Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Mastozoología neotropical
versión impresa ISSN 0327-9383versión On-line ISSN 1666-0536
Mastozool. neotrop. vol.26 no.1 Mendoza jun. 2019
ARTÍCULO
Activity pattern and habitat selection by invasive wild boars (Sus scrofa) in Brazilian agroecosystems.
Fernando. I. Martins1, Guilherme M. Mourão1, Zilca Campos1, Aiesca Pellegrin1 and Virgínia S. Silva2
1 Embrapa Pantanal, Rua 21 de Setembro, nº 1880, Aeroporto, CEP 79320-900, Corumbá, MS, Brazil. [Correspondence: Fernando I. Martins <fimquelonio@hotmail.com>]
2 Embrapa Suínos e Aves, Rodovia BR-153, s/n, CEP 89700-991, Concórdia, SC, Brazil.
ABSTRACT
Wild boar populations are rapidly spreading through midwest Brazil, causing crop damage, impacting biodiversity and, possibly, spreading diseases to livestock. Despite their threat, little is known about introduced populations in Brazilian agricultural landscapes. The critical lack of knowledge about basic aspects of wild boar spatial ecology led us to evaluate home range sizes, activity pattern and habitat selection in interface regions of agricultural crops and natural riparian forests. We captured and monitored seven individuals using global position system collars throughout 19 to 77 days. The home range sizes calculated by Minimum Convex Polygon and Biased Random Bridge Kernel ranged from 273 to 1253 ha (median = 497 ha) and from 129 to 779 ha (median = 235 ha), respectively. Animals were more active at night, with an activity peak during the first half of the night (between 19:00-03:00 h) and less activity during the hottest hours of day (12:00 h). Wild boars positively selected riverine forests both during the day and night. They also avoided open areas (like crops and pastures), mainly during daylight. Our study provides information to improve control plans, directing capture efforts towards periods of high activity in the preferred habitats used by wild boars in the studied region.
RESUMO
Padrão de atividade e seleção de habitat por javalis invasivos (Sus scrofa) em agroecossistemas brasileiros.
As populações de javali estão se espalhando rapidamente pelo centro-oeste do Brasil, causando dano às lavouras, impactando a biodiversidade e, possivelmente, carreando doenças para os animais de criação. Apesar do seu perigo, pouco se conhece sobre as populações introduzidas em áreas agrícolas do país. A crítica falta de conhecimentos sobre aspectos básicos da ecologia espacial dos javalis na região nos levou a capturar e monitorar sete javalis com colares equipados com sistema de posicionamento global de modo a avaliar seus padrões de atividade, características das áreas de vida e padrões de seleção de habitat em áreas de interface entre zonas agrícolas e matas ciliares. Capturamos e rastreamos os animais usando coleiras GPS entre 19 e 77 dias. As áreas de vida estimadas através dos métodos Mínimo Polígono Convexo e Kernel de Pontes Aleatórias Tendenciosas variaram de 273-1253 ha (mediana = 497 ha) e 129-779 ha (mediana = 235 ha) respectivamente. Os animais monitorados foram mais ativos no período noturno, com pico de atividade durante a primeira metade da noite (entre 19:00-03:00 h) e menor atividade nas horas mais quente do dia (12:00 h). Os javalis tiveram clara preferência por áreas de mata ciliar tanto em períodos diurnos quanto noturnos. Eles também evitaram áreas abertas (como lavouras e pastagens), principalmente no período diurno. Nosso estudo fornece informações importantes para aumentar a eficiência dos planos de controle, direcionando esforços de captura para os períodos de maior atividade e para os habitats preferidos pelos javalis na região estudada.
Key words: Agricultural landscape; GPS collar; Habitat preference; Home range.
Palavras chave: Área de vida; Coleira GPS; Paisagem agrícola; Preferência de habitat.
Recibido 13 diciembre 2017.
Aceptado 18 agosto 2018.
Editor asociado: M. L. Guichón
INTRODUCTION
Sus scrofa is one of the most prolific mammals of the world (Heise-Pavlov et al. 2009; West et al. 2009). Their free-ranging populations can increase fast when the conditions are suitable (Heise-Pavlov et al. 2009). Wild boars and their hybrids with domestic pigs cause negative effects in almost every region where feral populations are known to occur (Hegel & Marini 2013). Their negative effects are extensive and range from environmental damages to agricultural losses, and because of that, it is considered a problematic species in many countries (Hegel & Marini 2013; Cuevas et al. 2016). For the International Union for Conservation of Nature (IUCN) and the Invasive Species Specialist Group (ISSG), S. scrofa is one of the 100 species world’s worst invasive alien species. Besides, there is no sufficient number of natural predators of free-ranging wild boars in most regions outside their native range (Lowe et al. 2000).
Favorable environmental conditions, such as large food supply, may lead wild boar populations to increase uninterruptedly (Bieber & Ruf 2005). Since its diet is dominated by plant material (Schley & Roper 2003), the agroecosystems from the Brazilian midwest provide large amount of food for wild boars, including monocultures of corn (Zea mays), soybean (Glycine max) and sugarcane (Saccharum sp.). These systems also provide sheltering areas in the remnant riverine forests and its adjacent wetlands. Wild boars are expanding rapidly their distribution in the midwest Brazil. For example, in 2007 wild boars were found in rural areas of seven municipalities of the state of Mato Grosso do Sul - MS (Deberdt & Scherer 2007). However, by 2015 they already occurred in rural areas of at least 46 municipalities in MS (Pedrosa et al. 2015), a fast and unforeseen invasion in the state.
