Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Biocell
Print version ISSN 0327-9545
Biocell vol.26 no.3 Mendoza Aug./Dec. 2002
Water relations and leaf growth rate of three agropyron genotypes under water stress
María G. García, Carlos A. Busso, Pablo Polci, Norberto L. García Girou, and Vivivan Echenique
Departamento de Agronomía y CERZOS (CONICET), Universidad Nacional del Sur. (8000) Bahía Blanca, Argentina.
Address correspondence to: Dr. Carlos A. Busso. Dpto. de Agronomía, Universidad Nacional del Sur, Altos del Palihue.
(8000) Bahía Blanca, Buenos Aires, ARGENTINA. Fax: (+54- 291) 459 5127. E-mail: cebusso@criba.edu.ar
Key words: Agropyron scabrifolium (Döell) Parodi, Agropyron elongatum (Host) Beauv., water stress, water relations, leaf growth rate.
ABSTRACT: The effects of water stress on leaf water relations and growth are reported for three perennial tussock grass genotypes under glasshouse conditions. Studies were performed in genotypes El Palmar INTA and Selección Anguil of Agropyron scabrifolium (Döell) Parodi, and El Vizcachero of A. elongatum (Host) Beauv. Agropyron scabrifolium El Palmar INTA is native to a region with warm-temperate and humid climate without a dry season, and an average annual precipitation of 900 mm. Agropyron scabrifolium Selección Anguil comes from a region with a sub-humid, dry to semiarid climate and a mean annual precipitation of 600 mm. Agropyron elongatum is a widespread forage in semiarid Argentina with well-known water stress resistance. A mild water stress treatment was imposed slowly; plants reached a minimum pre-dawn leaf water potential of about -1.83 MPa by day 21 after watering was withheld. In all genotypes, water stress led to a reduction of leaf growth. There was a tendency for a greater epicuticular wax accumulation on water-stressed plants of A. scabrifolium Selección Anguil and A. elongatum than on those of A. scabrifolium El Palmar INTA. This may have contributed to obtain greater turgor pressures and relative water contents in the first two than in the later genotype. In turn, this may have contributed to determine smaller leaf growth rate reductions in A. scabrifolium Selección Anguil and A. elongatum than in A. scabrifolium El Palmar INTA under water stress. This study demonstrated variation in water stress resistance between genotypes in A. scabrifolium, and between A. scabrifolium Selección Anguil and A. elongatum versus A. scabrifolium El Palmar INTA, which was related to their differential responses in water relations.
Introduction
Water potential and its components are generally considered a reliable measurement of the water status of plant tissue (Brown, 1995). By the mid eightys, relative water content was proposed as a better indicator of water status than water potential (Sinclair and Ludlow, 1985). This is because relative water content, through its relation to cell volume, may more closely reflect the balance between water supply to the leaf and transpiration rate. Measurements of total leaf water potential, leaf osmotic potential and relative water content relate directly or indirectly to plant response to water stress (Brown, 1995). All of these are, in theory, potential water stress resistance screening criteria (Matin et al., 1989).
The importance of plant water for the maintenance of turgidity required for plant growth is widely recognized. Many workers (e.g., Levitt, 1972; Brown, 1995) have shown that water stress-resistant plants have smaller water deficit per unit decrease in leaf water potential than more water-stress susceptible plants. Work of Carter and Patterson (1985) on Glycine max and Schonfeld et al. (1988) on Triticum aestivum indicated that cultivars with higher relative water content are more water stress resistant.
Many workers have suggested that total water potential of plant tissue may differentiate between water stress-resistant and -susceptible cultivars. More water stress-resistant lines of Glycine max (L.) Merr (Cortes and Sinclair, 1986), Hordeum vulgare L. (Hanson et al., 1977; Matin et al., 1989) and Oryza sativa L. (Novero et al., 1985) have been shown to maintain higher total leaf water potentials. Genotypic total water potential variation in large populations of plants has been obtained by Sammons et al. (1978) in Glycine max, Blum (1974) in Sorghum bicolor (L.) Moench, and Quarrie and Jones (1979) in Triticum aestivum. These studies concluded that higher total leaf water potentials in specific cultivars within the populations indicated increased water stress resistance. Additionally, various physiological indices have been used in the past to differentiate genotype response to water stress. For example, Quarrie and Jones (1979) reported genotypic differences in Triticum aestivum L. leaves in the rate of water potential decrease.
