Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Mastozoología neotropical
versão impressa ISSN 0327-9383versão On-line ISSN 1666-0536
Mastozool. neotrop. vol.26 no.1 Mendoza jun. 2019
NOTA
New records of the peruvian crevice-dwelling bat Tomopeas ravus (Chiroptera: Molossidae): what do they mean for its conservation?
Horacio Zeballos1, 2, Kateryn Pino4, 2, Jean P. Ludeña2, Alain C. Escóbar2, Alexander Pari3, 2, César E. Medina2 and Gerardo Ceballos5
1 Instituto de Ciencias de la Naturaleza, Territorio y Energías Renovables; Pontificia Universidad Católica del Perú, San Miguel, Lima, Perú
2 Universidad Nacional de San Agustín de Arequipa, Museo de Historia Natural, Colección Científica (MUSA), Arequipa, Perú. [Correspondence: Kateryn Pino <katerynpino@gmail.com>]
3 Programa de Magíster en Ciencias, Mención en Zoología, Facultad de Ciencias Naturales y Oceanográficas, Universidad de Concepción, Concepción, Chile.
4 Programa de Doctorado en Sistemática y Biodiversidad, Facultad de Ciencias Naturales y Oceanográficas, Universidad de Concepción, Concepción, Chile.
5 Instituto de Ecología, Universidad Nacional Autónoma de México, México D.F., México.
ABSTRACT
The Peruvian crevice-dwelling bat, Tomopeas ravus, is a rare, threatened, and endemic species to the coastal desert from Peru. Recently, new records of the species after almost 30 years of absence in inventories were made, in the north and center of its distribution at localities from Ica and Piura departments, which opens discussions about the knowledge of its rarity, conservation status, and natural history. Here we present new records for this taxon and extend its geographic range by 260 km southwards of its known distributional range renewing the discussion on the conservation status of this species and its habitats, and proposing actions for its conservation.
RESUMEN
Nuevos registros del murciélago peruano de grietas Tomopeas ravus (Chiroptera: Molossidae), ¿qué significan para su conservación?
Tomopeas ravus, el murciélago peruano de grietas, es una especie rara, amenazada y endémica de la costa del desierto peruano. Recientemente, y después de 30 años, se reportaron nuevos registros de la especie, al norte y centro de su distribución, abriendo una nueva discusión acerca de la rareza, estado de conservación e historia natural de esta especie. Aquí se reportan nuevos registros para la especie, extendiendo su rango de distribución 260 km al sur de su distribución conocida. Asimismo, se discute el estado de conservación de la especie y sus hábitats y se proponen acciones para su conservación.
Key words: Bat conservation; Neotropical bats; Peruvian desert.
Palabras clave: Conservación de murciélagos; Desierto peruano; Murciélagos neotropicales.
Recibido 26 setiembre 2018.
Aceptado 5 diciembre 2018.
Editor Asociado: L. Aguirre
The free-tailed bats of the family Molossidae Gervais, 1856 are broadly distributed in tropics and subtropics around the world and comprise one of the larger families of bats in species richness (Simmons 2005; Burgin et al. 2018). Currently, two subfamilies are recognized (Simmons 1998; Simmons 2005; Eger 2008; Gregorin & Cirranello 2016), Tomopeatinae, which contains the monotypic genus Tomopeas, and Molossinae which include all the remaining molossid species (~116 spp.).
Tomopeas ravus Miller, 1900, the sole member of the genus Tomopeas, is one of the most enigmatic species of the family Molossidae. The species is endemic to the Peruvian Pacific Coast of South America, with the elevational range from near sea level to near 2300 m (Velazco et al. 2013; Velazco 2016). This species is one of the smallest bats in the world (2-3.5 g), and it is adapted to the extreme temperature variation and light radiation in the Peruvian Coastal Desert (Davis 1970). It has morphological characters typical for Molossidae and Vespertilionidae species (Miller 1900; Aellen 1966; Davis 1970). Sudman et al. (1994), based on molecular evidence, assessed the phylogenetic position of Tomopeas ravus, and placed it as a member of the family Molossidae, as a sister genus of a clade including other Neotropical molossid species. Nevertheless, the phylogenetic position of this species is still uncertain, because it was not included in recent phylogenetic studies of the family, which assessed the relationships of Paleotropical and Neotropical molossid species (Lamb et al. 2011; Ammerman et al. 2012). Moreover, until sequence data of Tomopeas ravus is included in a complete phylogenetic reconstruction, it is difficult to draw conclusions regarding the earliest origins of the family Molossidae and distinguish among the different biogeographic hypothesis proposed (Barkley 1984; Legendre 1984; Lim 2008; Ammerman et al. 2012; Ammerman et al. 2013).