According to the local farmers, the invasion in agricultural areas of south MS occurred in the 90’s, when the first individuals escaped from a wild boar farm located at Dourados River’s margins, where pure lineages were raised for commercial purposes and started to breed with pigs that have fled the sty. The invasion in south MS clearly differs from that observed in the Pantanal, a large and pristine floodplain in the center of South America. In the Pantanal, pigs became feral about 300 years ago (Oliveira-Santos et al. 2016) and are locally known as “porco-monteiro”. To date, the Brazilian regulation makes no distinction between “pure” wild boars and hybrid lineages of feral pigs, which are subject to management for population control in Brazil (IBAMA 2013), but protect the “porco-monteiro”. Because of this, hereafter we will use “wild boars” to refer to pure wild boars and to their hybrid lineages from most of Brazil, and “porco-monteiro” to refer to feral-pigs from the Pantanal.
The space use of free ranging pigs was seldom studied in Brazil (Oliveira 2012). Recently, Oliveira-Santos et al. (2016) supplied data for spatial ecology of “porco-monteiro”, describing the influence of spatial memory on space use for animals inhabiting the Pantanal of Brazil. However, to date there is a lack of information about the space use of wild boars occurring in agricultural areas of Brazil. Information on habitat use and selection of invasive species may have direct application in management and population control (Saunders & Kay 1991).
Our main goals were to evaluate the activity patterns, home range characteristics, time budget and habitat selection by invasive wild boars living in agricultural regions of MS, midwest Brazil.
MATERIALS AND METHODS
We conducted this study in two sites (farms) in the Rio Ivinhema catchment in midwest Brazil (Fig. 1). The sites are about 23 km distant to each other: Site 1 (21º 54¢ 32² S, 54º 13¢ 14² W) is close to the Brilhante River, a tributary of the Ivinhema River and Site 2 (21º 41¢ 48² S, 54º 14¢ 12² W) is close to the Vacaria River, another Ivinhema¢s tributary. The soil is rich and the topography favors the use of agricultural machinery. Therefore, this region is an important producer of agricultural commodities, such as soybean, corn, and recently, sugarcane (Oliveira et al. 2000).

Fig. 1. Location and land use of the study sites. Upper right: South America indicating the position of the Mato Grosso do Sul state (MS) (light grey) in midwestern Brazil. Center: Rio Brilhante city in MS. Left: Satellite image of the two study sites (Universal Transverse Mercator system (UTM), zone 21k).
In the studied area there is an intersection of tropical and subtropical humid climates, according to Köppen classification. The average monthly temperature ranges from 18 to 27 ºC and the monthly mean rainfall ranges from 56 to 175 mm while the annual rainfall is about 1500 mm (Embrapa 2018). The original vegetation included a mosaic of savannas, semi-decidual forests, marshes and riverine forests. Nowadays, grain crops and sugarcane plantations dominate the landscape but strips of riverine forests and some marshes are still present, as they are protected under Brazilian Law as “Permanent Preservation Areas.”
To capture the wild boars, we used two corral traps and one box trap in each study site from October 2014 to December 2015. The traps were baited with fermented corn and inspected daily (in the morning) during 36 trapping campaigns of about 7 days each, totaling 1584 trap nights. Captured boars were chemically immobilized with an intra-muscle injection of 5.0 mg/kg of a combination of tiletamine hydrochloride and zolazepan hydrochloride (Zoletil, Virbac, Carros-Cedex, France). After immobilization the animals were sexed and classified as adults, subadults or juveniles according to teeth eruption and body weight (Matschke 1967; Anezaki 2009). Boars were weighted with 200 kg spring scale (precision 5 kg) and only animals above 20 kg were fitted with GPS collars. Handling time was less than 40 minutes and we waited for animals recovery in the proximities of the release site. This study and the handling procedures were authorized under licenses SISBIO Nº: 36636-4; 36636/5; 51134/3 of Brazilian Institute of Environment and Renewable Natural Resources and sanctioned by the Committee of Ethical Considerations and Animals Use from the Universidade Federal de Mato Grosso do Sul (CEUA-UFMS 01/2012).
We trapped wild boars for 264 days, totaling 1584 trap-nights and 56 captured individuals. Most of the captured individuals (n = 40) were piglets < 10 kg. Only 16 wild boars were large enough (25-92 kg) to fit the GPS-collars without suffocation risk. We used two types of GPS-collars: ATS collars (G2110B, Advanced Telemetry Systems, Isanti, Minnesota) and EP collars (adapted collars following Oliveira-Santos et al. 2016), using a modified commercial GPS unit used by runners attached to a VHF transmitter). Of the 16 boars fitted with GPS-collars (eight with ATS collars and eight with EP collars), only seven (four ATS collars and three EP collars) were successfully recovered in the field and provided sufficient data for spatial analysis (Table 1). The nine remaining collars were damaged by the wild boars or destroyed by hunters that are very active in this area. The main hunting method in the study area is ground shooting (with or without dogs), in some occasions hunters use cars or trucks to cover large areas.
Table 1
Summary information of the seven wild boars monitored in agroecosystems of midwest Brazil, from October 2014 to December 2015. ID = identification number of the animal; Site = Study site (see Fig. 1); Sex: M = male, F = female; W = body mass in kg; Collar = collar type: EP = assembled in Embrapa Pantanal, ATS= Advanced Telemetry Systems; Start= monitoring start date; days = number of monitoring days; fixes = number of relocations obtained from the GPS; dt = programmed time interval between relocations; CV= coefficient of variation, defined as the standard deviation of measured time interval between locations divided by the programmed dt; MCP = range (ha) estimated by the Minimum Convex Polygon; BRB-K= range (ha) estimated by the Biased Random Bridge Kernel.

We programmed the ATS collars to attempt fixes every 5 hours, with fixes attempts lasting 10 minutes, while the EP collars were programmed to acquire fixes every 5 minutes. The expected lifespan was 200 days for the ATS collars and about 100 days for the EP collars under the programmed schedules. We programmed ATS and EP collars differently because we intended to compare tracks from large and short periods. However, since the wild boars destroyed the collars in periods shorter than 100 days, this comparison was not feasible. We radiotracked the animals within the study area by car or walking with a Yagi antenna connected to a digital receiver (RS4500S, Advanced Telemetry Systems, Isanti, Minnesota). Boars’ signals were monitored once a day, in the morning, during the trapping period.