It is well known that water deficit in plants can induce a lowering of osmotic potential in some species and cultivars which contributes to cell turgor maintenance at low leaf water potentials (Brown, 1995). Maintenance of turgor pressure helps the plant in maintaining stomatal opening, photosynthesis, and more water uptake from the soil (Brown, 1995). Genotypic differences in turgor maintenance have been reported on several grasses such as wheat (Morgan, 1977; Fischer and Sanchez, 1979), sorghum (Ackerson et al., 1980) and pearl millet (Henson et al.,1982).
Agropyron scabrifolium (Döell) Parodi is a perennial tussock grass native to the northeast and central Argentina growing mainly close to water courses. This species has also established successfully in the semiarid region of this country showing resistance to water stress conditions (Kade and Cardielo, 1983). Although this species can then show resistance to water stress, the mechanisms underlying this response are not well understood. In central Argentina, it has replaced to some extent to Agropyron elongatum (Host) Beauv., another common perennial, water-resistant, less palatable forage grass in this region (García et al., 1992). Although water stress resistance make these species valuable forages in arid and semiarid regions of Argentina (Covas and Ballari, 1969), research is limited on their water stress resistance and physiological responses to water stress.
We sought to determine the effects of water stress during the vegetative stage of development on leaf water relations and leaf growth rates in two genotypes of A. scabrifolium coming from regions with different ecological characteristics, and in one genotype of A. elongatum. Leaf water and osmotic potentials, turgor pressure, and relative water content, and their relationship to leaf growth, were measured in an attempt to differentiate genotypes differing in apparent water stress resistance.
Materials and Methods
Experimental procedures
Studies were performed in genotypes El Palmar INTA and Selección Anguil of A. scabrifolium, and El Vizcachero of A. elongatum. Agropyron scabrifolium El Palmar INTA is native to a region with warm-temperate and humid climate without a dry season, and an average annual precipitation of 900 mm. Agropyron scabrifolium Selección Anguil comes from a region with a sub-humid, dry to semiarid climate and a mean annual precipitation of 600 mm. Agropyron elongatum is a widespread forage in semiarid Argentina with well-known water stress resistance.
Seeds of the three Agropyron genotypes were sown during mid-summer (6 February) in 10 l pots filled with soil under glasshouse conditions with natural sunlight and photoperiod. Plants were kept under these conditions during the study. Seeding was carried out at a time when commercial seeding usually takes place under field conditions. Thinning was thereafter conducted leaving 12 plants on each pot. Two water treatments were imposed on a total of 96 pots (50% irrigated, 50% water-stressed): irrigated plants were maintained near field capacity by irrigating daily during the experiment while water was withheld from water-stressed plants from day 120 to day 141 after sowing. Four pots (replicates) were randomly assigned to each genotype and water treatment combination, and different pot sets were sampled 3, 14, 17 and 21 days after withholding water. When pots were sampled more than once, determinations were effected on different plants. On each pot, 9 to 12 plants were destructively sampled for determining plant-water relation parameters while another group of 3 plants was used for conducting leaf growth measurements. These later measurements, however, were conducted only on those pots assigned to the last sampling date. Glasshouse temperatures during the measurement period ranged between 15 and 18ºC by day and between 10 and 12ºC by night.