Tomopeas ravus is a rare and threatened species of Peru, with a few records throughout its distribution (Ortiz de la Puente 1951; Tuttle 1970; Koopman 1978). However, new records of the species to the north and center of its distribution, in Ica and Piura departments, respectively, were recently published after almost 30 years of absence in inventories (Velazco et al. 2013; Zamora et al. 2013). Herein, we document new records for this taxon and extend the range of distribution of the species.
The cited specimens are deposited in the Scientific Collection of the Natural History Museum of Universidad Nacional de San Agustín (MUSA), Arequipa, Peru, preserved in ethanol with the skull removed and in good condition. The identification of the specimens was based on the diagnostic characteristics provided by Miller (1900) and Davis (1970), and we compared external and cranial measurements, taken in the field and laboratory respectively, described in the literature (Miller 1900; Aellen 1966; Davis 1970; Velazco et al. 2013) (Table 1). Specimens were measured with calipers to an accuracy of 0.01 mm. The measurements were as follows: Total length, TL; Length of tail, LT; Head and body length, HB; Hindfoot length, HFL; Ear length, EL; Forearm, FA; Tibia, Tb; Calcar, Ca; Thumb, Th; Metacarpal III, M3; Phalanx 1 of III, P1-3; Phalanx 2, of III, P2-3; Dorsal hair length, HL; Weight in grams, W; Greatest length of skull, GSL; Condyle basal length, CBL, Basilar length, BL; Zygomatic breadth, ZB; Interorbital breadth, IO; Greatest breadth of brain-case above roots of zygomatic, GBS; Depth of braincase, DB; Upper toothrow, LHMS; Mastoidal breadth, MB; Width across M3-M3, M3-M3; Length of palate, LP; Mandibular length, ML; Lower toothrow, LHMI.
Table 1
External and cranial measurements (mm), and weights (g) of specimens of Tomopeas ravus from literature (1Davis 1970, 2Miller 1900, 3Aellen 1965, 4Zamora et al. 2013) and new records from Piura and Arequipa (this study). Measurement definitions are described in main text

Order Chiroptera Blumenbach 1779
Family Molossidae Gervais, 1856
Tomopeas ravus Miller, 1900
Specimens examined (3).— Adult male, MUSA 14256, collected by Kateryn Pino at Quebrada San Juan, Talara Province, Piura Department (4° 20¢ 21.48² S, 81° 14¢ 48.48² W, 45 m; Fig. 1) on December 2012; adult male, MUSA 14392, collected by Jean Paul Ludeña in Ispacas, Condesuyos Providence, Arequipa Department (15° 50¢ 17.45² S, 73° 53¢ 40.45² W, 585 m) on March 2012; adult female, MUSA 14500, collected by Alain Escóbar in Puglle, La Unión Providence, Arequipa Department (15° 33¢ 33.84² S, 73° 07 01.92¢¢ W, 940 m).
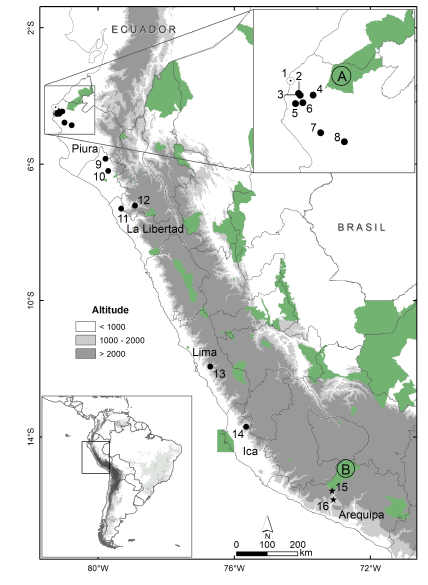
Fig. 1. Map showing known localities of Tomopeas ravus. Previous records (black dots) (Velazco et al. 2013); new records in the north, Alto Talara, Piura (white dot); and in the south, Ocoña Valley, Arequipa (stars). The Peruvian protected natural areas pointed out in green. Protected natural areas nearest to T. ravus records: A. Coto de Caza El Angolo; B. Reserva Paisajística Sub Cuenca del Cotahuasi. The name of localities is given in the Appendix 1.
Distribution.— Tomopeas ravus is endemic to the coastal desert of central and northern Peru, except for some records from the Cajamarca Department, in the highlands of northern Peru (Velazco et al. 2013). We report the two southernmost records of Tomopeas ravus, which were captured in the Ocoña valley in Arequipa department of southern Peru, more than 260 km from its nearest locality, at Quebrada San Juan, Pisco, in Ica department (Zamora et al. 2013). Moreover, a new locality in northern Peru, in Piura department (Fig. 1).