We used two different methods to estimate wild boars’ home range: the Minimum Convex Polygon with 100% of the locations (MCP 100%) (Mohr 1947), and the Biased Random Bridge Kernel with 95% of the locations (BRB 95%) (Benhamou & Cornelis 2010; Benhamou 2011). The MCP 100% is estimated by the smallest possible convex polygon encompassing all locations of an individual (Mohr 1947). This method is frequently used as home range estimator (Row & Blouin-Demers 2006; Nielsen et al. 2008; Boyle et al. 2009) and allowed comparisons of our results with previous studies. The BRB method considers the paths between locations and the locations distributions, providing a more accurate home range estimate.
We used the package adehabitatHR (Calenge 2006) of the R software (R Core Team 2017) to estimate the MCP100% and BRB 95% for the seven monitored wild boars. For the BRB estimates we defined Tmax (maximum time threshold to consider connected two successive locations) = 10 hours, Dmin (minimum distance threshold) = 50 meters and Emin (minimum GPS error) = 20 meters. We evaluated the asymptotic sizes of home ranges, considering the MCP method, using a bootstrap estimation (Kranstauber et al. 2017). The analyses of asymptotic sizes are showed in Fig. S1 (Supplementary Material 1).
To describe the hourly activity pattern of wild boars, we used the information obtained from the three boars monitored by EP collars. We could not use the information provided by ATS-collars since they were programmed to acquire fixes every five hours. Therefore, two males (individuals J 2 and J 8) and one female (individual J 5) were tracked during spring 2014 (J 2) and summer 2014-2015 (J 5 and J 8). We used the square root of the distance between locations as an index of the animal’s activity. This transformation allows to detect the slow movement usually associated with foraging animals (Attias et al. 2018) and it also resulted in residuals that conformed better to the assumption of normality. Since the time of day (TOD) has a cyclic nature, we represent the values of this variable as points on a unit circle, given by four harmonics of this value, calculated as: s1 = sine (2π*TOD/24); c1 = cosine (2π*TOD/24); s2 = sine (4π*TOD/24); c2 = cosine (4π*TOD/24) (Forester et al. 2009). Then, we used a linear mixed effects model (nlme) in the package nlme (Pinheiro et al. 2017) to adjust the activity index as a function of the harmonics (fixed variables), including the animals as a random variable. Finally, we plot the predicted output of the resulting model against TOD.
We used LANDSAT TM satellite images (30 m resolution) recorded in 2013, Google earth images, and field observations to categorize the study sites in four habitat types that we consider relevant to wild boar biology: Permanent Preservation Areas (PPA) - riverine forests, riparian zones and adjacent wetlands; Grain Crops (GC) - corn and soybean crops; Sugarcane Crops (SCC), and Pasture (P). Although GC and SCC are both crops, they were classified in distinct categories due to strong difference in dynamics and physical characteristics between them. Furthermore, soybean and corn crops occupy the same space but they are cultivated in different periods (soybean is cultivated from September to February while corn is cultivated from February to September). We classified the habitat types using Spring v.5.5.1 software (Câmara et al. 1996).
To show the time budget of wild boars, we plotted the average hourly proportion of habitat use (proportion of locations in each habitat category throughout the day) of the three wild boars monitored by the EP collars (two individuals from site 1and one from site 2, Table 1).
We understood habitat as a “category of physical environment that occurs in a determined area and that is available to an organism or its group” (Leuchtenberger et al. 2013). Studies on habitat selection commonly evaluated the habitat use and availability for some organisms (Calenge & Dufour 2006). In this study, we evaluated resource selection using design II analyses (Manly et al. 2002; Thomas & Taylor 2006). Habitat use was measured individually but habitat availability was measured at population level. We used the approach proposed by Manly et al. (2002) which use Chi-square analysis to test the hypothesis that animals are using resources in proportion to their availability and to test the hypothesis of identical use of habitat by all monitored individuals. We defined use as the location of an animal within a specific habitat category and availability as the proportion of each habitat type within each study site. Study sites were defined as rectangles that encompassed all locations of tracked animals (Thomas & Taylor 2006).
We determined whether selection was positive, negative or neutral for habitat categories using the package adehabitatHS (Calenge 2006) in the R 2.12 program (R Development Core Team 2012). Selection ratios were calculated as the ratio use/availability, and the selection at the population level was calculated by averaging individual selection ratios (Manly et al. 2002). According to Manly et al. (2002), habitat selection was evaluated based on the selection ratio (ŵ) and whether it differed significantly from 1 calculating its confidence interval (CI) and interpreting positive selection for a habitat category if the lower limit of the CI was >1, negative selection if the upper limit was <1, or neutral selection if the confidence interval contained the value 1. We used Eigen analysis of selection ratios and the graphical approach to analyze the variation in habitat selection among wild boars (Calenge & Dufour 2006). To explore differential habitat selection between day and night periods we separated the locations acquired during day (06:00-18:00 h) and night (18:01-05:59 h). We defined day and night periods according to the sunrise and sunset times recorded in the study area during the study period. For this analysis, we considered as daytime the period in which the sun was visible above the horizon, therefore, twilight periods were considered as night time.
RESULTS
Seven radio-collars were successfully recovered in the field and allowed to download series of relocations that varied from 19 to 77 days (Table 1). In total, we obtained 9881 fixes from four wild boars tracked in site 1 and three in site 2.
As expected, the home ranges estimated by the MCP 100% (median = 558 ha) were larger in average than those estimated by the BRB 95% method (median = 326 ha) (Wilcoxon signed ranks test Z= 2.19, p= 0.03) (Table 1). When we plotted the number of fixes versus cumulative home range sizes, the home range of only one wild boar (J 2) reached the asymptotic size (400 ha with 2000 fixes) (Fig. S1, SM 1). All home ranges were associated to PPA and agricultural areas (Figs. 2 and 3).
