Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Mastozoología neotropical
versão impressa ISSN 0327-9383versão On-line ISSN 1666-0536
Mastozool. neotrop. vol.26 no.1 Mendoza jun. 2019
ARTÍCULO
Changes in bat diversity in agrosystems in the Atlantic Rain Forest, Brazil
Alan D. Pereira1, Isaac P. de Lima2, Nelio R. dos Reis2
1 Programa de Pós-Graduação em Ciências Biológicas, AC: Biodiversidade e Conservação de Habitats Fragmentados. Universidade Estadual de Londrina, Centro de Ciências Biológicas, Rodovia Celso Garcia Cid, PR 445, Km 380, CEP 86.057-970 - Londrina, Paraná, Brazil. [Correspondence: Alan D. Pereira <alandeivid_bio@live.com>]
2 Laboratório de Ecologia de Mamíferos, Departamento de Biologia Animal e Vegetal, Universidade Estadual de Londrina, Londrina, Paraná, Brazil.
ABSTRACT
Modifications in habitats cause several alterations in the functional structure of communities. It seems that, to date, there are no studies on the functional diversity of bats concerning habitat changes in the phytophysionomy of the Brazilian Semideciduous Seasonal Forest (SSF). Current analysis compares richness, abundance and functional diversity of bats captured within the matrix and within rural and urban fragments. Samples were retrieved from five forest fragments of the SSF in the northern region of the state of Paraná, Brazil, between 2015 and 2016. Richness rates within the forest fragments were higher than in the matrix, with significant differences between sites. The abundance of captured specimens did not differ between matrix and the interior of the forest. Parameters of functional diversity were greater within the forest than in the matrix. Results show that habitat type affects species abundance and richness in the SSF. Heterogeneous matrices provide several resources, including complementary ones, for certain bat species, in food or in havens, mirroring the community´s functional diversity
RESUMO
Mudanças na diversidade de morcegos em agroecossistemas da mata atlântica, Brasil.
Modificações no habitat podem causar alterações na estrutura funcional das comunidades. Até o presente momento não se tem estudos que abordem a variação na diversidade funcional de morcegos em relação às alterações do habitat na fitofisionomia Floresta Estacional Semidecidual (FES) do Brasil. O objetivo deste estudo foi comparar a riqueza, abundância e diversidade funcional de morcegos capturados na matriz e interior de fragmentos rurais e urbanos. As coletas ocorreram em cinco fragmentos florestais de FES, localizados na região norte do estado do Paraná, região do Brasil, entre 2015 a 2016. Em todos os fragmentos a riqueza no interior da floresta foi maior que na matriz ocorrendo diferenças significativas entre os ambientes. A abundância de indivíduos capturados não diferiu entre a matriz e interior das matas. Os parâmetros de diversidade funcional foram maiores no interior da floresta ao comparado com a matriz. Nossos resultados indicam que o tipo de habitat influencia na abundância e na riqueza de espécies na Floresta estacional Semidecidual sendo que as matrizes heterogêneas ofertam uma variedade de recursos até complementares para algumas espécies de morcegos, seja na forma de alimento ou seja na forma de refúgios refletindo na diversidade funcional da comunidade.
Key words: Chiroptera; Functional diversity; Functional traits; Habitat fragmentation; Urban fragments.
Palavras chave: Chiroptera; Conservação de espécies; Fragmentação de habitat; Fragmentos urbanos; Traços funcionais.
Recibido 13 diciembre 2017.
Aceptado 18 agosto 2018.
Editor asociado: M. L. Guichón
INTRODUCTION
Habitat fragmentation caused by human factors is the process by which a continuous stretch of habitats is divided into several smaller units (Cerqueira et al. 2003). Deforestation in Brazil has always been succeeded by the fragmentation of natural areas, especially in littoral regions, the site of the Atlantic Rainforest biome. The Atlantic Rainforest is perhaps one of the most disturbed and fragmented ecosystem in the Americas (Ribeiro et al. 2009, 2011). Although the estimated area of the original Atlantic Rainforest may have reached 1.5 million km², comprising large sections of east and south Brazil, eastern Paraguay and northeastern Argentina, recent estimates indicate between 8.5 and 12.5% of the original covering (SOS Mata Atlântica & INPA 2014).
Habitat transformation and fragmentation are the main threats in biodiversity degradation (Fahrig 2003; Meyer et al. 2016; Wilson et al. 2016). The surface, naturally covered by the Semideciduous Seasonal Forest (SSF) in the region focused in current study, the northern area of the state of Paraná, Brazil, was drastically reduced during decades of anthropic activities. Few fragments, smaller than 100 hectares each and surrounded by intensive agriculture, have survived (Ribeiro et al. 2009).
Several studies identify impacting alterations on bat communities in ecosystems within the neotropical region (Klingbeil & Willig 2009; Williams-Guillén et al. 2016). Investigations on the effects of habitat fragmentation on the richness and abundance of bat species have been undertaken in SSF in the state of Paraná (e.g.Reis & Muller 1995; Félix et al. 2001; Ortêncio-Filho et al. 2009; Reis et al. 2012; Rodrigues et al. 2015). Nevertheless, there are no studies that discuss the effects of pressure related to surrounding matrix type of fragments on the functional diversity of bats in SSF.