Description.— The examined specimens show all the diagnostic characteristics and the measurements are within the size range of Tomopeas ravus as described by Miller (1900), Davis (1970), Barkley (1984) and Velazco et al. (2013). Coloration is yellowish brown with whitish underparts, but specimens from Arequipa are paler (Fig. 2). The pelage between the shoulders is long (mean = 7.13 mm, SD = 0.72 mm). The wings are thin, and the tail membrane is long. Dorsal hairs are tricolored with creamy base (10%), the middle light grayish brown (60%) and the distal yellow (30%), and there are colored hairs around the face and patagium. The ears are large, triangular shaped, and widely separated in the forehead. The pelage extends to the base of the wing membrane, dorsally, and the inner side of the proximal propatagium. The patagium is brown to dark brown, which clearly highlights the pelage color. However, southern specimens are paler. In an external appearance, the upper lip is longer than the lower lip so that the face appears long (Fig. 2). Nostrils are dark brown or blackish with a tubular appearance, separate, and divergent (Fig. 2). The face has few pale hairs and abundant granulations. In the upper lips, a fringe of hairs directed downwards is observed; mystacial vibrissae are long and there are three intrarramal vibrissae (Fig. 2). The tail is long and has a distal projection protruding from the uropatagium edge by 5.66 mm on average (SD = 1.73). The skull is slightly depressed, with the front portion inflated and supraoccipital portion directed up and back (Fig. 3); the rostrum is wide and the nasal region is anteriorly depressed. The zygomatic arch is fragile and straight, with a massive maxillary root showing the roots of molars. The auditory bulla is well developed, averaging length of 2.54 mm (SD = 0.18). In occlusal view the maxillary toothrow in curved canines are well developed, straight or slightly arched, and the first premolar is larger than the second.
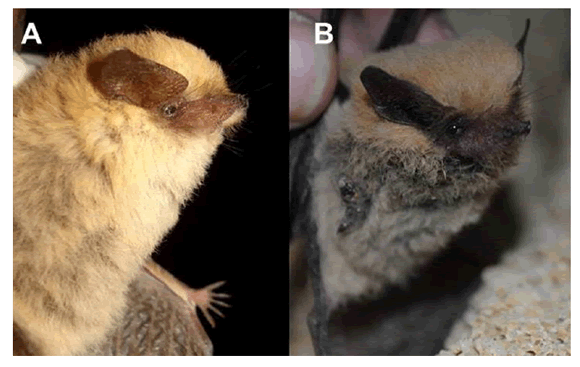
Fig. 2. Tomopeas ravus showing color variation: A) A pale female in Ocoña (MUSA 14500); B) A dark male in Alto Talara (MUSA 14256). Photograph by A. Escóbar and K. Pino.

Fig. 3. Dorsal and ventral views of the cranium and lateral view of the cranium and mandible of an adult male Tomopeas ravus (MUSA 14392), collected in Ocoña Valley, Arequipa, Peru. Scale bar = 6 mm.
Natural history.— Specimens from southern Peru were captured using mist nets in a cultivated valley adjacent to the Ocoña River in a deeper canyon with columnar cacti, between 18:00 and 20:00 hours. Whereas the two specimens (one released) captured in Piura were collected in sandy and clayish ground in a drier ravine with few trees of the families Fabacea and Capparaceae (Prosopis pallida, Acacia macracantha, and Capparis sp.) using a mist net. In both areas, there are rocky formations with cavities and crevices. Besides, the areas have extremely dry conditions (Fig. 4). However, average annual precipitation is higher in the north (125 mm) than in the south (20 mm) (Marquet et al. 2002; Pari et al. 2015). Tomopeas ravus was captured with bats of the families Phyllostomidae and Vespertilionidae, Glossophaga soricina and Histiotussp., in Piura, and with Platalina genovensium, Glossophaga soricina, Myotis atacamensis, and Histiotus montanus in Arequipa.

Fig. 4. Landscapes of new records of Tomopeas ravus: A. Ocoña valley, Arequipa, southern Peru; B. Alto Talara, Piura, northern Peru. Photograph by A. Escóbar and K. Pino.
