Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Mastozoología neotropical
versión impresa ISSN 0327-9383versión On-line ISSN 1666-0536
Mastozool. neotrop. vol.26 no.1 Mendoza jun. 2019
ARTÍCULO
Macro and microhabitat patterns of habitat use and selection by wild boar in Los Alerces National Park
Antonella Panebianco1, Roberto Fabián Bó2, Pablo Francisco Gregorio1 and Alejandro Vila3
1 Grupo de Investigación en Ecofisiología de Fauna Silvestre (GIEFAS), INIBIOMA-AUSMA - CONICET - UNCo, San Martín de los Andes, Neuquén, Argentina. [Correspondence: Antonella Panebianco <apanebianco@comahue-conicet.gob.ar>]
2 Grupo de Investigaciones en Ecología de Humedales (GIEH), IEGEBA – CONICET -Departamento de Ecología, Genética y Evolución, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Ciudad Autónoma de Buenos Aires, Argentina.
3 Wildlife Conservation Society Argentina, Ciudad Autónoma de Buenos Aires, Argentina.
ABSTRACT
The wild boar (Sus scrofa) is an invasive species and currently considered as one of the most dangerous species around the world. Although its negative impacts on ecosystems have been broadly described, little is known about its patterns of habitat use in protected areas of South America. In this study, we assessed macro and microhabitat patterns of habitat use and selection of wild boar in Los Alerces National Park, Argentina, during two contrasting study periods (spring-summer and autumn-winter). We surveyed 115 sampling plots to estimate use and availability of macrohabitat and microhabitat variables. Our results showed that Lomatia hirsuta forest was selected at the macrohabitat scale in both study periods, while Nothofagus pumilio forest and Valdivian evergreen rainforest were used significantly less than their availability, and therefore, they were avoided. At a microhabitat scale, we found that wild boars used low slopes and high canopy covers. The use of L. hirsuta forest was also associated with SE exposure and intermediate proportion of ground cover. This study is one of the first attempts to assess wild boar habitat use and selection at two scales in protected areas dominated by temperate forest in Patagonia and the results obtained might be helpful to design management actions to control this invasive species and understand its ecological role.
RESUMEN
Patrones de uso y selección de macro y microhábitat del jabalí en el Parque Nacional Los Alerces.
El jabalí (Sus scrofa) es una especie invasora que actualmente está considerada como una de las especies más dañinas del mundo. Aunque sus impactos negativos en los ecosistemas han sido ampliamente descritos, poco se sabe sobre sus patrones de uso y selección de hábitat en las áreas protegidas de América del Sur. En este estudio, se evaluaron los patrones de uso y selección de hábitat del jabalí a escalas de macro y micro-hábitat en el Parque Nacional Los Alerces, Argentina, durante dos periodos de estudio contrastantes (primavera-verano y otoño-invierno). Se relevaron 115 parcelas de muestreo para estimar el uso y la disponibilidad de variables de macrohábitat y de microhábitat. Nuestros resultados mostraron que, a escala de macrohábitat, el bosque de Lomatia hirsuta fue seleccionado en ambos periodos de estudio, mientras que el bosque de Nothofagus pumilio y la selva valdiviana se usaron significativamente menos que su disponibilidad, y por lo tanto, fueron seleccionados negativamente. A escala de microhábitat, se observó que el jabalí utilizó pendientes bajas y altas coberturas de dosel. El uso del bosque de Lomatia hirsuta también se asoció con la exposición SE y una proporción intermedia de la cobertura vegetal. Este estudio representa uno de los primeros intentos por evaluar el uso y la selección de hábitat del jabalí a dos escalas en áreas protegidas dominadas por bosques templados en la Patagonia, y los resultados obtenidos pueden ser útiles para diseñar acciones de manejo tendientes a controlar a esta especie invasora y comprender su rol ecológico.
Key words: Argentina; Invasive species; Sus scrofa; Use versus availability studies; Wildlife management.
Palabras clave: Argentina; Especies invasoras; Manejo de fauna silvestre; Sus scrofa; Uso y selección de hábitat.
Recibido 2 marzo 2018.
Aceptado 3 setiembre 2018.
Editor asociado: M. L. Guichón
INTRODUCTION
The abundance and distribution of animal populations vary in space and time according to the quality and availability of resources and habitat conditions (Litvaitis et al. 1994). The resource use refers to the quantity of the resource that is utilized by an animal or population in a certain time interval, while availability is estimated as the amount of certain resource accessible to the animal or population during that period of time (Manly et al. 2002; Thomas & Taylor 2006). According to Johnson (1980), use is selective if habitats or resources are exploited disproportionately in relation to their availability. Therefore, at a given scale, to determine if a habitat or resource is selected, use should be compared to its availability. In this sense, wildlife habitat can be considered from a multiscale context as a hierarchically nested organization of conditions and resources required by an organism (Kolasa & Waltho 1998).
Selection occurs first at the level of the geographic range, then at the home-range level; third, at the level of specific sites or habitat types within a particular area, also known as macrohabitat, and finally, according to how animals choose particular components within a habitat, considered as microhabitat (Johnson 1980; Senft et al. 1987; Litvaitis et al. 1994; Manly et al. 2002). Factors that determine habitat selection within each scale of analysis can vary (Johnson 1980). For example, climatic conditions may determine geographic range, while habitat structure may influence the size and shape of the home-range. On the other hand, intraspecific competition may influence the occupied territory within the range, while food distribution and shelter become more important at lower levels or scales of selection (Litvaitis et al. 1994). Therefore, the understanding of how animals exploit a particular set of resources at different scales of analysis is essential to implement management actions that contribute to the control of invasive species (Litvaitis et al. 1994).