The development of water stress was monitored by measuring pre-dawn leaf water and osmotic potentials, and relative water content of youngest, fully expanded leaves. These measurements were conducted using 4 replicate leaves, one per pot, on each genotype and water treatment. Leaf water potentials were measured by the pressure chamber technique following Turner (1981). Leaf osmotic potentials were measured thereafter on the same leaves after freezing in liquid nitrogen and measuring osmotic potential in the expressed sap using a
vapour pressure osmometer (Wescor model 5500, USA). Osmotic potentials, which were not corrected for the dilution from apoplastic water, were calculated according to the following formula: osmotic potential (J kg -1)=-RCT, where R is the gas constant (J K -1 mol -1 ), T the absolute temperature (ºK) and C the osmotic concentration (mol kg -1 ); values of osmotic potential were multiplyied by 0.001 to obtain this parameter in MPa. Turgor pressures were calculated as the difference between leaf water and osmotic potentials (Turner, 1981). Measurements of leaf relative water content were effected following Turner (1981).
At the beginning of the study, one tiller on each of 3 plants per pot was permanently marked using a wire loop at its base; 4 replicates were used per genotype and water treatment. The leaf apex of the youngest expanding leaf on each selected tiller was daily marked using a water-proof pen; daily growth was calculated as the distance between two adjacent points (Cutler et al., 1980). Leaf growth rates were calculated by dividing the amount of new leaf length produced during any period by the number of days of that period.
At the last sampling date, three to four wholly expanded leaves were taken from each of four replicate pots per genotype and water treatment. Total leaf area was determined on those leaves used for wax extraction. This was accomplished by weighing leaf heliographic copies and then relating this weight to that of known surface areas. Leaves were immersed in chloroform during 15 sec. The resulting solutions were then filtered using filter paper Whatman N o 43 and evaporated to near dryness in a chamber with nitrogen to prevent oxidation of organic compounds. Wax content was determined following Ebercon et al. (1977). In addition,
wax was extracted from 30 leaves of A. scabrifolium and 40 leaves of A. elongatum to obtain a calibration curve; details of this procedure are given by García et al. (1992). Wax content will be expressed in g cm -2 leaf area.
TABLE 1.Three-way analysis of variance examining the effects of water level, plant genotype and harvest date on
total leaf and osmotic potentials, turgor pressure and relative water content for Agropyron scabrifolium cv
Selección Anguil, A. scabrifolium cv El Palmar INTA and A. elongatum. Data are presented in Figs 1 and 2.

Statystical analysis
A completely randomized block experimental design was used in this study. Plant water relation variables were analyzed using three-way (2 water levels x 3 genotypes x 8 sampling dates) ANOVA. Differences in leaf growth rate between irrigated and water-stressed plants (3 genotypes x 12 sampling dates) or the wax data (2 water levels x 3 genotypes) were analyzed using two-way ANOVA. Student-Newman-Keuls test was utilized for mean separation when F tests were significant at the 0.05 level. Linear regression analysis was used to investigate relationships between leaf water potential, osmotic potential, turgor pressure or relative water content following Neter and Wasserman (1974).
Results and Discussion
Plant water relations
Genotype and date interacted significantly (P<0.05) with water level when studying leaf water and osmotic potentials, turgor pressure and relative water content (Table 1). By day 12 after watering was withheld, the predawn leaf water potential was lower (P<0.05) than at previous sampling dates and reached the lowest (P<0.05) value by the end of the study in the water-stressed plants of the three genotypes (mean??1 s.e.; A. scabrifolium El Palmar INTA= -1.83?0.15 MPa, A. scabrifolium Selección Anguil= -1.23?0.33 MPa, A. elongatum= -1.34?0.18 MPa; Fig. 1). In agreement with other reports (e.g., Sobrado, 1986), this degree of water deficit was considered mild and slowly achieved.