Remarks.— Recent reports (Zamora et al. 2013; Velazco et al. 2013) present new records of Tomopeas ravus after three decades (Barkley 1984). These records and the present here could be the result of small population size over the past, with a recent recovery. However, it’s difficult to know with the information available. In that sense, a long-term study is necessary to explain the temporal population dynamics. Until that, other explanations could be plausible as well, like scarcity of field studies. Nevertheless, this is rebuttable because many areas of Tomopeas distribution have been studied without success in recording it (Graham & Barkley 1984; Hernández-Ríos & Velásquez 1996; AEDES 1998; Zeballos et al. 2000; Zeballos et al. 2002; Mena & Williams 2002; Cadenillas 2003; Pacheco et al. 2007; Montero-Commisso et al. 2008).
Also molossids are known to fly high, foraging in open space above forest canopies, or over open landscapes (Mora et al. 2004; Kalko et al. 2008), and because of this, they are notably difficult to capture with conventional methods (mist nets at ground-level), being this another plausible explanation for the scarcity of T. ravus records. In fact, Piura’s specimens were captured with high mist nets (6 m from the ground), and likewise, other recent records of molossid species (i.e. Cynamops abrasus, Eumops patagonicus, Molossus coibensis) in Peru (Medina et al. 2016). Although, previous records from Piura and Ica Departments (Velazco et al. 2013; Zamora et al. 2013) were done with ground-level nets. These nets were located near potential roosts, rocky outcrops in Ica record (Zamora et al. 2013). Furthermore, Davis (1970) points out that several (~10) specimens of T. ravus captured at Cerro La Vieja and Cerro Amotape localities in Lambayeque and Piura Departments, respectively, were done by hand in roosts under the exfoliations of granite boulders and outcroppings. In addition, Barkley (1984), notes that most specimens, 30 of 50 examined by her, were captured alike. Therefore, future studies to focus on this species should consider additional methods to the traditional mist nets, like the search for roosts, and acoustic’s methods (Jung et al. 2014; Voss et al. 2016).
Conservation.— Tomopeas ravus is considered a rare species (Pari et al. 2015), because it fits all criteria for rarity (Rabinowitz 1981; Rabinowitz et al. 1986): a) small population size, based on occasional captures (Velazco 2016); b) reduced habitat specificity, it is an exclusively desert bat (Davis 1970; Barkley 1984); and c) it has a limited distribution range and is endemic from the Peruvian desert (Velazco et al. 2013). Moreover, it is one of the most endangered bat species of Peru, listed as Vulnerable by Peruvian legislation (Ministerio de Agricultura y Riego de la República del Perú 2014) and as Endangered by the IUCN red list, because of its population reduction and small and fragmented geographic range (Velazco 2016). It seems to depend on vegetated riparian habitats and streams, which restricts its habitat to mainly coastal valleys below 1900 m but associated with rock crevices in granitic boulders used as roosts (Davis 1970; Barkley 1984). Nevertheless, this kind of habitats is being very impacted by agriculture and urban expansion and other anthropogenic activities, since the last decades (Zamora et al. 2013; Velazco 2016). In fact, the Peruvian coastal desert, throughout which Tomopeas ravus is distributed, is an area inhabited by 13.5 million people (around 45% of the total Peruvian population). The main population centers in the area are the city of Lima (Peru’s capital) with 9 million people, and the cities of Piura, Chiclayo, and Trujillo (north of Peru) with more than 3 million (Marquet et al. 2002). Coincidentally, most records of this species were around these cities (See Fig. 1), at least until the 80s. In the case of Cerro La Vieja, at 1968 five specimens were captured (Davis 1970); in 1981, 17 specimens were captured (Barkley 1984; Duszynski & Barkley 1985); whereas during the surveys in January and May 2006, made by HZ in this locality and others to the north (La Manga-Embalse Pechos, Tranca), despite 800 net-hours and 102 individuals caught of 12 bat species of the families Mormoopidae, Phyllostomidae, Furipteridae, Molossidae, and Vespertilionidae (Mormoops megalophylla, Pteronotus gymnonotus, Desmodus rotundus, Glossophaga valens, Choeroniscus minor, Lonchophylla hesperia, Artibeus fraterculus, Sturnira bogotensis, Amorphochilus schnablii, Molossus molossus, Myotis nigricans, Eptesicus innoxius), not a single individual of T. ravus was captured. On the other hand, the recent reports (Velazco et al. 2013; Zamora et al. 2013) and the one we present here were made in areas with good conservation status, due to the restrictions to human presence by the oil concessions in Talara, Piura department (Velazco et al. 2013), and the remoteness of the southern localities, Quebrada San Juan, in Ica department, and Ocoña River basin, in Arequipa department.