Biological invasions are considered one of the main threats to natural ecosystems (Vitousek et al. 1997). The wild boar (Sus scrofa) is a broad-niche species with most of the attributes of a successful invader (Oli & Dobson 2003; Bieber & Ruf 2005). Consequently, it is considered one of the “worst” invasive species (Sodeikat & Pohlmeyer 2003; Lowe et al. 2004). Several factors have been identified as affecting its abundance and distribution, including climatic and ecological factors, human activities, and topography (Jedrzejewska et al. 1997; Acevedo et al. 2006). It also represents one of the best-known cases of introduced species worldwide (Mc Graw & Mitchell 1998; Rosell et al. 2001; Novillo & Ojeda 2008; Barrios-Garcia & Ballari 2012). In Argentina, it was introduced in 1906 for hunting purposes and is currently found in a wide geographic range, occupying most of the terrestrial ecoregions in this country (Novillo & Ojeda 2008; Ballari et al. 2015). It scurrent range in Patagonia is extended from 36° to 52° S (Pescador et al. 2009), including Lanin, Nahuel Huapi, Lago Puelo, and Los Alerces National Parks (Novillo & Ojeda 2008; Pescador et al. 2009). The negative effects of wild boar on invaded habitats have been broadly reported and include changes in soil properties and nutrient dynamics (Singer et al. 1984; Cuevas et al. 2012), modification in the composition and structure of plant communities (Kotanen 1995; Siemann et al. 2009; Sanguinetti & Kitzberger 2010; Cuevas et al. 2012; Barrios-Garcia & Simberloff 2013) and predation on small vertebrates (Howe et al. 1981; Wilcox et al. 2009; Jolley et al. 2010; Barrios-Garcia & Ballari 2012).
Despite the potential impacts produced by this invasive species in protected areas, few studies on its ecology have been conducted in Argentina assessing distribution and abundance (Daciuk 1978; Bonino 1995; Jaksic et al. 2002; Merino & Carpinetti 2003; Novillo & Ojeda 2008; Pescador et al. 2009; Ballari et al. 2015). Understanding patterns of habitat use and selection by invasive species can be critical when making management and conservation decisions (Simberloff et al. 2005). However, studies assessing habitat use in temperate forests of Patagonia are also scarce (Schiaffini & Vila 2012; Gantchoff et al. 2013; Gantchoff & Belant 2015). Therefore, the aims of this study are to: 1) assess habitat use and selection of wild boar at a macrohabitat scale, and 2) determine the eventual selection of foraging sites at a microhabitat scale, on a seasonal basis, in Los Alerces National Park, Argentina.
Taking into account that wild boar requires dense canopy cover (Groves & Giles 1989; Mc Graw & Mitchell 1998) and plant species in open understory forests to feed (Skewes et al. 2007; Sanguinetti & Kitzberger 2010), it is expected that it would select this kind of habitat types and resources in the study area. Also, since ungulates of mountain environments tend to perform seasonal altitudinal movements (Rosell et al. 2001; Schiaffini & Vila 2012), it is expected that wild boar would select habitats located at low elevations during the winter and at high elevations during the summer.
MATERIALS AND METHODS
Study Site
Los Alerces National Park (LANP) was created in 1937 and covers 263 000 hectares. It is located in the Andean region, in the NW of Chubut Province, Argentina (Fig. 1). It includes two protected areas management categories: National Park and National Reserve (Martín & Chehébar 2001). The climate is temperate-cold. The mean maximum temperature in summer is 14.7 °C and the mean minimum temperature in winter is 1.8 °C (APN 1997). Mean annual precipitation decreases abruptly from west to east, from more than 3000 mm/year on the western side to 800 mm/year at the eastern border of the National Park (APN 1997). This gradient determines the distribution of xeric vegetation towards the east end and different forest formations in its western wet portion (APN 1997).
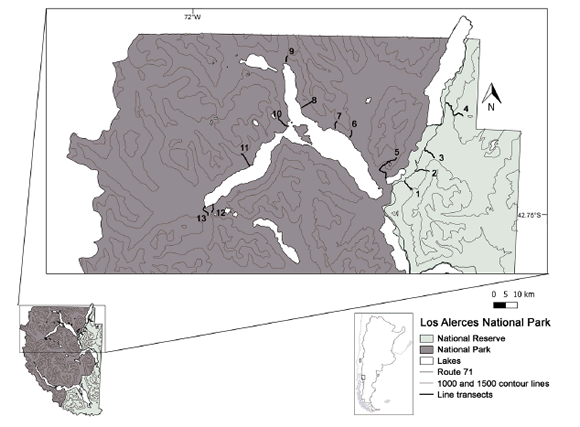
Fig. 1. Location of the study area and line transects surveyed in Los Alerces National Park (LANP) between 2011 and 2012. Transects were distributed perpendicular to contour lines, covering the following habitat types: 1) radal and ñire forests; 2) radal, maitén, and ñire forests; 3) radal and ñire forests, and high Andean steppe; 4) coihue, ñire, radal, and lenga forests; 5) coihue, radal, and lenga forests; 6), 7), 8) and 10) coihue and lenga forests; 9) and 13) Valdivian evergreen forest; 11) Valdivian evergreen and coihue forests; and 12) Valdivian evergreen, coihue, and lenga forests.
According to APN (1997), wild boar probably colonized this protected area from the north in 1931. Schiaffini and Vila (2012) indicated that they are present in 47% of the area of this national park. The study area is located in the northern sector of LANP, covering all types of habitats and management categories present in this protected area (Fig. 1). LANP encompasses two phytogeographical provinces: Subantarctic and High Andean (Cabrera 1976). Seven major habitat types were identified and described in the field, according to their altitudinal location and cover of dominant and co-dominant plant species. Coihue forest (Nothofagus dombeyi, COF), valdivian evergreen forest (VAF), maitén forest (Maytenus boaria, MAF), ñire forest (Nothofagus antarctica, NIF), radal forest (Lomatia hirsuta, RAF) and lenga forest (Nothofagus pumilio, LEF) are located in the Subantarctic portion of the study area, while the High Andean steppe (HAE) is located in the High Andean province.