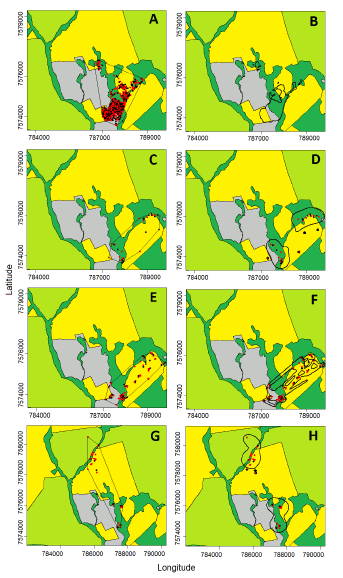
Fig. 2. Estimated ranges and relocations of four wild boars in study area 1, in midwest Brazil, from October 2014 to August 2015. Left column: ranges estimated using the Minimun Convex Polygon method. Right column: ranges estimated using the Biased Random Bridge Kernel method. Black dots represent locations taken during daylight period while red dots represent night locations. We omitted the dots in panel B to facilitate the visualization. Panels A and B refer to the subadult male J 2, panels C and D to the female J 4, panels E and F to the female J 5, and panels G and H to the male J 10 (see Table 1). Dark green represents riverine forests and their adjacent wetlands, light green represents sugarcane plantations, yellow represents grain crops, and grey represents pastures. Grids are presented in Universal Transverse Mercator system (UTM), zone 21k.
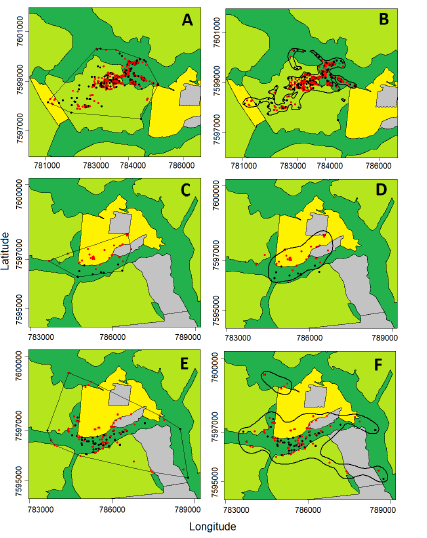
Fig. 3. Estimated ranges and relocations of three wild boars in study area 2, in midwest Brazil, from February to December 2015. Left column: ranges estimated using the Minimun Convex Polygon method. Right column: ranges estimated using the Biased Random Bridge Kernel method. Black dots represent locations taken during daylight period while red dots represent night locations. Panels A and B refer to the male J 8, panels C and D to the male J 12, panels E and F to the female J 13 (see Table 1). Dark green represents riverine forests and their adjacent wetlands, light green represents sugarcane plantations, yellow represents grain crops, and grey represents pastures. Grids are presented in Universal Transverse Mercator system (UTM), zone 21k.
All four harmonics that represented time of the day affected the activity index (t(s1) = -3.89, t(c1) = 18.81, t(s2) = -4.04, t(c2) = -6.93, df = 9545 and p < 0.001 for every harmonics). When considering all the three individuals, boars were more active at night, especially during the first half of the night, and less active during the daytime, especially at the warm hours around noon (Fig. 4).
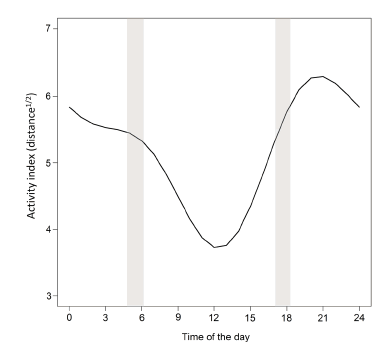
Fig. 4. Estimated activity of three wild boars, indexed by the square root of the distance moved between consecutive locations, as a function of the time of the day. These wild boars were GPS-monitored for 19-39 days from October 2014 to January 2016 in agroecosystems of Midwest Brazil. Grey bands represent the range of sunset and sunrise hours throughout the study period (see text).
Wild boars monitored with EP collars used PPA habitats (riverine forests and their adjacent wetlands) all day long, however, the use of this habitat decreased in the first half of the night, when animals used more grain crops and pasture habitats (Fig. 5). In lower proportion than PPA, sugarcane plantations were regularly used all day long.

Fig. 5. Time budget displaying the proportion of locations among four habitat categories throughout the hours of day for the three wild boars monitored with GPS collars for 19-39 days from October 2014 to January 2015 in agroecosystems of Midwest Brazil.
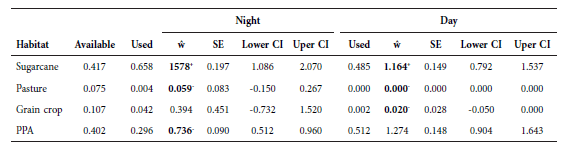
The monitored wild boars did not randomly select the habitat in proportion to their availability at both sites, both at night (study area 1: χ2 = 6956, df = 3, p < 0.001; study area 2: χ2 = 360.7, df = 3, p < 0.001) and day time (study area 1: χ2 = 9785.4, df = 3, p < 0.001; study area 2: χ2 = 344.3, df = 3, p < 0.001). The habitat selection was not identical for all animals at night (study area 1: χ2 = 123.6, df = 9, p < 0.001; study area 2: χ2 = 287.7, df = 6, p < 0.001) and day (study area 1: χ2 = 123.3, df = 9, p < 0.001; study area 2: χ2 = 89.2, df = 6, p < 0.001). The habitat availability was clearly distinct between the two study sites; the site 1 had lower availability of PPA than site 2 (Tables 2 and 3). The tracked boars showed distinct selection patterns between the sites. In the site 1, during the night time (high activity period) they positively selected PPA habitats and negatively selected SCC habitats, meanwhile, at daytime they positively selected PPA habitats and negatively selected all other habitat categories. In the site 2, during the night time, boars positively selected SCC habitats and negatively selected P and PPA habitats, meanwhile, at daytime they positively selected SCC habitats and negatively selected P and GC habitats (Tables 2 and 3).
