores nor granivores (Fernandez 2003), they build large hard-packed soil nest mounds and farmers must incur high economic costs to destroy them (Folgarait et al. 2002, 2004).
Additionally, other arthropods such as arachnids, carabid coleopterans and staphylinid insects had low FO and ICI values. These invertebrates are considered important in agroecosystems as predators of other insect pests (Murrell 2017). Foraging by these armadillos thus do not affect other species of natural controllers.
In view of the high degree of toxicity and loss of diversity generated by agrochemicals used to exterminate arthropod pests, the presence of opportunistic vertebrates, such as armadillos, as putative pest controllers should be considered as a valuable characteristic of agroecosystems.
ACKNOWLEDGMENTS
We would like to thank G. T. Navone, M. C. Ezquiaga and T. A. Rios for providing the stomach samples, to L. G. Pagano for his valuable help in the field and to J. Barneche for his help with identifying arthropods. We appreciate the improvements in English usage made by R. Wilcox.
LITERATURE CITED
1. Abba, A. M., E. Zufiaurre, M. Codesido, & A. D. N. Bilenca. 2016. Habitat use by armadillos in agroecosystems of central Argentina: does plot identity matter? Journal of Mammalogy 20:1-7. https://doi.org/10.1093/jmammal/gyw100 [ Links ]
2. Abba, A. M., G. H. Cassini, G. Valverde, M. K. Tilak, S. F. Vizcaíno, M. Superina, & F. Delsuc. 2015a. Systematics of hairy armadillos and the taxonomic status of the Andean hairy armadillo (Chaetophractus nationi). Journal of Mammalogy 96:673-689. https://doi.org/10.1093/jmammal/gyv082 [ Links ]
3. Abba, A. M., E. Zufiaurre, M. Codesido, & D. N. Bilenca. 2015b. Burrowing activity by armadillos in agroecosystems of central Argentina: Biogeography, land use, and rainfall effects. Agriculture, Ecosystems and Environment 200:54-61. https://doi.org/10.1016/j.agee.2014.11.001 [ Links ]
4. Abba, A. M., G. H. Cassini, M. H. Cassini, & S. F. Vizcaíno. 2011a. Historia natural del piche llorón Chaetophractus vellerosus (Mammalia: Xenarthra: Dasypodidae). Revista Chilena de Historia Natural 84:51-64. https://doi.org/10.4067/S0716-078X2011000100004 [ Links ]
5. Abba, A. M., G. H. Cassini, & F. C. Galliari. 2011b. Nuevos aportes a la historia natural de la mulita pampeana Dasypus hybridus (Mammalia, Dasypodidae). Iheringia, Série Zoologia 101:325-335. https://doi.org/10.1590/S0073-47212011000300007 [ Links ]
6. Abba, A. M., & M. H. Cassini. 2010. Ecological differences between two sympatric species of armadillos (Xenarthra, Mammalia) in a temperate region of Argentina. Acta Theriologica 55:35-44. https://doi.org/10.4098/j.at.0001-7051.083.2008 [ Links ]
7. Abba, A. M., M. J. Nabte, & D. E. U. Sauthier. 2010. New data on armadillos (Xenarthra: Dasypodidae) for Central Patagonia, Argentina. Edentata 11:11-17. https://doi.org/10.1896/020.011.0103 [ Links ]
8. Abba, A. M., & M. Superina. 2010. The 2009 / 2010 Armadillo Red List Assessment. Edentata 11:135-184. [ Links ]
9. Abba, A. M., S. F. Vizcaíno, & M. H. Cassini. 2007. Effects of land use on the distribution of three species of armadillos in the Argentinean Pampas. Journal of Mammalogy 88:502-507. https://doi.org/10.1644/06-MAMM-A-006R1.1 [ Links ]
10. Abba, A. M., D. E. Udrizar, & S. F. Vizcaíno. 2005. Distribution and use of burrows and tunnels of Chaetophractus villosus (Mammalia, Xenarthra) in the Eastern Argentinean pampas. Acta theriologica 50:115-124. https://doi.org/10.1007/BF03192624 [ Links ]
11. Aguiar, M. R., J. M. Paruelo, O. E. Sala, & W. K. Lauenroth. 1996. Ecosystem responses to changes in plant functional type composition: An example from Patagonian steppe. Journal of vegetation science 7:381-390. https://doi.org/10.2307/3236281 [ Links ]
12. Altieri, M. A. 1999. The ecological role of biodiversity in agroecosystems. Agriculture, Ecosystems and Environment 74:19-31. https://doi.org/10.1016/S0167-8809(99)00028-6 [ Links ]