Recently, several measurements of functional diversity have been employed in many ecology studies on different taxonomic groups (Scheiner et al. 2017). In fact, they establish a link between biodiversity and ecosystem functions (Trindade-Filho et al.2011). These gauges are different from traditional indexes of diversity and richness (Poos et al.2009), or groupings, such as functional and trophic groups (Petchey & Gaston 2002). In fact, they take into account a single set of physical, behavioral and feeding characteristics of each species of a single community. Loss of biodiversity may cause the loss of several functional traits which potentially affect the ecosystem and the processes that structure sets of species (Petchey & Gaston 2002; Hooper et al. 2005; Scheiner al. 2017).
The matrix´s quality and permeability in fragmented landscapes (for instance, vegetation structure, succession stages and spatial extension) and landscape characteristics (for instance, forest covering and connectivity of fragments) form an environmental filter that determines species persistence through distinct functional traits (Faria et al. 2006; Meyer & Kalko 2008; Quesnelle et al. 2014). Consequently, aspects of the forest fragment and of its surrounding matrix prove to be relevant for the maintenance of the diversity of bat species within the neotropical region (Faria et al. 2006; Faria & Baumgarten 2007; Farneda et al. 2015; Rocha et al. 2017).
In the case of bats, there is no consensus on matrix permeability. According to Williams-Guillén et al. (2016), the intensification of agriculture at landscape level should cause a less permeable matrix due to decrease in natural resources and structural factors, such as trees, which affects the survival of bats in fragmented landscapes. However, the matrix is a greater asset to species when compared to urban matrices (Faria et al. 2006; Faria & Baumgarten 2007). The current analysis aimed to test variations in richness, abundance and functional diversity of bats captured in the matrix and within rural and urban forest fragments in the SSF in South Brazil. Therefore, two hypotheses were tested. 1: If bat species present morphological and behavioral variations, they will affect the richness and the abundance of species in the two different landscapes. 2: If the amount of resources offered to bats is higher in the interior of forest than in the matrix, then functional diversity rates will be higher in this environment.
MATERIAL AND METHODS
Study area
The studied area in the north of the state of Paraná, Brazil, features a humid subtropical mesothermal climate (Cfa). The predominant vegetation in the region is the Seasonal Semideciduous Forest (SSF), a type of Atlantic Forest that occurs mainly in south and southeast Brazil (Medeiros et al. 2015). SSF has been systematically transformed into sugarcane, soybean and eucalyptus monocultures, pastureland and urban areas. Remnants total only about 7% of the total covering (Ribeiro et al. 2009). Four forest fragments in Londrina were selected, measuring between 10 and 690 ha, with distances ranging from 1.5 km to 16 km between fragments, plus one fragment measuring 288 ha in the municipality of Arapongas on the boundary with Londrina.
The 690-ha State Park Mata do Godoy (PEMG), tagged A1(23°26¢53²S; 51°15¢21²W) is the biggest fragment under analysis. PEMG is a totally protected unit, a haven and priority area for the conservation of biodiversity in the northern region of the state of Paraná. The Fazenda Bule, tagged A2 (23°24¢21.55²S; 51°19¢25.94²W), featuring 288 ha, is a fragment of native forest in the municipality of Arapongas, some 7 km from PEMG. The Jardim Botânico de Londrina, tagged A3 (23°21¢42.61²S; 51°10¢50.61²W), measuring 110 ha, lies within the urban perimeter of Londrina, coupled to the Instituto Agronômico do Paraná (IAPAR). It is composed of experimental forests at different stages of ecological succession, featuring native and exotic species. The Mata Versalhes, tagged A4 (23°24¢16.96²S; 51°19¢40.04²W), with 31 ha, is a small remnant of native forest, within the urban perimeter of Londrina. Selective cutting of high-priced trees occurred in the area, with the occupation of several pioneer species in the subsequent forest clearings. The Horto of the Universidade Estadual de Londrina, tagged A5 (23°19¢42.35²S; 51°12¢26.44²W), featuring 10 ha, is the smallest fragment analyzed. Due to its small size and rectangular shape, the fragment is wholly affected by the surrounding matrix, with boundary influences throughout (Fig. 1). PEMG and the Fazenda Bule may be tagged fragments with a rural matrix; the other areas are fragments with an urban matrix.
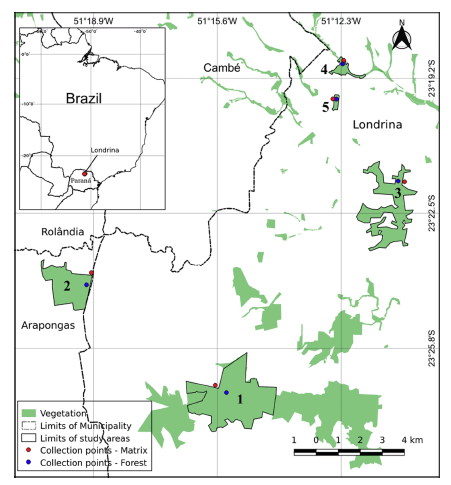
Fig. 1. Sites of fragments of the Atlantic Rainforest in the north of the state of Paraná, Brazil. (1) State Park Mata dos Godoy, (2) Fazenda Bule, (3) Jardim Botânico de Londrina, (4) Mata Versalhes, (5) Horto of the Universidade Estadual de Londrina.
Bat sampling
Captures occurred between April 2015 and March 2016, authorized by ICMbio (n. 48420-1) for the capture, retrieval and transport of specimens of wild animals, totaling 60 nights of sampling effort. Four mist nets were used, measuring (9 m long x 3 m high = 108 m²), placed 50 cm above the ground. The sampling effort was calculated according to Straube & Bianconi (2002).