Table 2
Estimated selection ratios (ŵ), standard errors (SE), lower and upper confidence intervals (CI) of habitat selection for wild boars in study area 1 during day and night periods. Bolded values refer to significant selection, values marked with + refer to positive selection (> 1) and values marked with – refer to negative selection (< 1), non bolded values of ŵ refer to neutral selection. Animals were monitored from October 2014 to August 2015 in agroecosystems of Midwest Brazil (Rio Brilhante city, Mato Grosso do Sul state).

Table 3
Estimated selection ratios (ŵ), standard errors (SE), lower and upper confidence intervals (CI) of habitat selection for wild boars in study area 2 during day and night periods. Bolded values ofŵ refer to significant selection, values marked with + refer to positive selection (> 1) and values marked with – refer to negative selection (< 1), non bolded values refer to neutral selection. Animals were monitored from February to December 2015 in agroecosystems of Midwest Brazil (Rio Brilhante city, Mato Grosso do Sul state).
In site 1, all boars preferred PPA habitats and the selection of this habitat category was stronger during daytime (Fig. 6). In site 2, boars selected habitat categories in different ways (Fig. 7). Two individuals (12 and 13) preferred agricultural habitats during the night and PPA habitats during daytime; meanwhile, one individual strongly selected SCC habitats both during night and daytime.
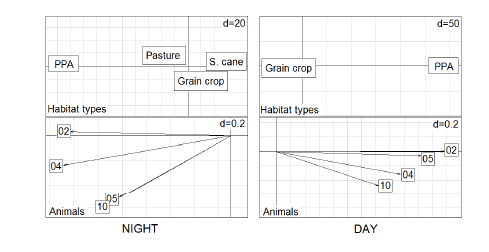
Fig. 6. Eigenanalysis of selection ratios conducted to determine day and night habitat selection by four wild boars from study area 1 considering four habitat categories in agroecosystems of Midwest Brazil (Rio Brilhante city, Mato Grosso do Sul state). Above: habitat types loadings on the first two factorial axes, which combined explain 97% of data variability for night period and 99% for day. Below: animal scores (represented as vectors) in the first factorial plane. PPA = riverine forests assigned in Brazilan law as Permanent Protected Areas; grain crop = soybean and corn crops; s.cane = sugarcane plantation. The “d” value is a parameter related to grid size inside the panels.

Fig. 7. Eigenanalysis of selection ratios conducted to determine day and night habitat selection by three wild boars from study area 2 on four habitat variables in agroecosystems from Midwest Brazil (Rio Brilhante city, Mato Grosso do Sul state). Above: habitat types loadings on the first two factorial axes, which together explain 98% of data variability for night and 99% for day. Below: animal scores (represented as vectors) in the first factorial plane. PPA = riverine forests assigned in Brazilan law as Permanent Protected Areas; grain crop = soybean and corn crops; s.cane = sugarcane plantation. The “d” value is a parameter related to grid size inside the panels.
DISCUSSION
Monitoring wild boars in the agroecosystems of midwest Brazil proved to be a challenging task. As in other studies (e.g., Singer et al. 1981; Baber & Coblentz 1986; Saunders & Kay 1991, 1996) we had to deal with the rusticity of the wild boars that often damaged the collars and with the rapid turnover of the population likely caused by a high hunting pressure, leading to short monitoring periods of the marked individuals. The short term data could have underestimated the real home ranges of wild boars in our study area. However, our estimates of home ranges, which varied from 273 to 1253 ha (MCP 100%) and 129 to 779 ha (BRB 95%), were within the range observed in previous studies. For example, a study in a tropical area developed by Oliveira-Santos et al. (2016) tracked feral pigs in the Pantanal of Brazil over 45 to 233 days and found MCP 100% home ranges ranging from 24.4 to 2388.5 ha ( = 822.8 ha), and BRB home ranges from 50.2 to 908.7 ha (![]() = 266 ha). Our home range estimations are also within values obtained for wild boars in temperate regions. In Italian Mediterranean coast, Massei et al. (1997) estimated wild boar’s MCP 100% home ranges ranging from 118 to 284 ha. Keuling et al. (2008) in a revision about females’ wild boar home ranges, pointed out that seasonal (two-three months) MCP 100% varied from 190 to 5140 ha. However, since the home range of only one of the boars we tracked reached the asymptotic size, more data are needed to accurately estimate wild boar home ranges in our study area. Furthermore, the absence of sex-related differences in boars’ home range areas in our study sites needs further investigation.
= 266 ha). Our home range estimations are also within values obtained for wild boars in temperate regions. In Italian Mediterranean coast, Massei et al. (1997) estimated wild boar’s MCP 100% home ranges ranging from 118 to 284 ha. Keuling et al. (2008) in a revision about females’ wild boar home ranges, pointed out that seasonal (two-three months) MCP 100% varied from 190 to 5140 ha. However, since the home range of only one of the boars we tracked reached the asymptotic size, more data are needed to accurately estimate wild boar home ranges in our study area. Furthermore, the absence of sex-related differences in boars’ home range areas in our study sites needs further investigation.
Although the activity pattern of free ranging wild boars varies within and among populations (e.g. Singer et al. 1981; Russo et al. 1997; Keuling et al. 2008; Oliveira-Santos et al. 2013; Morelle et al. 2015) and between sexes (Boitani et al. 1994), many authors have observed more nocturnal than diurnal movements. Usually the movements start to increase around sunset (Lemel et al. 2003; Keuling 2008; Oliveira-Santos et al. 2013) in association to foraging behavior (Boitani et al. 1994; Cahill et al. 2003). Our results also indicated an activity increase at night. According to Keuling et al. (2008), exceptionally when hunting pressure is low, diurnal activity should increase. This is not the case in our study area, where local farmers actively hunt the boars throughout the year in an attempt to protect their crops.