13. Alvarado, L. 1980. Sistemática y bionomía de los estados inmaduros de coleópteros Scarabaeidae que habitan en el suelo. Tesis de doctorado. Fac. Cs. Naturales y Museo. UNLP. La Plata, Argentina. [ Links ]
14. Arriagada, A., L. Baessolo, C. Saucedo, J. E. Crespo, J. Cerda, L. Parra, D. Aldridge, J. Ojeda, & A. Hernández. 2017. Hábitos alimenticios de poblaciones periféricas de Zaedyus pichiy y Chaetophractus villosus (Cingulata, Chlamyphoridae) en la Patagonia chilena. Iheringia. Série Zoologia 107:1-8. https://doi.org/10.1590/1678-4766e2017013 [ Links ]
15. Barlow, J. C. 1965. Land mammals from Uruguay: ecology and zoogeography. University of Kansas, Lawrence. [ Links ]
16. Bezerra, A. M. R. 2001. Predation of rodents by the yellow armadillo (Euphractus sexcinctus) in Cerrado of the Central Brazil. Mammalia 65:86-88. [ Links ]
17. Biondi, L. M., M. S. Bó, & M. Favero. 2005. Dieta del chimango (Milvago chimango) durante el periodo reproductivo en el sudeste de la provincia de Buenos Aires, Argentina. Ornitología Neotropical 16:31-42. [ Links ]
18. Bohan, D. A. et al. 2013. Networking agroecology. integrating the diversity of agroecosystem interactions. Advances in Ecological Research 49:1-67. https://doi.org/10.1016/B978-0-12-420002-9.00001-9 [ Links ]
19. Boito, G. T., J. A. Giuggia, J. A. Ornaghi, U. A. Gerardo, & D. Giovanini. 2009. Uso de trampas “Barber” para determinar la diversidad de coleópteros epígeos asociados al cultivo de maní (Arachis hypogaea L.). Córdoba, Argentina. Revista de la Facultad de Ciencias Agrarias, UNCuyo 41:23-31.
20. Bolković, M. L., S. M. Caziani, & J. J. Protomastro. 1995. Food habits of the three-banded armadillo (Xenarthra: Dasypodidae) in the dry Chaco, Argentina. Journal of Mammalogy 76:1199-1204. https://doi.org/10.2307/1382612 [ Links ]
21. Bruno, N., & E. Cuéllar. 2000. Hábitos alimenticios de cinco armadillos en el chaco boliviano. Manejo de Fauna Silvestre en Amazonía y Latinoamérica 401-411. [ Links ]
22. Buckner, C. H. 1966. The role of vertebrate predators in the biological control of forest insects. Annual Review of Entomology 11:449-470. https://doi.org/10.1146/annurev.en.11.010166.002313 [ Links ]
23. Carevic, F. S. 2011. Rol del pequén (Athene cunicularia) como controlador biológico mediante el análisis de sus hábitos alimentarios en la Provincia de Iquique, norte de Chile. IDESIA 29 15:21. https://doi.org/10.4067/S0718-34292011000100003 [ Links ]
24. Casanave, E. B., M. C. Manfredi, & E. M. Luengos Vidal. 2003. Ecología comportamental de los armadillos en un pastizal serrano. Actas de las II Jornadas del SO Bonaerense. EDIUNS. [ Links ]
25. Cingolani, A. M., I. Noy-Meir, D. D. Renison, & M. Cabido. 2008. La ganadería extensiva, ¿es compatible con la conservación de la biodiversidad y de los suelos? Ecología Austral 18:253-271. [ Links ]
26. Ciuccio, M. 2014. Ecología comportamental de los dasipódidos en el pastizal pampeano, con particular consideración de los hábitos alimenticios. Enfoque eco-morfo-fisiológico. Tesis de doctorado, Universidad Nacional del Sur, Bahía Blanca, Agentina. [ Links ]
27. Clausen, A., M. Ferrer, & M. Formica. 2008. Informe nacional sobre el estado de los recursos fitogenéticos para la agricultura y la alimentacion en Argentina. FAO. [ Links ]
28. Cuéllar, E. 2008. Biology and ecology of armadillos in the Bolivian Chaco. The Biology of the Xenarthra (W. J. Vizcaíno, S. F., Loughry, ed.). University of Florida Press, Gainesville. [ Links ]
29. Daguerre, J. B. 1945. Hormigas del género Atta fabricius de la Argentina. Revista de la Sociedad Entomológica Argentina 12:438-460. [ Links ]
30. De Wysiecki L. M., & N. E. Sánchez. 1992. Dieta y remoción de forraje de Dichroplus pratensis (Orthoptera, Acrididae ) en un pastizal natural de la provincia de La Pampa, Argentina. Ecología Austral 2:19-27. [ Links ]