Unfortunately, this species is not recorded in any protected area of Peru (See Fig. 1). Nevertheless, its presence in the Reserva Paisajística Sub Cuenca del Cotahuasi in Arequipa department and the Coto de Caza El Angolo in Piura department is to be expected, given the proximity to the Puglle (1.5 km) and Monte Grande (16 km) localities records, respectively (Fig. 1). Tomopeas ravus has been recorded along one of the driest deserts of the world, Sechura desert (Mostacero et al. 1996; Marquet et al. 2002). Nevertheless, this desert has a remarkable mammal diversity (Müller 1973; Pacheco et al. 2009), with 46 species (17 of them endemic, Pacheco et al. 2009). Even new additions of mammalian species have been made recently, from the description of new species and taxonomic studies (Gregorin & Chiquito 2010; Velazco & Cadenillas 2011; Medina et al. 2014; Zeballos et al. 2014; Do Prado & Percequillo 2017). In spite of its diversity and endemism, this area is the scenario of constant economic development and presents the highest population density in the country, which is endangering the survival of many species and habitats (Marquet et al. 2002). These ecosystems have been modified and reduced, especially coastal valleys (Weberbauer 1945; Koepcke 1954) and the Lomas formation (Rundel et al. 1991; Ferreyra 1993; Dillon 1997). Moreover, the coastal deserts are among the most poorly represented ecosystems between Peru’s national parks, reserves, and sanctuaries (Rodríguez & Yung 2000, See Fig. 1). Notwithstanding, one alternative to protect this threatened and endemic species could be to expand the Reserva Paisajística Sub Cuenca del Cotahuasi. Furthermore, other threatened species inhabit the high and middle areas of the Ocoña River, like Platalina genovensium, Amorphochilus schnablii, Myotis atacamensis, Lontra felina, Telmatobius sp., Cryphiops caementarius. Consequently, the enlargement of this protected area enables the conservation of Tomopeas ravus and other threatened species; besides it would prevent the spreading of illegal mining and agriculture expansion in the area.
Acknowledgements
We are grateful to Evaristo López, director of Museo de Historia Natural de la Universidad Nacional de San Agustín, for reviewing an earlier version of the manuscript; Paul Velazco for making important contributions and comments to improve the quality of the manuscript. We thank the Intendencia Forestal y Fauna Silvestre del Ministerio de Agricultura y Riego de la República del Perú for the collect authorization (Nº 062-2006-INRENA-IFFS-DCB); Koepcke Grants of APECO and Conservation International for the financial support for the expedition at Cerro La Vieja. KP appreciates the support of EDPG LPR-161 project of Dirección de Investigación of Universidad de Concepción and CONICYT doctoral fellowship number 21181388. AP was funded by fellowship from the Escuela de Graduados of the Universidad de Concepción. We would like to thank to Luis Aguirre and two anonymous reviewers for their critical comments and suggestions.
LITERATURE CITED
1. Aedes. 1998. Estudio de la biodiversidad vegetal y animal Cuenca del Cotahuasi: Riqueza Faunística, Zona II. Asociación Especializada para el Desarrollo, Arequipa. [ Links ]
2. Aellen, V. 1966. Sur une petite colection de Chiroptères du nord-ouest du Peru. Mammalia 29(4):563-571. https://doi.org/10.1515/mamm.1965.29.4.563 [ Links ]
3. Ammerman, L. K., D. N. Lee, & T. M. Tipps. 2012. First molecular phylogenetic insights into the evolution of tree-tailed bats in the subfamily Molossinae (Molossidae, Chiroptera). Journal of Mammalogy 93:12-28. https://doi.org/10.1644/11-mamm-a-103.1 [ Links ]
4. Ammerman, L. K., W. A. Brashear, & S. N. Bartlett. 2013. Further evidence for the basal divergence of Cheiromeles (Chiroptera: Molossidae). Acta Chiropterológica 15(2):307-312. https://doi.org/10.3161/150811013x678946 [ Links ]
5. Barkley, L. J. 1984. Evolutionary relationships and natural history of Tomopeas ravus (Mammalia: Chiroptera). M.S. thesis. Louisiana State University, Baton Rouge. [ Links ]
6. Burgin, C. J., J. P. Colella, P. L. Kahn, & N. S. Upham. 2018. How many species of mammals are there? Journal of Mammalogy 99(1):1-14. https://doi.org/10.1093/jmammal/gyx147 [ Links ]
7. Cadenillas, R. 2003. Especies del suborden Microchiroptera en el departamento de Piura, y sus posibles relaciones filogenéticas. Tesis Biólogo, Universidad Nacional de Piura, Perú [ Links ].