In the Temperate Forests of Patagonia, temperature, light and precipitation patterns strongly influence plant physiology (Donoso Zegers 1993). Consequently, it is possible to distinguish two well-marked periods: a warm-growing season (spring-summer) and a cold-dormant season (autumn-winter). During the cold period, annual plants die and biennials and perennials cease active growth, thus deciduous plants lose their leaves and evergreens curtail all new growth (Cabrera 1976; Schmaltz 1991; Donoso Zegers 1993; Veblen et al. 1995).
Sampling design
A total of 13 line transects, both previously existing or opened especially for this study with park rangers, were distributed perpendicular to contour lines. The area covered by these transects was identified by park rangers as potentially occupied by wild boar. They ran continuously from low (500-700 m a.s.l.) to high elevation (up to 1400 m a.s.l.), covering all the available habitat types described. Their average wide and lengths were three meters and 2.73 km (range = 1.40-5.04 km) respectively. The starting points of these transects were defined at random and the minimum distance between them was one km. Every 50 m in increasing elevation, sampling plots of 3 x 5 m were placed on the line transect (N = 115) to assess biotic and abiotic habitat features along the altitudinal gradient. In each plot, we determined the habitat type present, to perform macrohabitat analysis, and evaluated elevation, aspect, slope, canopy cover, total cover of shrubs and forbs, dominant species cover, leaf litter cover, and snow cover, to carry out microhabitat analyses.
These transects were surveyed both in the warm and cold seasons to estimate the use and availability of macrohabitat and microhabitat variables between 2011 and 2012. A total of 142.16 km were surveyed during 60 days of fieldwork, 34 days during the cold season (May to August) and 25 days during the warm season (November to January). As wild boar is a secretive and shy species in forest habitats, we chose an indirect method to evaluate habitat use (Litvaitis et al. 1994). We used rooting signs as an indicator of wild boar presence and feeding activity because they can be clearly seen, are visually unmistakable in any type of substrate, and show similar detectability in all seasons (Schiaffini & Vila 2012). As was described by Kotanen (1995), most of the observed signs of rooting in our study area were not higher than 1 m2. Recent rooting activity is easily distinguishable by the lack of mosses and plant buds. Moreover, almost no debris is observed on them and remaining herbaceous vegetation is still green (Anderson & Stone 1993). Given the differences between fresh and old rooting, it was assumed that the observations recorded in each survey were not surveyed previously.
Macrohabitat analyses
We used the methodological approach proposed by Marcum and Loftsgaarden (1980) to assess macrohabitat selection. We compared the frequency of occurrence of each habitat type (estimated from the sampling plots) with the proportion of rooting activity occurring in each habitat using a χ2 test. When a significant χ2 value was obtained, the availability proportions were compared with the Bonferroni confidence intervals of use proportions to determine the selection, avoidance, or random use of each vegetation type (Neu et al. 1974; Marcum & Loftsgaarden 1980). Considering that this test is sensitive to the observer decision on which and how many habitat types are actually available, it was also conducted using both all available habitats and excluding those highly available and poorly used (Thomas & Taylor 1990).
Microhabitat analyses
We evaluated elevation, aspect, slope, and cover of canopy, shrubs and forbs, dominant species, leaf litter, and snow. These variables were measured in both sampling plots and whenever we found rooting activity. The elevation was obtained using a GPS Garmin 12 (Olathe, Kansas, USA). The slope was classified as zero (0°), low (up to 25°), medium (25°-45°) or high (45°-60°), while aspect was determined using the Google Earth program Plus 6.0.1.2032 (beta). Six pictures of canopy and understory were taken perpendicular to the ground with a Canon Power Shot A560 digital camera. These pictures were superimposed digitally with a 100 squares grid to estimate canopy, shrub and forb cover.
In those habitat types where the sampling size was appropriate, a discriminant analysis (Härdle & Simar 2015) with a progressive forward stepwise type analysis (Tobler 2002) was used to determine which combination of variables maximized the difference between used and unused sites. We first analyzed variables related to the structural attributes of the habitat type, such as elevation, slope, and aspect. In cases where there were significant differences related to vegetation cover (e.g. shrub cover), the analysis was performed again considering plant species cover.
RESULTS
A total of 166 rooting signs were found during the surveys, 69.3% of these signs were recorded during the cold season and the remaining 30.7% during the warm season. Most of the rooting activity was registered on transects located in RAF (n = 92 and 39 in the cold and warm seasons, respectively) and COF habitat types (21 signs in the cold season and 10 in the warm season), in the eastern portion of the study area.
Macrohabitat analyses
At the macrohabitat-level, we observed that the frequency of rooting signs was significantly different from the expected use of habitat types in both seasons (cold season: χ2 = 346.25, df = 6, p < 0.05; warm season: χ2 = 137.67, df = 6, p < 0.05). During the cold season, wild boar used RAF in a greater proportion than its availability, and therefore, the species selected this habitat (Table 1). By contrast, NIF, LEF and VAF were avoided (Table 1). The remaining habitat types were used in proportion to their availability (Table 1). The results obtained by applying the correction suggested by Thomas and Taylor (1990) were similar except for the COF, which was avoided by wild boar (χ2: 153.82, df = 4, p < 0.05, Fig. 2a).
Table 1
Availability of habitat types, estimated from the sampling plots and observed frequencies of wild boar use in Los Alerces National Park during both cold and warm seasons. Selection was computed using the χ2-tests and Bonferroni 95% simultaneous confidence intervals (SCI). U = Use and A = Availability.

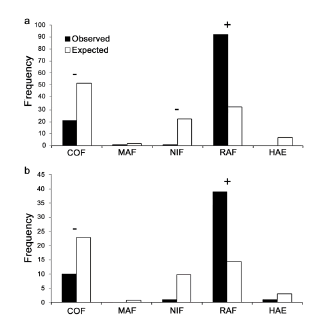
Fig. 2. Observed and expected frequency of wild boar rooting in each habitat type, excluding those highly available and poorly used during a) the cold season, and b) the warm season. BAF: coihue forest; MAF: maiten forest; NIF: ñire forest; HAE: high Andean steppe; RAF: radal forest. Habitat selection (+) and avoidance (-) are indicated above the bars.