When predawn leaf water potential of water-stressed plants was taken as the average of all values from day 1 to the end of measurements, it was lower (P<0.05) in A. scabrifolium El Palmar INTA (mean=-0.89 MPa) than in A. scabrifolium Selección Anguil (mean=-0.56 MPa) and A. elongatum (mean=-0.51 MPa). Several authors have reported that more water stress resistant crop plants can maintain higher total leaf water potentials than those susceptible (Hanson et al., 1977; Quarrie and Jones, 1979; Cortes and Sinclair, 1986; Matin et al., 1989). From 9 to 13 days after withholding water, predawn leaf water potentials in water-stressed plants decreased from -0.28?0.01 to -0.83?0.14 MPa in A. scabrifolium Selección Anguil, -0.36?0.04 to -1.49?0.25 MPa in A. scabrifolium El Palmar INTA and -0.21?0.02 to -0.74?0.20 MPa in A. elongatum (Fig. 1). At the same time, predawn leaf osmotic potentials in these plants only followed concomitant decreases in leaf water potentials by days 12-13 in A. scabrifolium Selección Anguil, and days 11-13 in A. scabrifolium El Palmar INTA and A. elongatum (Fig. 1). This determined reductions in turgor pressure in all three genotypes, although they were greater in A. scabrifolium El Palmar INTA than in A. scabrifolium Selección Anguil or A. elongatum because its earlier and greater decreases in leaf water potentials were not accompanied with parallel decreases in leaf osmotic potentials (Fig. 1). In fact, predawn leaf osmotic potentials on water-stressed plants of A. scabrifolium Selección Anguil and A. elongatum were only significantly lower (P<0.05) by 21 days after withholding water (mean=-1.47 MPa) than at previous sampling dates (mean=-1.20 MPa). Maximum rates of decline in predawn leaf water potential, osmotic potential or turgor pressure were -0.28, -0.13 or 0.15 MPa d -1 , respectively, from 9 to 13 days after withholding water in all three genotypes. Similar rates of decline for leaf and osmotic potentials, and turgor pressures, have been found in other studies after exposing grass plants to water stress (Westgate and Boyer, 1985; McCree, 1986; García Girou and Curvetto, 1988).

FIGURE 1. Predawn turgor pressure (s, water stress; ?, irrigation), and osmotic (n, water stress; q, irrigation) and water (l, water stress; m, irrigation) potentials in genotypes Selección Anguil and El Palmar INTA of A. scabrifolium and A. elongatum under increasing water stress and under irrigated conditions.
Each symbol is the average of n=4.
After 17 days of withholding water, and except by day 14 in A. elongatum, A. scabrifolium Selección Anguil and A. elongatummaintained turgor pressures greater than 0.43 MPa (Fig. 1). Turgor pressures in A. scabrifolium El Palmar INTA, however, only ranged from 0.19?0.13 to 0.36?0.12 MPa between 12-21 days after water was withheld (Fig. 1), and they were lower (P<0.05) in this genotype (mean=0.50 MPa) than in A. scabrifolium Selección Anguil (mean=0.69 MPa) and A. elongatum (mean=0.70 MPa). Turgor pressures were also lower (P<0.05) in A. scabrifolium El Palmar INTA (mean=0.83 MPa) than in A. elongatum (mean=0.91 MPa) on controls but differences were not as great as those on water-stressed plants. Predawn leaf water potentials also decreased (P<0.05) in unstressed plants of the Agropyron genotypes after 13 days of withholding water but they ranged on average from -0.16 to -0.33 MPa (Fig. 1); mean values for this parameter in these plants, however, were lower (P<0.05) in A. scabrifolium El Palmar INTA and A. scabrifolium Selección Anguil (-0.27 MPa in both genotypes) than in A. elongatum (-0.18 MPa). Mean pre-dawn leaf osmotic potentials were about -1.11 MPa in control plants of the three genotypes during the entire experiment.

FIGURE 2. Leaf relative water content in genotypes Selección Anguil and El Palmar INTA of A. scabrifolium and A. elongatum under increasing water stress (l) and under irrigated (m) conditions. Each symbol is the average of n=4.