8. Davis, W. B. 1970. Tomopeas ravus Miller (Chiroptera). Journal of Mammalogy 51(2):244-247. https://doi.org/10.2307/1378474 [ Links ]
9. Dillon, M. O. 1997. Lomas Formations-Peru. Centres of Plants Diversity. A Guide and Strategy for their Conservation (S. D. Davis, V. H. Heywood, O. Herrera-McBryde, J. Villa-Lobos & A. C. Hamilton, eds.). Oxford: WWF Information Press. [ Links ]
10. Do Prado, J. R., & A. R. Percequillo. 2017. Systematic studies of the genus Aegialomys Weksler et al., 2006 (Rodentia: Cricetidae: Sigmodontinae): geographic variation, species delimitation, and biogeography. Journal of Mammalian Evolution 25:71-118. https://doi.org/10.1007/s10914-016-9360-y [ Links ]
11. Duszynski, D., & L. J. Barkley. 1985. Eimeria from bats of the world: a new species in Tomopeas ravus from Peru. Journal of Parasitology 71(2):204-208. https://doi.org/10.2307/3281903 [ Links ]
12. Eger, J. L. 2008. Family Molossidae. Mammals of South America Volume 1: Marsupials, Xenarthrans, Shrews and Bats (A. L. Gardner, ed.). Washington D.C.: Smithsonian Institution Press. https://doi.org/10.7208/chicago/9780226282428.001.0001 [ Links ]
13. Ferreyra, R. 1993. Registros de la vegetación de la costa peruana en relación con el fenómeno del Niño. Bulletin d l’Institut Francais d’ Études Andines 22(1):259-266.
14. Graham, G. L., & L. J. Barkley. 1984. Noteworthy records of bats from Peru. Journal of Mammalogy 65(4):709-711. https://doi.org/10.2307/1380863 [ Links ]
15. Gregorin, R., & A. Cirranello. 2016. Phylogeny of Molossidae Gervais (Mammalia: Chiroptera) inferred by morphological data. Cladistics 32:2-35. https://doi.org/10.1111/cla.12117 [ Links ]
16. Gregorin, R., & E. A. Chiquito. 2010. Revalidation of Promops davisoni Thomas (Molossidae). Chiroptera Neotropical 16:648-660. [ Links ]
17. Hernández-Ríos, M., & V. Velázquez. 1996. Estudio de los murciélagos del Valle de Ica. Ecoregion 1:4-26. [ Links ]
18. Jung, K., J. Molinari, & E. K. V. Kalko. 2014. Driving factors for the evolution of species-specific echolocation call design in New World free-tailed bats (Molossidae). PLoS ONE 9(1):e85279. https://doi.org/10.1371/journal.pone.0085279 [ Links ]
19. Kalko, E. K. V., S. Estrada Villegas, M. Schmidt, M. Wegmann, & C. F. Meyer. 2008. Flying high - Assessing the use of aerosphere by bats. Integrative and Comparative Biology 48:60-73. https://doi.org/10.1093/icb/icn030 [ Links ]
20. Koepcke, M. 1954. Corte ecológico transversal en los Andes de Perú central con especial consideración de las aves. Memorias del Museo de Historia Natural Javier Prado 3:1-119. [ Links ]
21. Koopman, K. F. 1978. Zoogeography of Peruvian bats with special emphasis on the role of the Andes. American Museum Novitates 2651:1-33. [ Links ]
22. Lamb, J. M., T. M. C. Ralph, T. Naidoo, P. J. Taylor, F. Ratrimomanarivo, W. T. Stanley, & S. M. Goodman. 2011. Toward a molecular phylogeny for the Molossidae (Chiroptera) of the Afro-Malagasy Region. Acta Chiropterologica 13:1-16. https://doi.org/10.3161/150811011x578589 [ Links ]
23. Legendre, S. 1984. Etude odontologique des representants actuels du groupe Tadarida (Chiroptera, Molossidae). Implications phylogeniques, systematiques et zoogeographiques. Revue Suisse de Zoologie 91:399-442. https://doi.org/10.5962/bhl.part.81886 [ Links ]
24. Lim, B. K. 2008. Historical biogeography of New World emballonurid bats (tribe Diclidurini): taxon pulse diversification. Journal of Biogeography 35:1385-1401. https://doi.org/10.1111/j.1365-2699.2008.01888.x [ Links ]
25. Marquet, P. A., H. González, R. Pinto, C. Santoro, V. G. Standen, & H. Zeballos. 2002. Coastal deserts of Peru and Chile. Wilderness: Earth’s last wild places (R. A. Mittermeier, C. G. Mittermeier, P. Robles, J. Pilgrim, G. Fonseca, W. R. Konstant & T. Brooks, eds.). Chicago: University of Chicago Press.