Wild boars selected RAF during the warm season, while HAE, MAF, NIF and COF were used in proportion to its availability. In contrast, VAF and LEF were avoided (Table 1). When we used the Thomas and Taylor (1990) correction, COF was avoided and RAF was selected, while the other habitat types were used in proportion to their availability (χ2 = 58.99,df = 4, p < 0.05, Fig. 2b).
Microhabitat analyses
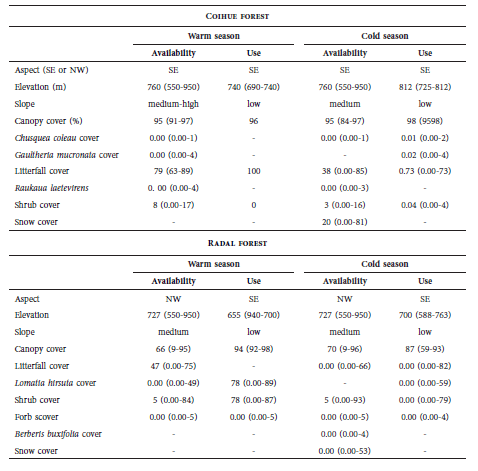
Due to the low number of signs registered in most of the habitat types (Table 1), only data obtained from RAF and COF were analyzed at a microhabitat-level. In these habitat types, wild boar used mid-elevations, low slopes and high canopy cover in both warm and cold seasons (Table 2). In addition, the patterns of microhabitat use were related to both low shrub and snow covers in the COF, and high radal cover in the RAF. The places where at least one sign was registered (Table 2) were also characterized by low slopes and were located at 700-800 m a.s.l. and 862-977 m a.s.l. during the cold and warm seasons, respectively.
Table 2
Summary of microhabitat variables (median and min-max) of coihue and radal forests in used and available sites by wild boar, during the cold and warm seasons, at Los Alerces National Park.
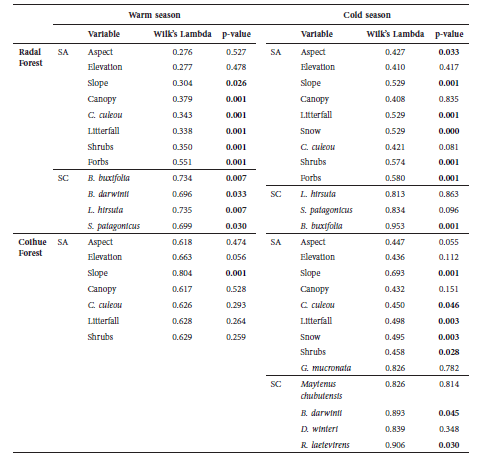
The results of the discriminant analysis showed that both RAF (cold season: Wilk’s λ = 0.41F(9.96) = 15.51, p < 0.001; warm season: Wilk’s λ = 0.27, F(8.49) = 16.24, p < 0.001) and COF (cold season: Wilk’s λ = 0.41, F(8.48) = 8.49, p < 0.001; warm season: Wilk’s λ = 0.61, F(7.45)=4.09, p < 0.001) had a combination of variables that differentiate used from not used sites. These variables were different in each season (p < 0.01 in all cases). During the cold season, the combination of variables at the RAF included southeast aspect and low slope, snow cover, vegetation cover (forbs and shrubs) and litterfall cover. Correct classification was achieved for 98.9% of the used sites and 64.2% of the unused sites. Litterfall cover was also relevant for wild boar at the COF, as well as low snow cover, low slopes and C. culeou cover. In this habitat type, correct classification was 100% and 85.7% for used and unused sites respectively.
During the warm season, the combination of variables that maximized the difference between used and unused sites at the RAF included low slopes, high canopy cover and C. culeou cover. Correct classification was achieved for 100% of the used sites and 77.7% of the unused sites. At the COF, only low slopes were significant to discriminate used and unused sites (p < 0.001).The used sites were discriminated in 100% of the cases, while those not used, in 90.9% of the cases (Table 3).
Table 3
Results from the discriminant analysis applied to the structural attributes of the habitat types and the dominant species that constitute the shrub cover in coihue and radal forests during the cold and warm seasons. p-values<0.05 were considered significant. SA: structural attributes. SC: shrub cover.
DISCUSSION
The introduction of wild boar has been recognized as a threat to the biodiversity of the temperate forests of the southern Andes (Vazquez 2002). In Argentina, several studies have shown that rooting activity reduces significantly the coverage of perennial plant species and the diversity of understory species, and increase soil compaction (Cuevas et al. 2010, 2012) and both establishment and biomass of exotic plant species (Barrios-Garcia & Simberloff 2013). According to the results obtained in this study, rooting activity could be strongly influenced by seasonality. As was observed in other protected areas in Patagonia, such as Nahuel Huapi National Park (Barrios-Garcia, pers. com.), most of the fresh rooting signs were found during the cold season. This is consistent with other studies conducted worldwide (Singer et al. 1984; Hone 1995; Welander 2000; Cahill et al. 2003)and could be explained by the differential food availability between seasons. During the cold season, wild boar feeds on roots, insects or fungi, while it probably feeds on fruits or other aerial parts of plants during the warm season (Welander 2000).