31. Devine, G. J., D. Eza, E. Ogusuku, & M. J. Furlong. 2008. Uso de insecticidas: contexto y consecuencias ecológicas. Revista Peruana de Medicina Experimental y Salud Publica 25:74-100. [ Links ]
32. Díaz, M. M., & R. M. Barquez. 2002. Los mamíferos de Jujuy, Argentina. LOLA (Literature of Latin America). Buenos Aires. [ Links ]
33. Douglas, J. 2008. Larvas de Lygirus villosus (COLEOPTERA: SCARABAEIDAE) en la dieta de la gaviota cáhuil (Larus maculipennis) (LARIDAE), en un valle interior de la región del Maule, Chile. Boletín chileno de ornitología 14:112-115. [ Links ]
34. Ezquiaga, M. C., T. A. Rios, A. M. Abba, & G. T. Navone. 2017. A new Rictulariid (Nematoda: Spirurida) in Xenarthrans from Argentina and new morphological data of Pterygodermatites (Paucipectines) Chaetophracti. Journal of Parasitology 103:727-735. https://doi.org/10.1645/16-74 [ Links ]
35. Ezquiaga, M. C., & G. T. Navone. 2014. A new species of Moennigia (Trichostrongylina: Molineidae) a parasite of Chaetophractus spp. (Xenarthra: Dasypodidae) from Argentina. Journal of Parasitology 100:500-503. https://doi.org/10.1645/13-337.1 [ Links ]
36. Ezquiaga, M. C., & G. T. Navone. 2013. Trichostrongylina parasites of Dasypodidae (Xenarthra) from Argentina: A new species of Macielia (Molineidae: Anoplostrongylinae) in Chaetophractus vellerosus and redescription of Trichohelix tuberculata. Journal of Parasitology 99:821-826. https://doi.org/10.1645/13-200.1 [ Links ]
37. Ezquiaga, M. C. Digiani, & G. T. Navone. 2012. A new Molineid (Nematoda: Trichostrongylina) parasite of Dasypus hybridus (Xenarthra: Dasypodidae) from Argentina. Journal of Parasitology 98:1156-1160. https://doi.org/10.1645/GE-3110.1 [ Links ]
38. FAO. 2004. Uso de fertilizantes por cultivo en Argentina. Roma. http://www.fao.org/tempref/agl/agll/docs/fertuseargent_s.pdf [ Links ]
39. Farji-brener, A. G. 1992. Modificaciones al suelo realizadas por hormigas cortadoras de hojas (Formicidae, Attini): Una revisión de sus efectos sobre la vegetación. Ecología Austral 2:87-94. [ Links ]
40. Fernandez, F. 2003. Introducción a las hormigas de la región neotropical. Instituto de Investigación de Recursos Biológicos Alexander von Humboldt, Bogotá, Colombia. [ Links ]
41. Folgarait, P. J., P. D. Adamo, & L. E. Gilbert. 2004. A grassland ant community in Argentina: the case of Solenopsis richteri and Camponotus punctulatus (Hymenoptera: Formicidae) attaining high densities in their native ranges. Annals of the Entomological Society of America 97:450-457. [ Links ]
42. Folgarait, P., S. Perelman, N. Gorosito, R. Pizzio, & J. Fernandez. 2002. Effects of Camponotus punctulatus ants on plant community composition and soil properties across land-use histories. Plant Ecology 163:1-13. https://doi.org/10.1023/A:1020323813841 [ Links ]
43. Foster, V. C., G. Porfirio, D. Viana, P. Sarmento, & E. Fischer. 2017. Yellow armadillos (Euphractus sexcinctus) can predate on vertebrates as large as a chicken. Mammalia 81:319-322. [ Links ]
44. Gallina-Tessaro, S. 2011. Técnicas para conocer la dieta. Manual de técnicas para el estudio de la fauna. Volúmen I. (S. Gallina & C. López-González, eds.). Universidad Autónoma de Querétaro-Instituto de Ecología, Querétaro, México. [ Links ]
45. Gardner, A. L. 2005. Order Cingulata. Mammal species of the world: a taxonomic and geographic reference (D. E. Wilson & D. M. Reeder, eds.). Johns Hopkins University Press, Baltimore, Maryland. [ Links ]
46. Ghys, M. I., & M. Favero. 2004. Espectro trófico de la gaviota capucho café (Larus maculipennis) en agroecosistemas del sudeste de la provincia de Buenos Aires, Argentina. Ornitologia Neotropical 15:493-500. [ Links ]
47. Greegor, D. H. 1980. Diet of the little hairy armadillo, Chaetophractus vellerosus, of northwestern Argentina. Journal of Mammalogy 61:331-334. https://doi.org/10.2307/1380058 [ Links ]
48. Grosman, F., G. González, P. Sanzano, & D. Agüería. 2002. Alimentación, nichos tróficos y competencia interespecífica de peces de la laguna de Monte, Argentina. I Congreso Iberoamericano Virtual de Acuicultura. [ Links ]