26. Medina, C. E., K. Pino, A. Pari, G. Llerena, H. Zeballos, & E. López. 2016. Mammalian diversity in the Savanna from Peru, with three new addictions from country. Papéis Avulsos de Zoologia 56(2):9-26. https://doi.org/10.1590/0031-1049.2016.56.02 [ Links ]
27. Medina, C. E., R. Gregorin, H. Zeballos, H. T. Zamora & L. M. Moras. 2014. A new species of Eumops (Chiroptera: Molossidae) from southwestern Peru. Zootaxa 3878(1):19-36. https://doi.org/10.11646/zootaxa.3878.1.2 [ Links ]
28. Mena, J. L., & M. Williams. 2002. Diversidad y patrones reproductivos de quirópteros en un área urbana de Lima, Perú. Ecología Aplicada 1(1):1-8. https://doi.org/10.21704/rea.v1i1-2.222 [ Links ]
29. Miller, G. S. 1900. A new bat from Peru. Annals and Magazine of Natural History 7(6):570-574. https://doi.org/10.1080/00222930008678426 [ Links ]
30. Ministerio de Agricultura y Riego de la República del Perú. 2014. Decreto Supremo No. 004-2014-MINAGRI. Decreto Supremo que aprueba la actualización de la lista de clasificación y categorización de las especies amenazadas de fauna silvestre legalmente protegidas. El Peruano, Normas legales, Lima: 276853–276855.
31. Montero-Commisso, F. G., C. Gazzolo-Navarro, & G. Gonzalez-Blacker. 2008. Nuevos registros de quirópteros para la Reserva Nacional de Paracas, Perú. Ecología Aplicada 7(2):183-185. https://doi.org/10.21704/rea.v7i1-2.374 [ Links ]
32. Mostacero, J., F. Mejía, & F. Peláez. 1996. Fitogeografía del Norte del Perú. Consejo Nacional de Ciencia y Tecnología, Lima. [ Links ]
33. Mora, E. C., S. Macías, M. Vater, F. Coro, & M. Kössl. 2004. Specializations for aerial hawking in the echolocation system of Molossus molossus (Molossidae, Chiroptera). Journal of Comparative Physiology A 190:561-574. https://doi.org/10.1007/s00359-004-0519-2 [ Links ]
34. Müller, P. 1973. The dispersal centres of terrestrial vertebrates in the Neotropical realm: A study in the evolution of the Neotropical biota and its native landscape. Biogeographica, vol. 2. Dr. W. Junk B. V., The Hague. [ Links ]
35. Ortiz De La Puente, J. O. 1951. Estudio monográfico de los quirópteros de Lima y alrededores. Museo de Historia Natural Javier Prado 7:1-48. [ Links ]
36. Pacheco, V., R. Cadenillas, S. Velazco, E. Salas, & U. Fajardo. 2007. Noteworthy bat records from the Pacific Tropical rainforest region and adjacent dry forest in northwestern Peru. Acta Chiropterologica 9(2):409-422. https://doi.org/10.3161/1733-5329(2007)9[409:nbrftp]2.0.co;2 [ Links ]
37. Pacheco, V., R. Cadenillas, E. Salas, C. Tello, & H. Zeballos. 2009. Diversidad y Endemismo de los Mamíferos del Perú. Revista Peruana de Biología 16:5-32. https://doi.org/10.15381/rpb.v16i1.111 [ Links ]
38. Pari, A., K. Pino, C. E. Medina, E. López, & H. Zeballos. 2015. Murciélagos de Arequipa, historia natural y conservación. Impresiones Juve, 179 págs. [ Links ]
39. Rabinowitz, D. 1981. Seven forms of rarity. The Biological aspects of rare plant conservation (H. Synge, ed.). New York: Wiley Press. [ Links ]
40. Rabinowitz, D., S. Cairns, & T. Dillon. 1986. Seven forms of rarity and their frequency in the flora of the British Isles. Conservation Biology: The Science of Scarcity and Diversity (M. E. Soule, ed.). Sunderland: Sinauer Associates, Inc. [ Links ]
41. Rodríguez, L. O., & K. R. Young. 2000. Biological diversity of Peru: Determining Priority Areas for Conservation. AMBIO: A Journal of the Human Environment 29(6):329-337. https://doi.org/10.1579/0044-7447-29.6.329 [ Links ]
42. Rundel, P. W., M. Dillon, B. Palma, H. Mooney, S. Gulmon, & J. Ehleringer. 1991. The phytogeography and ecology of the coastal Atacama and Peruvian deserts. Aliso 13:1-49. https://doi.org/10.5642/aliso.19911301.02 [ Links ]