Position of nets followed Zanon & Reis (2008), or rather, two nets were set within the landscape adjacent to the forest fragment (forest matrix) and two nets were set in the interior of the forests, at a distance of more than 25 m from the boundary in the case of the smaller fragments and more than 70 m in the case of bigger ones.
Morphometric measurements of captured animals were taken following identification keys (Gardner 2008; Miranda et al. 2011) and field guide (Reis et al. 2013). Captured specimens were identified, marked with washers to measure abundance and then released. The collection of bats at the Laboratory of Mammal Ecology of the Universidade Estadual de Londrina was also taken as reference. Taxonomy followed criteria by Nogueira et al. (2014). Confirmation of species occurring in the study area followed Muylaert et al. (2017).
Functional trait selection
Five functional traits were employed for each bat species to verify differences in functional composition with regard to the two environments analyzed, presuming that the following biological aspects should be taken into consideration for the differentiation in space employment by each species: (1) length of forearm; (2) weight; (3) wing load; (4) aspect ratio; (5) diet specialization, subdivided into five categories, or rather, invertebrates-general (Diet-Inv); blood of vertebrates (Diet-Vend); reptiles, snakes, amphibians, salamanders (Diet-Vect); fruit, drupes (Diet-Fruit); nectar, pollen, plant exudates, gums (Diet-Nect), following Wilman et al. (2014) (Table 1). Table S1 (Supplementary Material 1) shows mean rates of measured traits.
Table 1
Description of functional characteristics for the determination of the functional diversity of bats from the five fragments of the Atlantic Rainforest in south Brazil.

Body weight and size reflect the type and quantity of resources consumed by a species (Chillo & Ojeda 2012). To quantify body weight, we weighed each bat and averaged the values to estimate mean body weight per species, following García-Morales et al. (2016). For these measurements, we did not include pregnant females since their inclusion would introduce bias into the estimates of this functional trait. We measured bat size as mean forearm length per species. In the case of species with less than five collected specimens, data were complemented by the literature (see Table S1, SM 1).
Wing morphology is a functional characteristic since it is directly associated to the species´s ecological traits, such as sensitiveness to fragmentation, behavior and use of habitat (see Meyer et al. 2008; Marinello & Bernard 2014; Farneda et al. 2015). Measurements of specimens deposited in the Zoology Museum of the Universidade Estadual de Londrina, collected in the same region, were retrieved to quantify information on wing morphology. Measurements were based on photos of the right wing of each species from specimens at the Zoology Museum of the Universidade Estadual de Londrina. Following García-Morales et al. (2016), photos were analyzed by ImageJ 1.51j8 (National Institutes of Health, U.S.A.), calibrated at 24.23x18.17 unit (4000x3000 pixels) to measure span (E) and total area of the wing (A). Body weight (W) was calculated by multiplying mean mass by 9.81 ms-2, acceleration by gravity. Wing load was calculated as WL = W / A; aspect ratio as AR = E2 / A (Norberg & Rayner 1987) (see Table S1, SM 1)
Data analysis
Abundance, richness and functional diversity were measured (by multivariate measurements for the components of functional diversity) for the two environments within the five fragments. Functional richness index (FD) measures the total length of branches of a functional dendrogram generated by cluster analysis (Petchey & Gaston 2002; Mouchet et al. 2010). Functional evenness (FEve) measures the distribution regularity of functional traits within a functional space for a specific community (Villéger et al. 2008; Farias & Jaksic 2009). Rao´s Square Entropy (Q), a functional diversity measurement, measures mean functional distance between two randomly selected specimens within a functional space (Rao 1982; Botta-Dukát 2005; Mouchet et al. 2010).
Since continuous data are used, ecological distance between species was measured by Euclidean distance (Casanoves et al. 2010). All measurements of functional traits were standardized to guarantee equality in analyses by decostand of package Vegan (Oksanen et al. 2017). Indexes of functional diversity were calculated by dbFD of package FD (Laliberté et al. 2014).
The best grouping solution was qualitatively evaluated by inspecting all silhouette rates. Silhouette rate is the summarized distance of all points within the cluster. When mean rate of all clusters is extracted, the best solution is given by generating rates between -0.1 and 1.0. Cluster solutions with one silhouette over 0.5 are considered solid (Rousseeuw 1987). A set of different cluster solutions (2-8 clusters) was calculated and the silhouette rate for each solution was extracted. Categories description was subsequently calculated to verify which functional attributes weighed most for the formation of functional groups. Significance degree for each group was tested by employing the function catdes of package FactoMineR (Husson et al. 2010). Effect measurement of each functional trait employed was tested by Eta rate (η2). The latter is a measurement of effect size in variance analysis (Pierce et al. 2004).
In the case of data with normal distribution, t test was employed to measure differences between abundance and richness of species in the matrix and within the fragments. Wilcoxon´s test was employed for non-normal data (Gordon et al. 2015).