As in other studies, the wild boars used and positively selected the forested areas associated with riverine forests, which are close to water or food sources, and avoided open areas (Gerard et al. 1991; Dexter 1998; Thurfjell et al. 2009; Schiafini & Villa 2012; Oliveira-Santos et al. 2016). The wild boarpreference for PPA habitats likely reflects the trade-offs among thermal regulation, predation risk and forage quality. Wild boars lack sweat glands so they must rely on behavioral thermoregulation (e.g. staying in shadow areas or wallowing in mud or water) to avoid overheating (Baber & Coblentz 1986). As hunting pressure exists in our study area, boars may choose to stay in forested areas that provide shelter and reduce the chances of being shot by hunters. Finally, in our study areas PPA habitats are completely surrounded by agricultural fields where animals could easily find large amounts of food throughout the year. In fact, studies indicate that wild boar diet frequently include agricultural crops (Herrero et al. 2006; Ballari & Barrios-García 2013; Ballari et al. 2014) and we personally have observed the animals feeding on sugarcane, corn and soybean crops throughout the study period.
Wild boars used grain crops during night periods, however, no more than expected according to their availability. Conversely, they avoided the crops during day light. This is an expected pattern since wild boars tend to prevent the exposition to sun and hunters in agricultural fields (Hayes et al. 2009; Thurfjell et al. 2009). The sugarcane crop was negatively selected in site 1, regardless the period of the day. However, it was positively selected in site 2, where one individual stayed in the sugarcane plantation virtually 24 hours per day. Sugarcane was already described in wild boardiet (Ballari & Barrios-García 2013), and the physical structure of this crop provides a shelter environment, commonly used by medium size mammals in Brazil (Beca et al. 2017).
Herrero et al. (2006) suggested that the riparian zones of agroecosystems in Spain were not important foraging habitats for wild boars, but provided refuge sites adjacent to the crops, where food was abundant. Although food availability is known to limit most of wild boar populations (Matschke 1964; Baber & Coblentz 1986), the lack of shelters may retain the population growth, especially where the food is abundant.
Our study presents new information on space use and habitat selection by wild boars in agroecosystems from midwest Brazil, in a landscape dominated by monocultures of corn, soybean and sugarcane. Despite the relatively recent invasion, wild boars are spreading fast throughout Brazilian forests and agricultural zones. The efficacy of control actions in the studied region could be improved if hunters use our information to concentrate capture effort on habitats and time of the day when the animals are more vulnerable.
ACKNOWLEDGEMENTS
Special thanks to the owners of the two studied farms, its employees and to the farmers union of Rio Brilhante city. Our study received research grants from Fundação de Amparo à Pesquisa Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT; grant PRONEX- 006/2015) and Comitê Nacional de Pesquisa de Desenvolvimento Científico (CNPq), process nº 23/200.163/2014.
LITERATURE CITED
1. Anezaki, T. 2009. Estimating age at death in jomon Japanese wild boar (Sus scrofa leucomystax) based on the timing of molar eruption in recent comparative samples. Mammal Study 34:53-56. https://doi.org/10.3106/041.034.0201 [ Links ]
2. Attias, N., L. G. Oliveira-Santos, W. Fagan, & G. Mourão G. 2018. Effects of air temperature on habitat selection and activity patterns of two tropical imperfect homeotherms. Animal Behaviour 140:129-140. https://doi.org/10.1016/j.anbehav.2018.04.011 [ Links ]
3. Baber, D. W., & B. E. Coblentz. 1986. Density, home range, habitat use, and reproduction in feral pigs on Santa Catalina Island. Journal of Mammalogy 67:512-525. https://doi.org/10.2307/1381283 [ Links ]
4. Ballari, S. A., & M. N. Barrios-Garcia. 2013. A review of wild boar Sus scrofa diet and factors affecting food selection in native and introduced ranges. Mammal Review 44:124-134. https://doi.org/10.1111/mam.12015 [ Links ]
5. Ballari, S. A., M. F. Cuevas, R. A. Ojeda, & J. L. Navarro. 2014. Diet of wild boar (Sus scrofa) in a protected area of Argentina: the importance of baiting. Mammal Research, 60:81-87. https://doi.org/10.1007/s13364-014-0202-0 [ Links ]
6. Barrios-Garcia, M. N., & S. A. Ballari. 2012. Impact of wild boar (Sus scrofa) in its introduced and native range: a review. Biological Invasions 14:2283-2300. https://doi.org/10.1007/s10530-012-0229-6 [ Links ]
7. Beca, G. et al. 2017. High mammal species turnover in forest patches immersed in biofuel plantations. Biological Conservation 210:352-359. https://doi.org/10.1016/j.biocon.2017.02.033 [ Links ]
8. Benhamou, S., & D. Cornelis. 2010. Incorporating movement behavior and barriers to improve kernel home range space use estimates. Journal of Wildlife Management 74:1353-1360. https://doi.org/10.1111/j.1937-2817.2010.tb01257.x [ Links ]