43. Simmons, N. B. 1998. A reappraisal of interfamilial relationships of bats. Bat Biology and Conservation (T. H. Kunz & P. A. Racey, eds.). Washington, D.C.: Smithsonian Institution Press. [ Links ]
44. Simmons, N. B. 2005. Order Chiroptera. Mammal species of the World: a taxonomic and geographical reference, 3rd edition (D. E. Wilson & D. M. Reeder, eds.). Baltimore: The Johns Hopkins University Press. [ Links ]
45. Sudman, P. D., L. J. Barkley, & M. S. Hafner. 1994. Familial affinity of Tomopeas ravus (Chiroptera) based on protein electrophoretic and cytochrome B sequence data. Journal of Mammalogy 75(2):365-377. https://doi.org/10.2307/1382555 [ Links ]
46. Tuttle, M. 1970. Distribution and Zoogeography of Peruvian Bats, with Comments on Natural History. Kansas University Science Bulletin XLIX (2):45-86. [ Links ]
47. Velazco, P. 2016. Tomopeas ravus, in: IUCN 2016. IUCN Red List of Threatened Species. Version 2016.1. <www.iucnredlist.org>. Downloaded on 15 October 2017. https://doi.org/10.2305/iucn.uk.2016-1.rlts.t21982a21975053.en [ Links ]
48. Velazco, P. M., & R. Cadenillas. 2011. On the identity of Lophostoma silvicolum occidentalis (Davis & Carter, 1978) (Chiroptera: Phyllostomidae). Zootaxa 2962:1-20. https://doi.org/10.11646/zootaxa.2962.1.1 [ Links ]
49. Velazco, P. M., R. Cadenillas, O. Centty, L. Huamaní, & H. Zamora. 2013. New records of Platalina genovensium (Chiroptera, Phyllostomidae) and Tomopeas ravus (Chiroptera, Molossidae). Mastozoologia Neotropical 20:425-434. [ Links ]
50. Voss R. S., W. D. Fleck, R. E. Strauss, P. M. Velazco, & N. B. Simmons. 2016. Roosting Ecology of Amazonian Bats: Evidence for Guild Structure in Hyperdiverse Mammalian Communities. American Museum Novitates 3870:1-43. https://doi.org/10.1206/3870.1 [ Links ]
51. Weberbauer, A. 1945. El mundo vegetal de los Andes Peruanos. Editorial Lumen, Lima. [ Links ]
52. Zamora, H., C. E. Medina, A. Escobar, Y. Arteaga, R. Cadenillas, & P. M. Velazco. 2013. New distributional record of the rare endemic Peruvian Tomopeas ravus Miller, 1900 (Chiroptera, Molossidae, Tomopeatinae). Mammalia 78(2):257-260. https://doi.org/10.1515/mammalia-2013-0007 [ Links ]
53. Zeballos, H., L. Villegas, R. Gutiérrez, K. Caballero, & P. Jiménez. 2000. Vertebrados de las Lomas de Atiquipa y Mejía, Sur del Perú. Revista de Ecología de Latinoamérica 7(2):11-18. [ Links ]
54. Zeballos, H., E. López, & P. Jiménez. 2002. Registro de los murciélagos de Arequipa y clave de especies. Dilloniana 2(1):143-154. [ Links ]
55. Zeballos, H., R. E. Palma, P. A. Marquet, & G. Ceballos. 2014. Phylogenetic relationships of Calomys sorellus complex (Rodentia: Cricetidae), with the description of two species. Revista Mexicana de Mastozoología 4(1):1-23. [ Links ]
Summary of the known locality records of Tomopeas ravus. All records are shown in Fig. 1.
PERU: Piura: (1) Quebrada San Juan, Talara; (2) Fondo, 14 km N and 12 km E Talara; (3) Fondo [Quebrada Hondo], 14 km N and 13 km E Talara; (4) Monte Grande, 14 km N and 25 km E of Talara; (5) Talara, Pariñas, 9.6 km NE of Talara, Quebrada Pariñas; (6) Pariñas, 7 km N and 15 km E Talara (MVZ 135635–135637); (7) SE foot Cerro Amotape, 13 km N and 35 km W Sullana; (8) Mallares (Aellen, 1966).
Lambayeque: (9) 12 km N Olmos; (10) Cerro la Vieja, 7 km S Motupe.
La Libertad: (11) Tolón 30 km ENE Pacasmayo (Miller, 1900).
Cajamarca: (12) Llanllán (Miller, 1900).
Lima: (13) Chosica (Miller, 1900).
Ica: (14) Pisco, Quebrada San Juan.
Arequipa: (15) Puglle, La Unión; (16) Ispacas, Condesuyos.