RESULTS
A sample effort of 15 552 m2/ h in each fragment was achieved by the end of the study. We captured 638 specimens belonging to three families and 18 bat species (Table 2). Artibeus lituratus (Olfers, 1818), Sturnira lilium (É. Geoffroy, 1810) and Carollia perspicillata (Linnaeus, 1758) totaled 90% of captures, whilst Artibeus fimbriatus Gray, 1838, Artibeus planirostris (Spix, 1823), Pygoderma bilabiatum (Wagner, 1843), Epitesicus diminutus Osgood, 1915, and Epitesicus furinalis (d’Orbigny, 1847) were reported five or more times during the collection period. Nine species were registered only in one of the fragments, while A. lituratus, S. lilium and E. diminutus were reported in the five fragments.
Table 2
Richness and number of specimens registered in the matrix (M) and forest interior (W) of forest fragments in the region of Londrina PR Brazil: A1 (PEMG), A2 (Fazenda Bule), A3 (Jardim Botânico de Londrina), A4 (Mata Versalhes), A5 (Horto of UEL); ha = hectare.

The species Desmodus rotundus (E. Geoffroy, 1980), Molossus molossus (Pallas, 1766) and Molossus rufus (E. Geoffroy, 1805) were reported only in the matrix of the fragments. Seven species, Micronycteris microtis Miller, 1898, Phyllostomus hastatus (Pallas, 1767), Anoura caudifer (E. Geoffroy), Platyrrhinus lineatus (E. Geoffroy, 1810), Myotis nigricans (Schinz 1821), Myotis riparius (Handley, 1960) and Myotis ruber (E. Geoffroy, 1806) were registered exclusively within the forest (Table 2).
Richness within forest proved to be higher than in the matrix of the fragments (Table 3), with significant differences in fragments (t = 7.429, df = 4, p = 0.03). There were also significant differences between the interior of the forests and the matrix (t = 12.071, df = 1, p = 0.02). The abundance of specimens in the matrix was higher than that registered inside the forests. No significant differences were detected for this parameter (Table 3).
Table 3
Results of functional parameters of the community of bats captured in the forest interior and in the matrix of five forest fragments of the Atlantic Rainforest in the north of the state of Paraná, south Brazil.

There were differences in the functional index rates analyzed between within forests and the matrix of the fragments, one by one and after data summing (Table 3). Higher functional diversity richness index rates occurred in bigger-sized fragments and in rural matrices when compared to smaller fragments of urban matrices (Table 3). The best solution for functional grouping provided by the silhouette curve criterion consisted of four distinct functional groups for matrix and for the interior of the forest (Fig. 2 a-f). Functional traits with the best contribution for group formation in the interior of the forests were weight (η2 = 0.96, p = 0.001), length of forearm (η2 = 0.94, p = 0.001) and wing load (η2 = 0.84, p = 0.001). In the case of fragment matrix, weight (η2 = 0.97, p = 0.001), length of forearm (η2 = 0.95, p = 0.001) and Diet.Inv (η2 = 0.85, p = 0.001) were the functional traits with the greatest contribution for species grouping. Tables S2, S3 and S4 (Supplementary Material 1) show contribution rates of each variable for species grouped in the matrix of the fragments and within forests, and the rates of functional traits with their respective contribution for each dendrogram branch.

Fig. 2. Results of functional grouping analysis in forest interior and matrix. Respective silhouette (Sc) rates of each grouping are shown in graphs (a-b). Black circle indicates the best solution for the analysis of the discriminating function (DFA) represented in Figures (c-d). Final result of groupings is given in Figures (e-f).
DISCUSSION
The species richness within forest was significantly higher than in the matrix of the fragments. However, the difference was characterized less in fragments of urban matrices and more in fragments of rural matrices. Several research studies (Faria 2006; Klingbeil & Willig 2009; Heer et al. 2015; Muylaert et al. 2016) have already described changes in the structure of neotropical bat community caused by boundary effects. Investigations on Neotropical bats reveal that most species have a greater sensitiveness to boundary effects when the forest fragment lies within the urban matrix (Meyer et al. 2008; Klingbeil & Willig 2009; Lintott et al. 2016; Muylaert et al. 2016; Williams-Guillén et al. 2016). In the case of rural surrounding matrix of the fragment, the matrix has the potential of aiding the fragment, or rather, it is a support unit for certain bat species (Estrada et al. 2006; Faria & Baumgarten 2007; Heer et al. 2015).
Several studies on the analyzed region demonstrate that bat communities undergo alteration in species structures according to fragment characteristics such as size, shape and closeness to fragments of urban centers (e.g., Reis et al. 2003, 2006, 2012; Ortêncio-Filho & Reis 2009; Fregonezi et al. 2013; Ortêncio-Filho et al. 2014). Besides changes mentioned in community structuring, results indicate differentiation in the functional traits provided by the species and reflect the distinct functional structuring for each environment. Recent studies, focused on the effect of fragmentation on the functional structuring of bats, reached similar results as those in current study. They revealed that more mature forests with high vegetal cover rates had greater FD indexes when compared to degraded areas and man-disturbed matrixes (Farneda et al. 2015; García-Morales et al. 2016; Williams-Guillén et al. 2016).
The values of Functional Diversity (FD) were higher in the interior of forests in all fragments. When compared to matrices, a greater heterogeneous environment has a better capacity to harbor several species with different attributes and ecological functions. Rao’s Square Entropy (Q) was higher within fragments than in matrices. According to Mouchet et al. (2010), the index indicates functional richness and divergence. In other words, a community with high Q and FD tends to have a high functional diversity (the contrary is not true). On the other hand, FEve rates were higher in the matrix of fragments. According to Mouchet et al. (2010), FEve measures abundance distribution regularity in functional space. FEve will be maximized by a uniform distribution of species and their abundance in functional space. Consequently, FEve rates are lower when some parts of functional space are empty while others are densely populated (Mouchet et al. 2010).