9. Benhamou, S. 2011. Dynamic approach to space and habitat use based on Biased Randon Bridges. PLoSONE 6: e14592. https://doi.org/10.1371/journal.pone.0014592 [ Links ]
10. Bieber, C., & T. Ruf. 2005. Population dynamics in wild boar Sus scrofa: ecology, elasticity of growth rate and implications for the management of pulsed resource consumers. Journal of Applied Ecology 42:1203-1213. https://doi.org/10.1111/j.1365-2664.2005.01094.x [ Links ]
11. Boitani, L., L. Mattei, D. Nonis, & F. Corsi. 1994. Spatial and activity patterns of wild boars in Tuscany, Italy. Journal of Mammalogy, 75:600-612. https://doi.org/10.2307/1382507 [ Links ]
12. Boyle, S. A., W. C. Lourenço, L. R. da Silva, & A. T. S. Smith. 2009. Home range estimates vary with sample size and methods. Folia Primatologica 80:33-42. https://doi.org/10.1159/000201092 [ Links ]
13. Cahill, S., F. Llimona, & J. Gràcia. 2003. Spacing and nocturnal activity of wild boar Sus scrofa in a Mediterranean metropolitan park. Wildlife Biology 9:3-13. https://doi.org/10.2981/wlb.2003.058 [ Links ]
14. Calenge, C. 2006. The package adehabitat for the R software: a tool for the analysis of space and habitat use by animals. Ecological Modelling 197:516-519. https://doi.org/10.1016/j.ecolmodel.2006.03.017 [ Links ]
15. Calenge, C., & A. B. Dufour. 2006. Eigenanalysis of selection ratios from animal radio-tracking data. Ecology 87:2349-2355. https://doi.org/10.1890/0012-9658(2006)87[2349:EOSRFA]2.0.CO;2 [ Links ]
16. Câmara, G., R. M. C. Souza, U. M. Freitas, & J. Garrido. 1996. SPRING: integrating remote sensing and GIS by object-oriented data modelling. Journal of Computers & Graphics 20:395-403. https://doi.org/10.1016/0097-8493(96)00008-8 [ Links ]
17. Cuevas, M. F., R. A. Ojeda, & F. M. Jacksic. 2016. Ecological strategies and impact of wild boar in phytogeographic provinces of Argentina with emphasis on aridlands. Mastozoologia Neotropical 23:239-254. [ Links ]
18. Deberdt, A. J., & S. B. Scherer. 2007. O javali asselvajado: ocorrência e manejo da espécie no Brasil. Natureza & Conservação 5:31-44. [ Links ]
19. Dexter, N. 1998. The influence of pasture distribution and temperature on habitat selection by feral pigs in a semi-arid environment. Wildlife Research 25:547-559. https://doi.org/10.1071/WR97119 [ Links ]
20. Embrapa. 2008. Empresa Brasileira de Pesquisa Agropecuária, unidade Agropecuária Oeste. https://clima.cpao.embrapa.br/?lc=site/estatisticas/estatisticas [ Links ]
21. Forester, J. D., H. K. Im, & P. J. Rathouz. 2009. Accounting for animal movement in estimation of resource selection functions: sampling and data analysis. Ecology 90:3554-3565. https://doi.org/10.1890/08-0874.1 [ Links ]
22. Gerard, J-F., B. Cargnelutti, F. Spitz, G. Valet, & T. Sardin. 1991. Habitat use by wild boar in a French agroecosystem from late winter to early summer. Acta Theriologica 36:119-129. https://doi.org/10.4098/AT.arch.91-8 [ Links ]
23. Hayes, R., S. Rifftell, R. Minnis, & B. Holder. 2009. Survival and habitat use of feral hogs in Mississippi. Southeastern Naturalist 8:411-426. https://doi.org/10.1656/058.008.0304 [ Links ]
24. Hegel, C. G. Z. & Marini, M. A. 2013. Impact of the wild boar, Sus scrofa, on a fragment of Brazilian Atlantic Forest. Neotropical Biology and Conservation 8:17-24. [ Links ]
25. Heise-Pavlov, P. M., Heise-Pavlov, S. R. & Nelson, J. E. 2009. Sus scrofa: Population Structure, Reproduction and Condition in Tropical North-Eastern Australia. Acta Silvatica et Lignaria Hungarica 5:179-188. [ Links ]
26. Herrero, J., A. García-Serrano, S. Couto, V. M. Ortuño, & R. García-Gonzales. 2006. Diet of wild boar Sus scrofa L. and crop damage in an intensive agroecosystem. European Journal of Wildlife Research 52:245-250. https://doi.org/10.1007/s10344-006-0045-3 [ Links ]
27. Instituto Brasileiro de Meio Ambiente e dos Recursos Renováveis - IBAMA. 2013. Normative Instruction Nº 03/2013. Pp.: 1-4. [ Links ]
28. Kranstauber, B., M. Smolla, & A. K. Scharf. 2017. move: Visualizing and Analyzing Animal Track Data. R package version 3.0.2. https://cran.r-project.org/web/packages/move [ Links ]
29. Keuling, O., N. Stier, & M. Roth. 2008. Annual and seasonal space use of different age classes of female wild boar Sus scrofa L. European Journal of Wildlife Research 54:403-412. https://doi.org/10.1007/s10344-007-0157-4 [ Links ]
30. Lemel, J., Truvé, J. & Söderberg, B. 2003. Variation in ranging and activity behavior of European wild boar Sus scrofa in Sweden. Wildlife Biology 9:29-36. https://doi.org/10.2981/wlb.2003.061 [ Links ]
31. Leuchtenberger, C., L. G. R. Oliveira-Santos, W. Magnusson, & G. Mourão. 2013. Space use by giant otter groups in the Brazilian Pantanal. Journal of Mammalogy 94:320-330. https://doi.org/10.1644/12-MAMM-A-210.1 [ Links ]
32. Lowe, S., M. Browne, & S. Boudjelas. 2000. 100 of the world’s most invasive species: a selection from the global invasive species database. ISSG, Auckland.