At a macrohabitat level, our results supported the hypothesis that wild boar selects forests with dense canopy cover and open understory, and low elevations during the winter. This is consistent with seasonal movements towards lower altitudes during the winter season reported by Pescador et al. (2009) in Lanin National Park, as occurs in European mountain areas (Rosell et al. 2001). Both habitat types selected and those used yearly in proportion to their availability are located in the eastern portion of the study area. Given the history of colonization of the species in the LANP, which suggests that wild boar invaded the park from its northern limit, the presence of large lakes and dense understories in forests could have limited accessibility and, consequently, invasion of the western part of the park (Orellana et al. 2013). Nevertheless, Sanguinetti & Pastore (2016) suggest that the invasion process in Argentina has not finished and maximum potential densities have not been reached yet. They also suggest that wild boar could reach one of the highest densities in the more humid forests of Patagonia (Valdivian evergreen rainforest). Furthermore, the invasion pattern in Lanin National Park followed rivers and lakes from eastern steppes to the western hillsides (Pescador et al. 2009). Gantchoff et al. (2013) and Gantchoff & Belant (2015) also showed wild boar preference for more western humid areas in Nahuel Huapi National Park, where habitat types are similar to those found in LANP and Lanin.
By contrast, in the east portion of LANP, the available network of roads and paths, as well as the presence of open understories and cleared areas for public use (De Pietri 1993; Martín & Chehébar 2001) could explain the current distribution and abundance of the species in this area. In addition, this area also contains the higher proportion of the selected habitats by wild boar (radal forests). Wild boar occurrence was equally influenced by anthropogenic and environmental factors in Nahuel Huapi National Park (Gantchoff & Belant 2015).In addition, sites closer to roads had greater numbers of species detections, suggesting that boars are positively selecting roads and possibly using them as corridors (Gantchoff et al. 2013; Gantchoff & Belant 2015). Consequently, its distribution could be facilitated through human disturbance.
The avoidance or negative selection of habitat types located at higher elevations is consistent with previous studies conducted in this National Park (Schiaffini & Vila 2012) and in Nahuel Huapi National Park (Gantchoff & Belant 2015). This could be related to adverse climatic conditions, especially during the cold season, which affects negatively the availability of food (Vila et al. 2009; Vila & Borrelli 2011) and animal movements (Rosvold & Andersen 2008).
Lower slopes could facilitate movement and foraging at microhabitat level, while higher canopy cover could provide protection from high temperatures during the warm season. Since this species does not have sweat glands (Mysterud & Ostbye 1999; Dexter 2003), the effect of the canopy could modulate its habitat use and thermoregulatory behavior (Groves & Giles 1989; Dexter 1998, 2003). In addition, this high coverage could prevent soil freezing and snow accumulation during the cold season, thus facilitating rooting activities. These results are also consistent with studies conducted in other ungulates (Huot 1974; Peek et al. 1976; Armleder et al. 1994; Mysterud et al. 1997).
Taking into account both scales of analysis, RAF selection by wild boar could be important not only for its high percentage of shrub cover but also for its species composition, richness and relative abundance of species in the understory. Although other authors have suggested that the presence of bamboo C. culeou has an important role in defining wild boar habitat use (Schiaffini & Vila 2012), our results suggest that its importance depends on the combined presence of other species, such as B. darwinii and B. buxifolia.
In addition, L. hirsuta has got proteoid roots, characterized by dense clusters that enhance nutrient uptake (Diehl et al. 2008). This kind of roots could be potentially more attractive than others (Grosfeld, pers. com.) and could represent an important food source for wild boar. In addition, litterfall layer in RAF is thicker than in other types of forests (Mazzarino, pers.com.) and has lower decomposition rates (Diehl et al. 2008). These features could determine a greater presence of insects potentially included in wild boar diet (Ballari et al. 2014).
This study is one of the first attempts to assess wild boar habitat use and selection at multiple scales and the results obtained might be helpful to design management actions to control this invasive species in temperate forests of Patagonia. The emerging recommendations of our work include: 1) developing and implementing an early detection and a rapid response plan for the species (Genovesi et al. 2010; NYSDEC 2016) in the western part of the LANP; and 2) focusing control efforts on those habitats that are selected on a yearly basis (radal forests, low to mid elevations, low slopes, high canopy cover and open understory). It should be noted that both experiences gained and lessons learnt in wild boar management in other protected areas of Argentina (Gürtler et al. 2016, 2017)could be helpful for designing a control or eradication plan of this species in LANP. This plan must include long-term monitoring of populations and control activities combining different techniques, such as those used by Gürtler et al. (2016, 2017) and Sanguinetti & Pastore (2016).
ACKNOWLEDGMENTS
The National Park Service of Argentina (APN) provided the necessary permission to work in Los Alerces National Park. We thank Los Alerces National Park for providing logistical support for fieldwork and partially funding this research. We are particularly thankful to Marcelo Pietrobón, Martín Izquierdo, Gustavo Paramosz, Iván Hoermann, Aldana Calamari y Alejandro Rey for their assistance during fieldwork. We are also grateful to Lucía Mentesana, Laura Guichón, and two anonymous reviewers for their helpful comments on earlier versions of this paper. The fieldwork was also partially supported by Wildlife Conservation Society.