The current study registered the species M. microtis, P. hastatus, A. caudifer, P. lineatus, M. nigricans, M. riparius and M. ruber only in forest interiors and reinforces more and more the fragility and dependence on more heterogeneous areas of the species. The species mentioned above are considered rare or with low abundance for phytophysiognomy in several studies on the Seasonal Forest of the state of Paraná (Bianconi et al. 2004; Ortêncio-Filho & Reis 2009; Reiset al. 2012).
Species A. lituratus, C. perspicillata and S. lilium are opportunist frugivores (Muylaert et al. 2017). They are abundant not only in forest interiors but also in matrices. Several authors suggest the species have greater flexibility to fragmentation processes and habitat changes. Results by Muylaert et al. (2017) reveal a hyper-dominance of eight species that together comprise 80% of all captures for the Atlantic Rain Forest. In fact, A. lituratus, C. perspicillata and S. lilium occurred in more than 50% of all analyzed communities. According to these same authors, this hyper-dominance may be attributed to the method of capture (mist net studies). These species pinpoint disturbed areas since they are found more abundantly in altered areas and even in small fragments of forest in the midst of urban areas (Félix et al. 2001; Pedro et al. 2001; Estrada & Coates-Estrada 2002; Reis et al. 2003; Muylaert et al. 2017).
Species which are more tolerant to the matrix, or rather, those that exploit the matrix´s modified habitats, represent stable populations in the fragments and increase population size (Pires et al. 2006). Several sub-forest bats may use the matrix´s resources, especially secondary forests, agro-forests, coffee plantations, and river corridors, whereas insectivore bats use urban centers for a greater insect harvest due to great occurrence of light and greater concentration of insects (Duchamp et al. 2004).
Results show that habitat type affects species abundance and richness in the Semi-deciduous Seasonal Forest. In fact, the matrix´s composition and configuration are important for bats. A heterogeneous matrix provided different and even complementary resources for certain bat species in food and havens, reflecting the community´s functional diversity.
ACKNOWLEDGMENTS
We thank the Postgraduate Program in Biological Sciences of the Universidade Estadual de Londrina and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the logistic and financial support.
LITERATURE CITED
1. Bianconi, G. V., S. B. Mikich, & W. A. Pedro. 2004. Diversidade de morcegos (Mammalia, Chiroptera) em remanescentes florestais do município de Fênix, noroeste do Paraná. Revista brasileira de Zoologia 21(4):943-954. https://doi.org/10.1590/S0101-81752004000400032 [ Links ]
2. Botta-Dukát, Z. 2005. Rao’s quadratic entropy as a measure of functional diversity based on multiple traits. Journal of Vegetation Science 16(5):533-540. https://doi.org/10.1111/j.1654-1103.2005.tb02393.x
3. Casanoves. F., L. Pla, J. A. Di Rienzo, & S. Díaz. 2010. Diversity: a software package for the integrated analysis of functional diversity. Methods in Ecology and Evolution 3:233-237. [ Links ]
4. Cerqueira, R., A. Brant, M. T. Nascimento, & R. Pardini. 2003. Fragmentação: alguns conceitos. Fragmentação de ecossistemas: causas, efeitos sobre a biodiversidade e recomendações de políticas públicas (D. M. Rambaldi & D. A. S. de Oliveira, eds.). MMA/SBF, Brasília. [ Links ]
5. Chillo, V. O., & R. A. Ojeda. 2012. Mammal functional diversity loss under human-induced disturbances in arid lands. Journal of Arid Environments 87:95-102. https://doi.org/10.1016/j.jaridenv.2012.06.016 [ Links ]
6. Duchamp, J. E., D. W. Sparks, & J. O. Whitaker, Jr. 2004. Foraging-habitat selection by bats at an urban-rural interface: comparison between a successful and a less successful species. Canadian Journal of Zoology 82(7):1157-1164. https://doi.org/10.1139/z04-095 [ Links ]
7. Estrada, A., & R. Coates-Estrada. 2002. Bats in continuos forest, forest fragments and in an agricultural mosaic habitat-island at Los Tuxtlas, Mexico. Biological Conservation, Essex 103(2):237-245. https://doi.org/10.1016/S0006-3207(01)00135-5 [ Links ]
8. Estrada, C. G., A. Damon, C. S. Hernández, L. S. Pinto, & G. I. Núñez. 2006. Bat diversity in montane rainforest and shaded coffee under diferente management regimes in southeastern Chiapas, Mexico. Biological Conservation 132(2):351-361. https://doi.org/10.1016/j.biocon.2006.04.027 [ Links ]
9. Fahrig, L. 2003. Effects of habitat fragmentation on biodiversity. Annual Review of Ecology, Evolution and Systematics 34:487-515. https://doi.org/10.1146/annurev.ecolsys.34.011802.132419 [ Links ]