33. Manly, B., L. McDonald, D. Thomas, T. MacDonald, & W. Erickson. 2002. Resource selection by animals. Statistical design and analysis for field studies. Kluwer Academic Publisher. [ Links ]
34. Massei, G., P. V. Genov, B. W. Staines, & M. L. Gorman. 1997. Factors influencing home range and activity of wild boar (Sus scrofa) in a Mediterranean coastal area. Journal of Zoology 242:411-423. https://doi.org/10.1111/j.1469-7998.1997.tb03845.x [ Links ]
35. Matschke, G. H. 1964. The influence of oak mast on European wild hog reproduction. Proceedings of the Annual Conference of Southeastern Association of Game and Fish Comissioners 18: 35-39. [ Links ]
36. Matschke, G. H. 1967. Aging European wild hogs by dentition. Journal of Wildlife Management 31:109-113. https://doi.org/10.2307/3798365 [ Links ]
37. Mohr, C. 1947. Table of equivalent populations of North American small mammals. American Midland Naturalist 37:223-249. https://doi.org/10.2307/2421652 [ Links ]
38. Morelle, K., T. Podgórski, C. Prévot, O. Keuling, F. Lehaire, & P. Lejeune. 2015. Towards understanding wild boar Sus scrofa movement: a synthetic movement ecology approach. Mammal Review 45:15-29. [ Links ]
39. Nielsen, E. B., S. Pedersen, & J. D. C. Linnell. 2008. Can minimum convex polygon home ranges be used to draw biologically meaningful conclusions? Ecological Research 23:635-639. https://doi.org/10.1007/s11284-007-0421-9 [ Links ]
40. Oliveira, C. H. S. 2012. Ecologia e manejo de javali (Sus scrofa L.) na América do Sul. Doctoral thesis. Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brasil. [ Links ]
41. Oliveira, H. de, M. A. Urchei, & C. R. Fietz. 2000. Aspectos físicos e socioeconômicos da bacia hidrográfica do rio Ivinhema. Dourados: Embrapa Agropecuária Oeste, Documentos, 25. [ Links ]
42. Oliveira-Santos, L. G. R., C. A. Zucco, & C. Agostinelli. 2013. Using conditional circular kernel density functions to test hypotheses on animal circadian activity. Animal Behaviour 85:269-280. https://doi.org/10.1016/j.anbehav.2012.09.033 [ Links ]
43. Oliveira-Santos, L. G. R., J. D. Forester, U. Piovezan, W. M. Tomas, & F. A. S. Fernandez. 2016. Incorporating animal spatial memory in step selection functions. Journal of Animal Ecology 5:516-524. https://doi.org/10.1111/1365-2656.12485 [ Links ]
44. Pedrosa, F., R. Salerno, F. V. B. Padilha, & M. Galetti,. 2015. Current distribution of invasive feral pigs in Brazil: economic impacts and ecological aspects. Natureza & Conservação 13:84-87. https://doi.org/10.1016/j.ncon.2015.04.005 [ Links ]
45. Pinheiro, J., D. Bates, S. DebRoy, D. Sarkar, & R Core Team. 2017. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-131. https://cran.r-project.org/web/packages/nlme [ Links ]
46. R Core Team. 2017. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org [ Links ]
47. Row, J. R., & G. Blouin-Demers. 2006. Kernels are not accurate estimators of home-range size for herpetofauna. Copeia 4:797-802. https://doi.org/10.1643/0045-8511(2006)6[797:KANAEO]2.0.CO;2 [ Links ]
48. Russo, L., G. Massei, & P. V. Genov. 1997. Daily home range of wild boar in a mediterranean área free from hunting. Ethology, Ecology and Evolution 9:287-294. https://doi.org/10.1080/08927014.1997.9522888 [ Links ]
49. Saunders, G., & B. Kay. 1991. Movements of feral pigs (Sus scrofa) at sunny corner, New South Wales. Wildlife Research 18:49-61. https://doi.org/10.1071/WR9910049 [ Links ]
50. Saunders, G., & B. Kay. 1996. Movements and home ranges of feral pigs (Sus scrofa) in Kosciusko National Park, New South Wales. Wildlife Research 23:711-719. https://doi.org/10.1071/WR9960711 [ Links ]
51. Schiaffini, M. I., & A. R. Vila. 2012. Habitat use of the wild boar, Sus scrofa Linnaeus 1758, in Los Alerces National Park, Argentina. Studies on Neotropical Fauna and Environment 47:11-17. https://doi.org/10.1080/01650521.2012.657916 [ Links ]
52. Schley, L., & T. J. Roper. 2003. Diet of wild boar Sus scrofa in Western Europe, with particular reference to consumption of agricultural crops. Mammal Review 33:43-56. https://doi.org/10.1046/j.1365-2907.2003.00010.x [ Links ]
53. Singer, F. J., D. K. Otto, A. R. Tipton, & C. P. Hable. 1981. Home ranges, movements and habitat use of European wild boar in Tennessee. Journal of Wildlife Management 45:343-353. https://doi.org/10.2307/3807917 [ Links ]
54. Thomas, D. L., & E. J. Taylor. 2006. Study designs and tests for comparing resource use and availability II. Journal of Wildlife Management 70:324-336. https://doi.org/10.2193/0022-541X(2006)70[324:SDATFC]2.0.CO;2 [ Links ]
55. Thurfjell, H., J. P. Ball, P-A. Ahlén, P. Kornacher, H. Dettki, & K. Sjöberg. 2009. Habitat use and spatial patterns of wild boar Sus scrofa (L.): agricultural fields and edges. European Journal of Wildlife Research 55: 517-523. https://doi.org/10.1007/s10344-009-0268-1 [ Links ]
56. West, B. C., A. L. Cooper, & J. B. Armstrong. 2009. Managing wild pigs: A technical guide. Human-Wildlife Interactions Monograph 1:1-55. [ Links ]
Supplement 1
https://www.sarem.org.ar/wp-content/uploads/2019/07/SAREM_MastNeotrop_26-1_Martins-sup1.doc
Fig. S1. Boostrap estimation of the 25%, 50%, and 75% percentile of the 100% Minimum Convex Polygon (MPC) area with increasing number of locations per calculation.