Leaf relative water content was similar (P>0.05) among genotypes on control plants (mean=98.8%) and it declined slightly but significantly (P<0.05) from 10 (mean=99.43%) to 21 days (mean=98.31%) after study initiation in these plants (Fig. 2). Declines in relative water content were also significant (P<0.05) but much more pronounced on water-stressed plants of the three genotypes, and values ranged from 99.05 to 75.44% after 3 to 21 days of withholding water, respectively (Fig. 2). Relative water contents on water-stressed plants, however, were lower (P<0.05) in A. scabrifolium El Palmar INTA ( mean=88.69% ) than in A. scabrifolium Selección Anguil (95.70%) and A. elongatum (94.91%) (Fig. 2). Matin et al. (1989) found that relative water contents were usually significantly higher in the water stress resistant cultivars than on those susceptible of Hordeum vulgare after the development of enough plant stress. These results agree with Kirkham et al. (1980), Clarke and McCraig (1982), Carter and Patterson (1985) and Schonfeld et al. (1988), who found that cultivars believed to be more water stress resistant usually maintained higher leaf relative water contents under stress.

FIGURE 3. Relationship of relative water content (x) to total water potential (y) on leaves of water-stressed (l) or irrigated (m) plants of A. scabrifolium cv Selección Anguil (water-stressed, y=5.390-0.214x+0.002x2, R 2 =0.80, P<0.001; irrigated, y=-7.25+0.071x, R 2 =0.31, P=0.001), A. scabrifolium cv El Palmar INTA (water-stressed, y=1.270-0.112x+0.001x 2 , R 2 =0.83, P<0.001; irrigated, y=-0.056-0.002x, R 2 =0, P>0.8) or A. elongatum (water-stressed, y = 9.418 -0.300x + 0.002x 2 , R 2 =0.71, P<0.01; irrigated, y =-1.69 + 0.015x, R 2 =0.5, P>0.2). Linear regressions were fitted to the data when the curvature effect coefficient was not significant. Symbols are individual data points.
The trends of pre-dawn leaf water potentials versus relative water content were different for control and water-stressed plants of the Agropyron genotypes (Fig. 3): decreases in predawn leaf water potentials per unit decrease in relative water content were larger on water-stressed than on control plants. Similar results have been reported in other cultivated plants under water stress (Jones and Turner, 1978, 1980). These adjustments in the relationship between water potential and water content are determined by the interactions between osmotic concentration and wall elasticity (Tyree and Jarvis, 1982). Decreases in relative water content per unit decrease in total leaf water potential were similar (P>0.05) on water-stressed plants of the three genotypes (Fig. 3). Water stress-resistant plants have been shown to have smaller water deficit per unit decrease in leaf water potential than more water stress-susceptible plants (e.g. Levitt, 1972; Carter and Patterson, 1985), which suggests a similar resistance to water stress in both genotypes of A. scabrifolium and in A. elongatum. The relationship between osmotic potential and relative water content was also similar (P>0.05) among genotypes (Fig. 4).
Within each genotype, regression lines of the relationship between turgor pressure and total leaf water potential were similar (P>0.05) between water-stressed and control plants (Fig. 5). However, leaf turgor pressures tended to be higher in water-stressed than in control plants when leaf water potentials declined (Fig. 5), which suggests the ability of turgor maintenance in the face of declining leaf water potentials.

FIGURE 4. Relationship of osmotic potential (x) to relative water content (y) on leaves of water- stressed plants of A. scabrifolium cv Selección Anguil (y=15.2-0.444x, R 2 =0.54, P<0.001), A. scabrifolium cv El Palmar INTA (y=13.8-0.352x, R 2 =0.87, P<0.001) or A. elongatum (y=14.2- 0.379x, R 2 =0.71, P<0.001). Symbols are individual data points.
Leaf epicuticular wax
Wax content was not significantly different (P>0.05) among genotypes or water level treatments (Fig. 6). However, it was similar on water-stressed and irrigated plants of A. scabrifolium El Palmar INTA, but more than 50% greater on water-stressed than on irrigated plants in A. scabrifolium Selección Anguil and A. elongatum (Fig. 6). Increases of epicuticular wax production as a result of water stress have been shown in other Agropyron species (Jefferson et al., 1989). Brown (1995) reported that leaf cuticular waxes possibly lead to reduced cuticular loss of water when stomata are closed, and generally are felt to increase water conservation during water stress periods. Thus, although not significantly different (P>0.05), the tendency to a greater epicuticular wax content in A. scabrifolium Selección Anguil and A. elongatum than in A. scabrifolium El Palmar INTA may have contributed to determine greater turgor pressures in the first two than in the later genotype.