LITERATURE CITED
1. Acevedo, P., M. A. Escudero, R. Muñoz, & C. Gortázar. 2006. Factors affecting wild boar abundance across an environmental gradient in Spain. Acta Theriologica 51:327-436. https://doi.org/10.1007/BF03192685 [ Links ]
2. Anderson, S. J., & C. P. Stone. 1993. Snaring to control feral pigs Sus scrofa in a remote Hawaiian rain forest. Biological Conservation 63:195-201. https://doi.org/10.1016/0006-3207(93)90712-A [ Links ]
3. APN. 1997. Plan Preliminar de Manejo. Parque Nacional Los Alerces. [ Links ]
4. Armleder, H. M., M. J. Waterhouse, D. G. Keisker, & R. J. Dawson. 1994. Winter habitat use by mule deer in the central interior of British Columbia. Canadian Journal of Zoology 72:1721-1725. https://doi.org/10.1139/z94-232 [ Links ]
5. Ballari, S. A., M. F. Cuevas, S. Cirignoli, & A. E. J. Valenzuela. 2015. Invasive wild boar in Argentina: using protected areas as a research platform to determine distribution, impacts and management. Biological Invasions 17:1595-1602. https://doi.org/10.1007/s10530-014-0818-7 [ Links ]
6. Ballari, S. A., M. F. Cuevas, R. A. Ojeda, & J. L. Navarro. 2014. Diet of wild boar (Sus scrofa) in a protected area of Argentina: the importance of baiting. Mammal Research 60:81-87. https://doi.org/10.1007/s13364-014-0202-0 [ Links ]
7. Barrios-Garcia, M. N., & S. A. Ballari. 2012. Impact of wild boar (Sus scrofa) in its introduced and native range: A review. Biological Invasions 14:2283-2300. https://doi.org/10.1007/s10530-012-0229-6 [ Links ]
8. Barrios-Garcia, M. N., & D. Simberloff. 2013. Linking the pattern to the mechanism: How an introduced mammal facilitates plant invasions. Austral Ecology 38:884-890. https://doi.org/10.1111/aec.12027 [ Links ]
9. Bieber, C., & T. Ruf. 2005. Population dynamics in wild boar Sus scrofa: ecology, elasticity of growth rate and implications for the management of pulsed resource consumers. Journal of Applied Ecology 42:1203-1213. https://doi.org/10.1111/j.1365-2664.2005.01094.x [ Links ]
10. Boitani, L., L. Mattei, D. Nonis, & F. Corsi. 1994. Spatial and activity patterns of wild boars in Tuscany, Italy. Journal of Mammalogy 75:600-612. [ Links ]
11. Bonino, N. A. 1995. Proceedings of the first international wildlife considerations. Introduced mammals in Patagonia, southern Argentina: consequences, problems and management considerations (J. A. Bissonette & P. R. Krausman, eds.). Bethesda (MD): The Wildlife Society. [ Links ]
12. Cabrera, A. 1976. Regiones fitogeográficas argentinas. Enciclopedia Argentina de Agricultura y Jardinería 2:1-85. [ Links ]
13. Cahill, S., F. Llimona, & J. Gràcia. 2003. Spacing and nocturnal activity of wild boar Sus scrofa in a Mediterranean metropolitan park. Wildlife Biology 9:3-13. https://doi.org/10.2981/wlb.2003.058 [ Links ]
14. Cuevas, M. F., L. Mastrantonio, R. A. Ojeda, & F. M. Jaksic. 2012. Effects of wild boar disturbance on vegetation and soil properties in the Monte Desert, Argentina. Mammalian Biology 77:299-306. https://doi.org/10.1016/j.mambio.2012.02.003 [ Links ]
15. Cuevas, M. F., A. Novillo, C. Campos, M. A. Dacar, & R. A. Ojeda. 2010. Food habits and impact of rooting behaviour of the invasive wild boar, Sus scrofa, in a protected area of the Monte Desert, Argentina. Journal of Arid Environments 74:1582-1585. https://doi.org/10.1016/j.jaridenv.2010.05.002 [ Links ]
16. Cuevas, M. F., R. A. Ojeda, & F. M. Jaksic. 2013. Multi-scale patterns of habitat use by wild boar in the Monte Desert of Argentina. Basic and Applied Ecology 14:320-328. https://doi.org/10.1016/j.baae.2013.03.001 [ Links ]
17. Daciuk, J. 1978. Notas faunísticas y bioecológicas de Península Valdés y Patagonia. II. Evaluación preliminar de la fauna de vertebrados de la Isla Victoria (Parque Nacional Nahuel Huapi, prov. de Neuquén y Río Negro, Argentina). Anales de Parques Nacionales 14:87-95. [ Links ]
18. De Pietri, D. E. 1993. Dinámica de alteraciones por fuego y ganadería en un sistema forestal del Parque Nacional Los Alerces. Universidad de Buenos Aires. [ Links ]
19. Dexter, N. 1998. The influence of pasture distribution and temperature on habitat selection by feral pigs in semi-arid environment. Wildlife Research 25:547-559. https://doi.org/10.1071/WR97119 [ Links ]
20. Dexter, N. 2003. The influence of pasture distribution, and temperature on adult body weight of feral pigs in a semi-arid environment. Wildlife Research 30:75-79. https://doi.org/10.1071/WR01026 [ Links ]
21. Diehl, P., M. J. Mazzarino, & S. Fontenla. 2008. Plant limiting nutrients in Andean-Patagonian woody species: effects of interannual rainfall variation, soil fertility and mycorrhizal infection. Forest Ecology and Management 255:2973-2980. https://doi.org/10.1016/j.foreco.2008.02.003 [ Links ]
22. Donoso Zegers, C. 1993. Bosques templados de Chile y Argentina. [ Links ]
23. Gantchoff, M. G., & J. L. Belant. 2015. Anthropogenic and environmental effects on invasive mammal distribution in northern Patagonia, Argentina. Mammalian Biology 80:54-58. https://doi.org/10.1016/j.mambio.2014.10.001 [ Links ]
24. Gantchoff, M. G., J. L. Belant, & D. A. Masson. 2013. Occurrence of invasive mammals in southern Nahuel Huapi National Park. Studies on Neotropical Fauna and Environment 48:175-182. https://doi.org/10.1080/01650521.2013.875245 [ Links ]