49. Grosman, F., P. Sanzano, D. Agüería, & G. Gonzales. 2001. Gestión del pejerrey Odontesthes bonariensis en una pesquería periurbana de Argentina. AquaTIC 14:1-12. [ Links ]
50. Grosman, M. F. 1995. Variaciones estacionales en la dieta del pejerrey (Odonthes bonariensis). Revista de la Asociación de Ciencias Naturales del Litoral 26:9-18. [ Links ]
51. Guevara, L., & A. Sainoz Aguirre. 2012. Murcielagos controladores de plagas agricolas. ContactoS 83:29-35. [ Links ]
52. Hölldobler, B., & E. O. Wilson (Eds.). 1990. The ants. Harvard Univ. Press, Cambridge, Massachusetts. https://doi.org/10.1007/978-3-662-10306-7 [ Links ]
53. Luiselli, S., L. Beltrame, S. Zequin, S. Simioni, & C. Salto. 2002. Ciclo ninfal de tucuras (ORTHOPTERA: ACRIDIDAE) en agroecosistemas del centro oeste de Santa Fe y centro este de Córdoba. Revista FAVE - Ciencias Agrarias 1:37-45. https://doi.org/10.14409/fa.v1i1.56 [ Links ]
54. Magurran, A. E. 1988. Ecological diversity and its measurement. Princeton University Press. [ Links ]
55. Mancini, M., & F. Grosman. 1998. Aspectos poblacionales del pejerrey Odontesthes bonariensis en el Embalse Río Tercero, Córdoba. Natura Neotropicalis 29:137-143. [ Links ]
56. McDonough, C. M., & W. J. Loughry (Eds.). 2008. Behavioral ecology of armadillos. The Biology of the Xenarthra 26:281-293. [ Links ]
57. Murrell, E. G. 2017. Can agricultural practices that mitigate or improve crop resilience to climate change also manage crop pests? Current Opinion in Insect Science 23:81-88. https://doi.org/10.1016/j.cois.2017.07.008 [ Links ]
58. Nesbitt, S. A., W. M. Hetrick, L. E. Williams, & D. A. Austin. 1977. Foods of the nine-banded armadillo in Florida. Proceedings of the Annual Conference of the Southeastern Association of Fish & Wildlife Agencies. [ Links ]
59. Noss, A., M. Superina, & A. M. Abba. 2014. Tolypeutes matacus. The IUCN Red List of Threatened Species 2014. The IUCN Red List of Threatened Species 2014. [ Links ]
60. Paolasso, P., J. Krapovickas, & N. I. Gasparri. 2012. Deforestación, expansión agropecuaria y dinámica demográfica en el chaco seco argentino durante la década de los noventa. Latin American Research Review 47:35-63. https://doi.org/10.1353/lar.2012.0009 [ Links ]
61. Parera, A., & F. Erize. 2002. Los mamíferos de la Argentina y la región austral de Sudamérica. Editorial El Ateneo, Buenos Aires. [ Links ]
62. Paruelo, J., J. Guerschman, & S. Verón. 2005. Expansión agrícola y cambios en el uso del suelo. Ciencia hoy 15:14-23. [ Links ]
63. Pimentel, D. et al. 1991. Environmental and economic effects of reducing pesticide use. BioScience 41:402-409. https://doi.org/10.2307/1311747 [ Links ]
64. Poljak, S. et al. 2007. Un nuevo mamífero introducido en la Tierra del Fuego: el “peludo” Chaetophractus villosus (Mammalia, Dasypodidae) en Isla Grande. Revista Chilena de Historia Natural 80:285-294. https://doi.org/10.4067/S0716-078X2007000300003
65. R Core Team. 2016 R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ [ Links ]
66. Rader, M. J., T. W. Teinert, L. A. Brennan, F. Hernández, N. J. Silvy, & X. Ben Wu. 2007. Identifying predators and nest fates of bobwhites in southern Texas. Journal of Wildlife Management 71:1626-1630. https://doi.org/10.2193/2006-185 [ Links ]
67. Redford, K. H. 1985. Food habits of armadillos (XENARTHRA: DASYPODIDAE). The evolution and ecology of armadillos, sloths, and vermilinguas (G. Montgomery, ed.). Smithsonian Institution Press, Washington & London. [ Links ]
68. Riquelme Virgala, M. B. R., E. N. Botto, & C. Lafalce. 2006. Evaluación de algunos insecticidas para el control de la “polilla del tomate”, Tuta absoluta (Lepidoptera: Gelechiidae) y su efecto residual sobre el parasitoide Trichogrammatoidea bactrae (Hymenoptera: Trichogrammatidae). Revista de la Sociedad Entomológica Argentina 65:57-65.