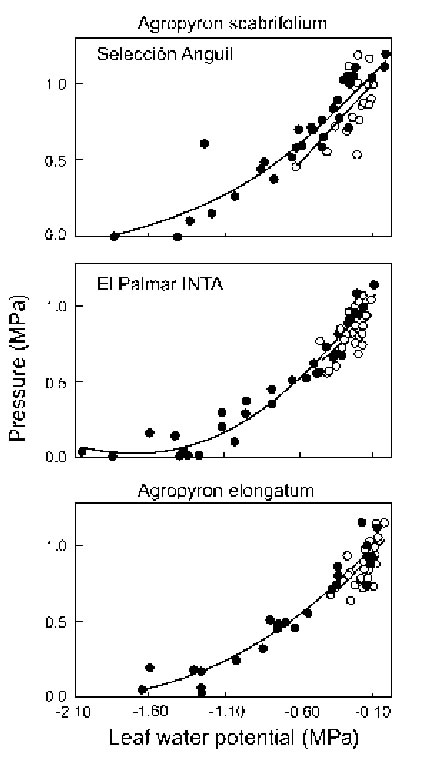
FIGURE 5. Relationship of total water potential (x) to turgor pressure (y) on leaves of water-stressed (l) or irrigated (m) plants of A. scabrifolium cv Selección Anguil (water-stressed, y=1.264+0.125x+0.003x 2 , R 2 =0.94, P<0.01; irrigated, y=1.18+0.109x, R 2 =0.38, P<0.001), A. scabrifolium cv El Palmar INTA (water-stressed, y=1.331+0.169x+0.006x 2 , R 2 =0.90, P<0.001; irrigated, y=1.09+0.092x, R 2 =0.32, P<0.001) or A. elongatum (water-stressed, y=1.162+0.115x+0.0031x 2 , R 2 =0.93, P<0.001; irrigated, y=1.11+0.111x, R 2 =0.40, P<0.001). Linear regressions were fitted to the data when the curvature effect coefficient was not significant. Symbols are individual data points.
Plant growth
Rates of leaf growth decreased in both water levels for all genotypes during the study period (Fig. 7). Leaf growth rates were always smaller in water-stressed than in irrigated plants of the three Agropyron genotypes (Fig. 7). Reduction of leaf growth because of water stress is well known in grasses (e.g., Brown, 1995), and it has been observed in other Agropyron species (Busso and Richards, 1995). Reductions in leaf growth because of water stress were greater (P<0.05) during days 9 to 13 after withholding water when compared to values at the beginning and end of the study period (Fig. 7). The smallest (P<0.05) differences in leaf growth rate between water-stressed and irrigated plants were observed in A. elongatum (mean= 0.27 cm d -1 ) in comparison to A. scabrifolium Selección Anguil (mean=0.62 cm d -1 ) and A. scabrifolium El Palmar INTA (mean=0.76 cm d -1 ) (Fig. 7). These differences tended to be the greatest in A. scabrifolium El Palmar INTA, although they were not significantly different (P>0.05) from those in A. scabrifolium Selección Anguil.
Based on the greater leaf growth reductions in A. scabrifolium El Palmar INTA than in A. scabrifolium Selección Anguil and A. elongatum in response to water stress, A. scabrifolium Selección Anguil and A. elongatum appeared more water stress resistant than A. scabrifolium El Palmar INTA. Agropyron scabrifolium Selección Anguil and A. elongatum maintained greater
turgor pressures, relative water contents and leaf water potentials than A. scabrifolium El Palmar INTA after withholding irrigation. It appeared to be a tendency for a greater epicuticular wax accumulation on the leaf surfaces of A. scabrifolium Selección Anguil and A. elongatum than on those of A. scabrifolium El Palmar INTA. Greater values for these parametes in A. scabrifolium Selección Anguil and A. elongatum may contribute to explain their smaller leaf growth reductions under water stress.Ê Similar relative water content per unit decrease in total leaf water potential; however, did not suggest differences on water stress resistance in these genotypes. More research is then needed to determine whether differences in resistance to water stress exist or not among genotypes of A. scabrifolium and A. elongatum.