25. Genovesi, P., R. Scalera, S. Brunel, W. Solarz, & D. Roy. 2010. Towards an early warning and information system for invasive alien species (IAS) threatening biodiversity in Europe. [ Links ]
26. Groves, C. P., & J. Giles. 1989. Siudae. Fauna of Australia, Mammalia Vol 1b. Australian. [ Links ]
27. Gürtler, R. E., V. Martín Izquierdo, G. Gil, M. Cavicchia, & A. Maranta. 2016. Coping with wild boar in a conservation area: impacts of a 10-year management control program in north-eastern Argentina. Biological Invasions 19:11-24. https://doi.org/10.1007/s10530-016-1256-5 [ Links ]
28. Gürtler, R. E., L. I. Rodríguez-Planes, G. Gil, V. M. Izquierdo, M. Cavicchia, & A. Maranta. 2017. Differential long-term impacts of a management control program of axis deer and wild boar in a protected area of north-eastern Argentina. Biological Invasions:1-17. https://doi.org/10.1007/s10530-017-1635-6 [ Links ]
29. Härdle, W. K., & L. Simar. 2015. Applied Multivariate Statistical Analysis. Fourth edition. Springer. [ Links ]
30. Hone, J. 1995. Spatial and temporal aspects of vertebrate pest damage with emphasis on feral pigs. The Journal of Applied Ecology 32:311-319. https://doi.org/10.2307/2405098 [ Links ]
31. Howe, T. D., F. J. Singer, & B. B. Ackerman. 1981. Forage relationships of European wild boar invading northern hardwood forests. Journal of Wildlife Management 45:748-753. [ Links ]
32. Huot, J. 1974. Winter habitat use of white-tailed deer at Thirty-One Mile Lake, Quebec. Canadian Field-Naturalist 88:293-301. [ Links ]
33. Jaksic, F. M., J. A. Iriarte, J. E. Jiménez, & D. R. Martínez. 2002. Invaders without frontiers: cross-border invasions of exotic mammals. Biological Invasions 4:157-173. https://doi.org/10.1023/A:1020576709964 [ Links ]
34. Jedrzejewska, B., W. Jedrzejewski, A. N. Bunevich, L. Milkowski, & Z. A. Krasinski. 1997. Factors shaping population densities and increase rates of ungulates in Bialowieza Primeval Forest (Poland and Belarus) in the 19th and 20th centuries. Acta Theriologica 42:399-451. https://doi.org/10.4098/AT.arch.97-39 [ Links ]
35. Johnson, D. H. 1980. The comparison of usage and availability measurements for evaluating resource preference. Ecology 61:65-71. https://doi.org/10.2307/1937156 [ Links ]
36. Jolley, D. B., S. S. Ditchkoff, B. D. Sparklin, L. B. Hanson, M. S. Mitchell, & J. B. Grand. 2010. Estimate of herpetofauna depredation by a population of wild pigs. Journal of Mammalogy 91:519-524. https://doi.org/10.1644/09-MAMM-A-129.1 [ Links ]
37. Kolasa, J., & N. Waltho. 1998. A hierarchical view of habitat and its relationship to species abundance. Ecological Scale: Theory and Applications (D. Peterson & V. T. Parker, eds.). Columbia University Press, New York., New York, USA. [ Links ]
38. Kotanen, P. M. 1995. Responses of vegetation to a changing regime of disturbance: effects of feral pigs in a Californian coastal prairie. Ecography 18:190-199. https://doi.org/10.1111/j.1600-0587.1995.tb00340.x [ Links ]
39. Litvaitis, J. A., K. Titus, & E. M. Anderson. 1994. Measuring vertebrate use of terrestrial habitats and foods. Research and management techniques for wildlife and habitats (T. A. Book). [ Links ]
40. Lowe, S., M. Browne, S. Boudjelas, & M. De Poorter. 2004. 100 of the world’s worst invasive alien species: a selection from the global invasive species database. Auckland.
41. Manly, F. J., L. L. McDonald, D. L. Thomas, T. L. McDonald, & W. Erickson. 2002. Resource selection by animals: statistical design and analysis for field studies. [ Links ]
42. Marcum, C. Les, & D. O. Loftsgaarden. 1980. A Nonmapping technique for studying habitat preferences. The Journal of Wildlife Management 44:963-968. https://doi.org/10.2307/3808336 [ Links ]
43. Martín, C. E., & C. Chehébar. 2001. The national parks of Argentinian Patagonia - Management policies for conservation, public use, rural settlements, and indigenous communities. Journal of the Royal Society of New Zealand 31:845-864. https://doi.org/10.1080/03014223.2001.9517680 [ Links ]
44. Mc Graw, C. C., & J. Mitchell. 1998. Feral pigs (Sus scrofa) in Queensland. Pest status Review series - Land Protection. Department of Natural Resources and Mines, Queensland. http://southeast.lls.nsw.gov.au/biosecurity/pest-control/feral-pigs [ Links ]
45. Merino, M. L., & B. N. Carpinetti. 2003. Feral pig Sus scrofa population estimates in Bahía Samborombón Conservation area, Buenos Aires Province, Argentina. Mastozoología neotropical 10:269-275. [ Links ]
46. Mysterud, A., B. H. Björnsen, & E. Ostbye. 1997. Effects of snow depth on food and habitat selection by roe deer Capreolus capreolus along an altitudinal gradient in south-central Norway. Wildlife Biology 3:27-33. https://doi.org/10.2981/wlb.1997.004 [ Links ]
47. Mysterud, A., & E. Ostbye. 1999. Cover as a habitat element for temperate ungulates: effects on habitat selection and demography. Wildlife Society Bulletin. http://www.jstor.org/stable/3783905 [ Links ]
48. Neu, C. W., C. R. Byers, & J. M. Peek. 1974. A technique for analysis of utilization-availability data. The Journal of Wildlife Management 38:541-545. https://doi.org/10.2307/3800887 [ Links ]
49. Novillo, A., & R. A. Ojeda. 2008. The exotic mammals of Argentina. Biological Invasions 10:1333-1344. https://doi.org/10.1007/s10530-007-9208-8 [ Links ]
50. NYSDEC. 2016. Rapid Response for Invasive Species: Framework for Response. [ Links ]