10. Faria, D. 2006. Phyllostomid bats of a fragmented landscape in the north-eastern Atlantic forest. Brazilian Journal Tropical Ecology 22(5):531-542. https://doi.org/10.1017/S0266467406003385 [ Links ]
11. Faria, D., & J. Baumgarten. 2007. Shade cacao plantations (Theobroma cacao) and bat conservation in southern Bahia, Brazil. Biodiversity and Conservation 16(2):291-312. https://doi.org/10.1007/s10531-005-8346-5 [ Links ]
12. Faria, D., R. R. Laps, J. Baumgarten, & M. Cetra. 2006. Bat and bird assemblages from forests and shade cacao plantations in two contrasting landscapes in the Atlantic rainforest of southern Bahia, Brazil. Biodiversity and Conservation 15(2):587-612. https://doi.org/10.1007/s10531-005-2089-1 [ Links ]
13. Farias, A. A., & F. M. Jaksic. 2009. Hierarchical determinants of the functional richness, evenness and divergence of a vertebrate predator assemblage. Oikos 118(4):591-603. https://doi.org/10.1111/j.1600-0706.2008.16859.x [ Links ]
14. Farneda, F. Z., R. Rocha, A. López-Baucells, M. Groenenberg, I. Silva, J. M. Palmeirim, P. E. D. Bobrowiec, & C. F. J. Meyer. 2015. Trait-related responses to habitat fragmentation in Amazonian bats. Journal of Applied Ecology 52:1381-1391. https://doi.org/10.1111/1365-2664.12490 [ Links ]
15. Félix, J. S., N. R. Reis, I. P. Lima, E. F. Costa, & A. L. Peracchi. 2001. Is the area of the Arthur Thomas Park, with its 82.72 ha, sufficient to maintain viable chiropteran populations? Chiroptera Neotropical 7(2):129-133. [ Links ]
16. Fregonezi, M. N., N. R. Reis, & B. K. Rossaneis. 2013. Richness, diversity and abundance of bats in small forest fragments of the Atlantic Forest in northern Parana, Brazil. Semina: Ciências Biológicas e da Saúde 34(2):149-158. https://doi.org/10.5433/1679-0367.2013v34n2p149 [ Links ]
17. García-Morales, R. et al. 2016. Deforestation Impacts on Bat Functional Diversity in Tropical Landscapes. Plos One, 11(12) e0166765. https://doi.org/10.1371/journal.pone.0166765 [ Links ]
18. Gardner, A. L. 2008. Order Chiroptera Blumenbach. Mammals of South America: Marsupials, Xenarthrans, Shrews, and Bats (A. L. Gardner, ed.). University of Chicago Press, Chicago. https://doi.org/10.7208/chicago/9780226282428.001.0001 [ Links ]
19. Gordon, A. F., S. N. Yankelevich, & V. J. Sosa. 2015. Ecological Statistics: Contemporary Theory and Application. Oxford University Press, Oxford. [ Links ]
20. Heer, K., M. H. Bonitz, R. Fernandez, M. A. R. Mello, & E. K. V. Kalko. 2015. Effects of land use on bat diversity in a complex plantation-forest landscape in Northeastern Brazil. Journal of Mammalogy 96(4): 720-731. https://doi.org/10.1093/jmammal/gyv068 [ Links ]
21. Hooper, D. U. et al. 2005 Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological monographs 75(1):3-35. https://doi.org/10.1890/04-0922 [ Links ]
22. Husson, F., S. Lê, & J. Pagès. 2010. Exploratory Multivariate Analysis by Example Using R. Chapman & Hall / CRC Press, Boca Raton. https://doi.org/10.1201/b10345 [ Links ]
23. Klingbeil, B. T., & M. R. Willig. 2009. Guild-specific responses of bats to landscape composition and configuration in fragmented Amazonian rainforest. Journal of Applied Ecology 46(1):203-213. https://doi.org/10.1111/j.1365-2664.2008.01594.x [ Links ]
24. Laliberté, E., P. Legendre, & B. Shipley. 2014. FD: measuring functional diversity from multiple traits, and other tools for functional ecology. R package version 1.0-12. [ Links ]
25. Lintott, P. R., K. Barlow, N. Bunnefeld, P. Briggs, C. Gajas Roig, & K. J. Park. 2016. Differential responses of cryptic bat species to the urban landscape. Ecology and Evolution 6(7):2044-2052. https://doi.org/10.1002/ece3.1996 [ Links ]
26. Marinello, M. M., & E. Bernard. 2014. Wing morphology of Neotropical bats: a quantitative and qualitative analysis with implications for habitat use. Canadian Journal of Zoology 92(2): 141-147. https://doi.org/10.1139/cjz-2013-0127 [ Links ]
27. Medeiros, H. R., G. M. Bochio, M. C, ribeiro, J. M. Torezan, & L. Anjos. 2015. Combining plant and bird data increases the accuracy of an Index of Biotic Integrity to assess conservation levels of tropical forest fragments. Journal for Nature Conservation 25:1-7. [ Links ]
28. Meyer, C. F. J., & E. K. V. Kalko. 2008. Assemblage-level responses of phyllostomid bats to tropical forest fragmentation: land-bridge islands as a model system. Journal of Biogeography 35(9):1711-1726. https://doi.org/10.1111/j.1365-2699.2008.01916.x [ Links ]
29. Meyer, C. F. J., J. Frund, W. P. Lizano, & E. K. V. Kalko. 2008. Ecological correlates of vulnerability to fragmentation in Neotropical bats. Journal of Applied Ecology 45(1):381-391. https://doi.org/10.1111/j.1365-2664.2007.01389.x [ Links ]