FIGURE 6. Epicuticular wax content on leaves of water-stressed (n) or irrigated (q) plants of A. scabrifolium cv Selección Anguil, A. scabrifolium cv El Palmar INTA or A. elongatum at the end of the study. Each histogram is the mean of n=4. Vertical bars represent one s.e.m.

FIGURE 7. Leaf growth rate on water-stressed (l) or irrigated (m) plants of A. scabrifolium cv Selección Anguil, A. scabrifolium cv El Palmar INTA or A. elongatum at different times after withhold-ing water. Each symbol is the mean of n=4.
References
ACKERSON RC, KRIEG DR, SUNG FJM (1980). Leaf conductance and osmoregulation of field-grown sorghum genotypes. Crop Sci 20: 10-14. [ Links ]
BLUM A (1974). Genotypic response in sorghum to drought stress. I. Response to soil moisture stress. Crop Sci 14: 361-364. [ Links ]
BROWN RW (1995). The water relations of range plants: Adaptations to water deficits. p. 291-413. In: Wildland Plants. Physiological Ecology and Developmental Morphology. D.J. Bedunah, R.E. Sosebee (Eds). Society for Range Management, Denver, CO. [ Links ]
BUSSO CA, RICHARDS JH (1995). Drougth and clipping effect on tiller demography and growth of two tussok grasses in Utah. J Arid Environ 29: 239-251. [ Links ]
CARTER JE Jr, PATTERSON RP (1985). Use of relative water content as a selection tool for drought tolerance in soybean. In: 1985 Agronomy Abstract. ASA. Madison, WI. [ Links ]
CLARKE JM, MCCRAIG TM (1982). Excised-leaf water retention capability as an indicator of drought resistance of triticum genotypes. Can J Plant Sci 62: 571-578. [ Links ]
CORTES PM, SINCLAIR TR (1986). Water relations of field grown soybean under drought. Crop Sci 26: 993-998. [ Links ]
COVAS G, BALLARI CP (1969). Agropiro criollo. Una buena gramínea perenne para integrar pasturas asociadas en la región semiárida pampeana. Circ. Ext. 27. INTA-EEA Anguil. [ Links ]
CUTLER JM, SHAHAN KW, STEPONKUS PL (1980). Influence of water deficits and osmotic adjustment on leaf elongation in rice. Crop Sci 20: 314-319. [ Links ]
EBERCON A, BLUM A, JORDAN WR (1977). A rapid colorimetric method for epicuticular wax content of sorghum leaves. Crop Sci 17: 179-180. [ Links ]
FISCHER RA, SANCHEZ M (1979). Drought resistance in spring wheat cultivars. II. Effects on plant water relations. Aust J Agric Res 30: 801-814. [ Links ]
GARCÍA MG, ECHENIQUE CV, GARCÍA GIROU NL, CURVETTO NR (1992). Effect of water stress on the content and morphology of epicuticular waxes in Agropyron. Micr Electr Biol Cel 16: 1-16. [ Links ]
GARCIA GIROU NL, CURVETTO NR (1988). Osmo-regulación en cultivares contrastantes de trigo y su relación con la etapa del desarrollo. An Edafol Agrobiol 46: 1261-1276. [ Links ]
HANSON AD, NELSEN CE, EVERSON EH (1977). Evaluation of free proline accumulation as an index of drought resistance using two contrasting barley cultivars. Crop Sci 17: 720-726. [ Links ]
HENSON IE, MAHALAKSHMI V, BIDINGER FR, ALAGARSWAMY G (1982). Osmotic adjustment to water stress in pearl millet [Pennisetum americanum (L.) Leeke] under field conditions. Plant Cell Environ 5: 147-154. [ Links ]