69. Riveros, F. 2002. El Gran Chaco. Available in http://www.fao.org [ Links ]
70. Rizzo, H. F. (Ed.) 1977. Catálogo de insectos perjudiciales en cultivos de la Argentina. Hemisferio Sur, Buenos Aires. [ Links ]
71. Rodríguez, M., A. France, & M. Gerding. 2004. Evaluación de dos cepas del hongo Metarhizium anisopliae var. Anisopliae (metsh.) para el control de larvas de gusano blanco Hylamorpha legans Burm. (COLEOPTERA: SCARABAEIDAE). Agricultura Técnica 64:17-24. https://doi.org/10.4067/S0365-28072004000100002 [ Links ]
72. Rusch, A., R. Bommarco, & B. Ekbom. 2016. Conservation biological control in agricultural landscapes. Advances in Botanical Research. Academic press. [ Links ]
73. Sagadin, I. M., & D. E. Gorla. 2002. Eficiencia de captura de adultos de Lepidoptera plagas de maíz (Zea mays) y de soja (Glicine max) en trampas de luz de vapor de mercurio y de luz negra en la región central de la provincia de Córdoba (Argentina). Ecologia Austral 12:99-104. [ Links ]
74. Soibelzon, E., G. Daniele, J. Negrete, A. Carlini, & S. Plischuk. 2007. Annual diet of the little hairy armadillo, Chaetophractus vellerosus (Mammalia, Dasypodidae), in Buenos Aires Province, Argentina. Journal of Mammalogy 88:1319-1324. https://doi.org/10.1644/06-MAMM-A-335R.1 [ Links ]
75. Staller, E. L., J. P. Carroll, R. P. Thornton, W. E. Palmer, & D. C. Sisson. 2005. Identifying predators at northern bobwhite nests. Journal of Wildlife Management 69:124-132. https://doi.org/10.2193/0022-541X(2005)069<0124:IPANBN>2.0.CO;2 [ Links ]
76. Stehr, F. W. (Ed.). 1987. Immature insects. Vol I. Kendall/Hunt publishing company, Dubuque, Iowa, USA. [ Links ]
77. Stehr, F. W. (Ed.). 1991. Immature Insects.Vol II. Kendall/Hunt publishing company, Dubuque, Iowa, USA. [ Links ]
78. Superina, M. 2007. Natural history of the pichi (Zaedyus pichiy) in Mendoza Province, Argentina. Tesis de doctorado. University of New Orleans, New Orleans. [ Links ]
79. Superina, M., & A. M. Abba. 2014. Mammalian Species: Zaedyus pichiy (Cingulata : Dasypodidae ). Mammalian Species 46:1-10. https://doi.org/10.1644/905.1 [ Links ]
80. Superina, M., F. Fernández Campó N, E. L. Stevani, & R. Carrara. 2009. Summer diet of the pichi Zaedyus pichiy (Xenarthra: Dasypodidae) in Mendoza Province, Argentina. Journal of Arid Environments:1-4. https://doi.org/10.1016/j.jaridenv.2009.01.011 [ Links ]
81. Torales, G. J., E. Laffont, & A. Manuel. 1995. Infestación de construcciones por Microcerotermes strunchii Söerensen (ISOPTERA: TERMITIDAE, NASUTITERMITINAE). Revista de la Asociación de Ciencias Natirales del Litoral 26:41-48. [ Links ]
82. Villani, M. G., S. R. Krueger, & J. P. Nyrop. 1990. A case study of the impact of the soil environment on insect/pathogen interaction: scarabs in turfgrass. Use of pathogens in scarabs pest management. (T. R. Glare & T. A. Jackson, eds.). Intercept Andover, Hampshire, England. [ Links ]
83. Vitti, D., C. Salto, & M. A. Sosa. 2008. Insectos en girasol. Polinizadores, fitófagos y entomófagos. Instituto Nacional de Tecnología Agropecuaria, Rafaela, Argentina. [ Links ]