30. Meyer, C. F. J., M. J. Struebig, & M. R. Willig. 2016. Responses of tropical bats to habitat fragmentation, logging, and deforestation. Bats in the Anthropocene: Conservation of Bats in a Changing World (C. Voigt, & T. Kingston, eds.). Springer, Cham. [ Links ]
31. Miranda, J. M. D., I. P. Bernardi, & F. C. Passos (eds.). 2011. Chave ilustrada para a determinação dos morcegos da Região Sul do Brasil. João M.D. Miranda, Curitiba. [ Links ]
32. Mouchet, M. A., S. Villéger, N. W. H. Mason, & D. Mouillot. 2010. Functional diversity measures: an overview of their redundancy and their ability to discriminate community assembly rules. Functional Ecology 24(4): 867-876. https://doi.org/10.1111/j.1365-2435.2010.01695.x [ Links ]
33. Muylaert, R. L., R. D. Stevens, & M. C. Ribeiro. 2016. Threshold effect of habitat loss on bat richness in cerrado-forest landscapes. Ecological Applications 26(6): 1854-1867. https://doi.org/10.1890/15-1757.1 [ Links ]
34. Muylaert, R. L. et al. 2017. ATLANTIC BATS: a dataset of bat communities from the Atlantic Forests of South America. Ecology, 98(12): 3227. https://doi.org/10.1002/ecy.2007 [ Links ]
35. Nogueira, M. R., I. P. Lima, R. Moratelli, V. C. Tavares, R. Gregorin, & A. L. Peracchi. 2014. Checklist of Brazilian bats, with comments on original records. Check List 10(4):808-821. https://doi.org/10.15560/10.4.808 [ Links ]
36. Norberg, U. M., & J. M. V. Rayner. 1987. Ecological morphology and flight in bats (Mammalia; Chiroptera): wing adaptations, flight performance, foraging strategy and echolocation. Philosophical Transactions of the Royal Society B 316:335-427. https://doi.org/10.1098/rstb.1987.0030 [ Links ]
37. Ksanen J. et al. 2017. Vegan: community ecology package R package version 2.4-2. [ Links ]
38. Ortêncio-Filho, H., & N. R. Reis. 2009. Species richness and abundance of bats in fragments of the stational semidecidual forest, Upper Paraná River, southern Brazil. Brazilian journal of biology 69(2):727-734. https://doi.org/10.1590/S1519-69842009000300026 [ Links ]
39. Ortêncio-Filho, H., T. E. Lacher Jr, & L. C. Rodrigues. 2014. Seasonal patterns in community composition of bats in forest fragments of the Alto Rio Paraná, southern Brazil. Studies on Neotropical Fauna and Environment 14(3):1-11. https://doi.org/10.1080/01650521.2014.950834 [ Links ]
40. Pedro, W. A., F. C. Passos, & B. K. Lim. 2001. Morcegos (Chiroptera: Mammalia) da Estação Ecológica dos Caetetus, estado de São Paulo. Chiroptera Neotropical 7(1-2):136-140. [ Links ]
41. Petchey, O. L., & K. J. Gaston. 2002. Functional diversity (FD), species richness and community composition. Ecology Letters 5(3):402-411. https://doi.org/10.1046/j.1461-0248.2002.00339.x [ Links ]
42. Pierce C. A., C. A. Block, & H. Aguinis. 2004. Cautionary note on reporting eta-squared values from multifactor anova designs. Educational and Psychological Measurement 64(6):916-924. https://doi.org/10.1177/0013164404264848 [ Links ]
43. Pires, A. S., F. A. S. Fernandez, & C. S. Barros. 2006. Vivendo em um mundo em pedaços: Efeitos da fragmentação florestal sobre comunidades e populações animais. Biologia da Conservação: Essências (C. F. D. Rocha, H. G. Bergallo, M. V. Sluys & M. V. Alves, eds.). RiMa, São Carlos. [ Links ]
44. Poos, M. S., S. C. Walker, & D. A. Jackson. 2009. Functional diversity indices can be driven by methodological choices and species richness. Ecology 90(2):341-347. https://doi.org/10.1890/08-1638.1 [ Links ]
45. Quesnelle, P. E., K. E. Lindsay, & L. Fahrig. 2014. Low reproductive rate predicts species sensitivity to habitat loss: a meta-analysis of wetland vertebrates. Plos One 9(3):e90926. https://doi.org/10.1371/journal.pone.0090926 [ Links ]
46. Rao, C. R. 1982. Diversity and dissimilarity coefficients: A unified approach. Theoretical Population Biology 21(1): 24-43. https://doi.org/10.1016/0040-5809(82)90004-1 [ Links ]
47. Reis, N. R., & M. F. Müller. 1995. Bat diversity of forests and open areas in a subtropical region of south Brazil. Ecologia Austral 5:31-36. [ Links ]
48. Reis, N. R., P. H. Gallo, A. L. Peracchi, I. P. Lima, & M. N. Fregonezi. 2012. Sensitivity of populations of bats (Mammalia: Chiroptera) in relation to human development in northern Paraná, southern Brazil. Brazilian Journal of Biology. Revista Brasileira de Biologia 72(3):511-518. [ Links ]
49. Reis, N. R., M. L. S. Barbieri, I. P. Lima, & A. L. Peracchi. 2003. O que é melhor para manter a riqueza de morcegos (Mammalia, Chiroptera): um fragmento florestal grande ou vários fragmentos de pequeno tamanho? Revista Brasileira de Zoologia 20(2):225-230. https://doi.org/10.1590/S0101-81752003000200009 [ Links ]