JEFFERSON PG, JOHNSON DA, RUMBAUGH MD, ASAY KH (1989). Water stress and genotypic effects on epicuticular wax production of alfalfa and crested wheatgrass in relation to yield and excised leaf water loss rate. Can J Plant Sci 69: 481-490. [ Links ]
JONES MM, TURNER NC. (1978). Osmotic adjustment in leaves of sorghum in response to water deficits. Plant Physiol 61: 122-126. [ Links ]
JONES MM, TURNER NC (1980). Osmotic adjustment in expanding and expanded leaves of sunflowers in response to water deficits. Aust J Plant Physiol 7: 181-192. [ Links ]
KADE M, CARDIELO R (1983). Introducción de especies forrajeras en el partido de Chascomús, Prov. de Bs. As. Gramíneas invernales. IDIA 413-416: 70-85. [ Links ]
KIRKHAM MB, SMITH EL, DHANASOBHON C, DRAKE TI (1980). Resistance to water loss of winter wheat flag leaves. Cereal Res Commun 8: 393-399. [ Links ]
LEVITT J (1972). Responses of plants to environmental stress. Academic Press, New York. [ Links ]
MATIN MA, BROWN JH, FERGUSON H (1989). Leaf water potential, relative water content, and diffusive resistance as screening techniques for drought resistance in barley. Agron J 81: 100-105. [ Links ]
McCREE KJ (1986). Whole-plant carbon balance during osmotic adjustment to drought and salinity stress. Aust J Plant Physiol 13: 33-43. [ Links ]
MORGAN JM (1977). Differences in osmoregulation between wheat genotypes. Nature 270: 234-235. [ Links ]
NETER J, WASSERMAN W (1974). Applied Linear Statistical Models. Regression, Analysis of Variance, and Experimental Designs. Richard D. Irwin, Inc., Homewood. [ Links ]
NOVERO RP, O'TOOLE JC, CRUZ RT, GARRITY DP (1985). Leaf water potential, crop growth response and microclimate of dry-land rice under line-source sprinkler irrigation. Agric For Meteorol 35: 71-82. [ Links ]
QUARRIE SA, JONES HG (1979). Genotypic variation in leaf water potential, stomatal conductance, and abscisic acid concentration in spring wheat subjected to artif icial drought stress. Ann Bot 44: 323-332. [ Links ]
SAMMONS DJ, PETERS DB, HYMOWITZ T (1978). Screening soybeans for drought resistance. I. Growth chamber procedure. Crop Sci 18: 1050-1055. [ Links ]
SCHONFELD MA, JOHNSON RC, CARVER BF, MORNHINWEG DW (1988). Water relations in winter wheat as drought resistance indicators. Crop Sci 28: 526-531. [ Links ]
SINCLAIR TR, LUDLOW MM (1985). Who taught plants thermodynamics?. The unfulfilled potential of plant water potential. Aust J Plant Physiol 12: 213-217. [ Links ]
SOBRADO MA (1986). Tissue water relations and leaf growth of tropical corn cultivars under water deficits. Plant Cell Environ 9: 451-457. [ Links ]
TURNER NC (1981). Techniques and experimental approaches for the measurement of the plant water status. Plant Soil 58: 339-366. [ Links ]
TYREE MT, JARVIS PG (1982). Water in tissues and cells. In: Encyclopedia of plant physiology Vol. 12B. Physiological Plant Ecology II. Water relations and carbon assimilation. O.L. Lange, P.S. Nobel, C.B. Osmond, H. Ziegler (Eds). Springer-Verlag, Berlín, pp. 35-77. [ Links ]
WESTGATE ME, BOYER JS (1985). Osmotic adjustment and the inhibition of leaf, root, stem and silk growth at low water potentials in maize. Planta 164: 540-549. [ Links ]
Received on May 3, 2001.
Accepted on July 26, 2002.