51. Oli, M. K., & F. S. Dobson. 2003. The relative importance of life‐history variables to population growth rate in mammals: Cole’s Prediction Revisited. The American Naturalist 161:422-440. https://doi.org/10.1086/367591
52. Orellana, Y., R. Príncipe, M. B. D., A. L., M. Izquierdo, & P. Tato. 2013. Informe final de comunidades vegetales y comunidades terrestres del Parque Nacional Los Alerces. Actualizaciones de los planes de manejo de los Parques Nacionales Lago Puelo y Los Alerces. APN, Esquel. [ Links ]
53. Peek, J. M., D. L. Urich, & R. J. Mackie. 1976. Moose habitat selection and relationships to forest management in northeastern Minnesota. Wildlife Monographs 48:1-65. [ Links ]
54. Pescador, M., J. Sanguinetti, H. Pastore, & S. Peris. 2009. Expansion of the introduced wild boar (Sus scrofa) in the Andean region, Argentinean Patagonia. Galemys 21:121-132. [ Links ]
55. Rosell, C., P. Fernández-Llario, & J. Herrero. 2001. El jabalí (Sus scrofa Linnaeus, 1758). Galemys 13:1-25. [ Links ]
56. Rosvold, J., & R. Andersen. 2008. Wild boar in Norway - Is climate a limiting factor? Natural History:28. [ Links ]
57. Sanguinetti, J., & T. Kitzberger. 2010. Factors controlling seed predation by rodents and non-native Sus scrofa in Araucaria araucana forests: Potential effects on seedling establishment. Biological Invasions 12:689-706. [ Links ]
58. Sanguinetti, J., & H. Pastore. 2016. Abundancia poblacional y manejo del jabalí (Sus scrofa): una revision global para abordar su gestión en Argentina. Mastozoología Neotropical 23:305-323. https://doi.org/10.1007/s10530-009-9474-8 [ Links ]
59. Schiaffini, M. I., & A. R. Vila. 2012. Habitat use of the wild boar, Sus scrofa Linnaeus 1758, in Los Alerces National Park, Argentina. Studies on Neotropical Fauna and Environment 47:11-17. https://doi.org/10.1080/01650521.2012.657916 [ Links ]
60. Schmaltz, J. 1991. Deciduous forests of Southern South America. Ecosystems of the World. 7. Temperate deciduous forests (E. Röhrig & B. Ulrich, eds.). Elsevier, Amsterdam. [ Links ]
61. Senft, R., M. Coughenoer, D. Bailey, L. Rittenhouse, O. Sala, & D. Swift. 1987. Large herbivore foraging and ecological hierarchies. Bioscience 37:789-796. https://doi.org/10.2307/1310545 [ Links ]
62. Siemann, E., J. A. Carrillo, C. A. Gabler, R. Zipp, & W. E. Rogers. 2009. Experimental test of the impacts of feral hogs on forest dynamics and processes in the southeastern US. Forest Ecology and Management 258:546-553. 553. https://doi.org/10.1016/j.foreco.2009.03.056 [ Links ]
63. Simberloff, D., I. Parker, & P. N. Windle. 2005. Introduced species policy, management, and future research needs. Frontiers in Ecology and the environment 3:12-20. 20. [ Links ]
64. Singer, F. J., W. T. Swank, & E. E. C. Clebsch. 1984. Effects of wild pig rooting in a deciduous forest. The Journal of Wildlife Management 48:464-473. https://doi.org/10.2307/3801179 [ Links ]
65. Skewes, O., R. Rodriguez, & F. M. Jaksic. 2007. Ecología trófica del jabalí europeo (Sus scrofa) silvestre en Chile. https://doi.org/10.4067/S0716-078X2007000300004 [ Links ]
66. Sodeikat, G., & K. Pohlmeyer. 2003. Escape movements of family groups of wild boar Sus scrofa influenced by drive hunts in Lower Saxony, Germany. Wildlife Biology 9:43-49. https://doi.org/10.2981/wlb.2003.063 [ Links ]
67. Thomas, D. L., & E. J. Taylor. 1990. Study designs and tests for comparing resource use and availability. National Wildlife 70:322-330. https://doi.org/10.2307/3809050 [ Links ]
68. Thomas, D. L., & E. J. Taylor. 2006. Study designs and tests for comparing resource use and availability II. Journal of Wildlife Management 70:324-336. [ Links ]
69. Tobler, M. W. 2002. Habitat use and diet of baird’s tapirs (Tapirus bairdii) in montane cloud forest of the Cordillera de Talamanca, Costa Rica. Biotropica 34:468-474.
70. Vazquez, D. P. 2002. Multiple effects of introduced mammalian herbivores in a temperate forest. Biological Invasions 4:175-191. https://doi.org/10.1023/A:1020522923905 [ Links ]
71. Veblen, T. T., B. Burns, T. Kitzberger, A. Lara, & R. Villalba. 1995. The ecology of the conifers of southern South America. Ecology of the Southern Conifers (S. Enright & N., Hill, ed.). Melbourne. [ Links ]
72. Vila, A. R., & L. Borrelli. 2011. Cattle in the Patagonian forests: Feeding ecology in Los Alerces National Reserve. Forest Ecology and Management 261:1306-1314. https://doi.org/10.1016/j.foreco.2011.01.009 [ Links ]
73. Vila, A. R., L. Borrelli, & L. Martínez. 2009. Dietary overlap between huemul and livestock in Los Alerces National Park, Argentina. Journal of Wildlife Management 73:368-373. https://doi.org/10.2193/2008-062 [ Links ]
74. Vitousek, P. M., C. M. D’Antonio, L. L. Loope, M. Rejmänek, & R. Westbrooks. 1997. Introduced species: a significant component of human-caused global change. New Zealand Journal of Ecology 21:1-16.
75. Welander, J. 2000. Spatial and temporal dynamics of wild boar (Sus scrofa) rooting in a mosaic landscape. Journal of Zoology 252:263-271. https://doi.org/10.1111/j.1469-7998.2000.tb00621.x [ Links ]
76. Wilcox, J. T., D. H. Van Vuren, & D. I. R. K. H. V. A. N. V Uren. 2009. Wild pigs as predators in Oak Woodlands of California. Journal of Mammalogy 90:114-118. https://doi.org/10.1644/08-MAMM-A-017.1 [ Links ]