50. Reis, N. R., I. P. Lima, & A. L. Peracchi. 2006. Morcegos (Chiroptera) da área urbana de Londrina, Paraná, Brasil. Revista Brasileira de Zoologia 19(3):739-746. https://doi.org/10.1590/S0101-81752002000300011 [ Links ]
51. Reis, N. R., M. N. Fragonezi, A. L. Peracchi, & O. A. Shibatta (eds.). 2013. Morcegos do Brasil: Guia de campo. Technical Books, Rio de Janeiro. [ Links ]
52. Ribeiro, M. C., J. P. Metzger, A. C. Martensen, F. Ponzoni, & M. M. Hirota. 2009. Brazilian Atlantic forest: how much is left and how is the remaining forest distributed? Implications for conservation. Biological Conservation 142(6):1141-1153. https://doi.org/10.1016/j.biocon.2009.02.021 [ Links ]
53. Ribeiro, M. C, A. C. Martensen, J. P. Metzer, M. Tabarelli, F. Scarano, & M. J. Fortin. 2011. The Brazilian Atlantic Forest: a shrinking biodiversity hotspot. Biodiversity hotspots: distribution and protection of conservation priority areas (F. E. Zachos, & J. C. Habel, eds.). Springer, Heidelberg. [ Links ]
54. Ricklefs, R. E (ed.). 2010. A Economia da Natureza. 6th edition. Guanabara Koogan, Rio de Janeiro. [ Links ]
55. Rocha, R., A. López-baucells, F. Z. Farneda, M. Groenenberg, P. E. D. Bobrowiec, & M. Cabeza. 2017. Consequences of a large-scale fragmentation experiment for Neotropical bats: disentangling the relative importance of local and landscape-scale effects. Landscape Ecology 32(1):31-45. https://doi.org/10.1007/s10980-016-0425-3 [ Links ]
56. Rodrigues, J., H. Ortêncio, & T. E. Lacher. 2015. Species richness and edge effects on bat communities from Perobas Biological Reserve. Studies on Neotropical Fauna and Environment 48(2):135-141. [ Links ]
57. Rousseeuw, P. J. 1987. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. Journal of Computational and Applied Mathematics 20:53-65. https://doi.org/10.1016/0377-0427(87)90125-7 [ Links ]
58. Scheiner, S. M., E. Kosman, S. J. Presley, & M. R. Willig. 2017. Decomposing functional diversity. Methods in Ecology and Evolution 8(7):809-820. https://doi.org/10.1111/2041-210X.12696 [ Links ]
59. SOS Mata Atlantica & INPA - Instituto Nacional de Pesquisas Espaciais. 2014. Atlas dos remanescentes florestais da Mata Atlântica, Período 2012-2013, Relatório técnico. São Paulo. [ Links ]
60. Straube, F. C., & G. V. Bianconi. 2002. Sobre a grandeza e a unidade utilizada para estimar esforço de captura com utilização de redes de neblina. Chiroptera Neotropica 8(1):150-152. [ Links ]
61. Trindade-Filho, J., F. L. Sobral, M. V. Cianciaruso, & R. D. Loyola. 2011. Using indicator groups to represent bird phylogenetic and functional diversity. Biological Conservation 146(1): 155-162. https://doi.org/10.1016/j.biocon.2011.12.004 [ Links ]
62. Villéger, S., N. W. Mason, & D. Mouillot. 2008. New multi- dimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89(8):2290-2301. [ Links ]
63. Williams-Guillén k., E. Olimpi E, B. Maas, P. J. Taylor, & R. Arlettaz. 2016. Bats in the anthropogenic matrix: challenges and opportunities for the conservation of chiroptera and their ecosystem services in agricultural landscapes. Bats in the Anthropocene: Conservation of Bats in a Changing World (C. Voigt & T. Kingston, eds.). Springer, Cham. [ Links ]
64. Wilson, M. C. et al. 2016. Habitat fragmentation and biodiversity conservation: key findings and future challenges. Landscape Ecology 31(2):219-227. https://doi.org/10.1007/s10980-015-0312-3 [ Links ]
65. Wilman, H., J. Belmaker, J. Simpson, C. de la Rosa, M. M. Rivadeneira, & W. Jetz. 2014. EltonTraits 1.0: Species-level foraging attributes of the world’s birds and mammals. Ecology 95:2027. https://doi.org/10.1890/13-1917.1
66. Zanon, C. M. V., & N. R. Reis. 2008. O efeito de borda sobre morcegos (Mammalia, Chiroptera) em um fragmento florestal - Fazenda Unidas - Mato Grosso do Sul. Ecologia de morcegos (N. R. Reis, A. L. Peracchi & G. A. S. D. Santos, eds.). Technical Books, Rio de Janeiro. [ Links ]
Supplement 1
https://www.sarem.org.ar/wp-content/uploads/2019/05/SAREM_MastNeotrop_26-1_Pereira-sup1.docx
Table S1. Values of the functional traits of the species of bats collected in five forest fragments of the Atlantic Forest in the southern region of Brazil, north of the state of Paraná
Table S2. Contribution values of each characteristic measured for the clustering formation corresponding to species collected in the within forests and in the matrix of the fragments studied
Table S3. Functional dendrogram of the species sampled in within forests studied in the present study
Table S4. Functional dendrogram of the species sampled in the matrix of forests studied in the present study














