Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell v.26 n.3 Mendoza ago./dic. 2002
Effects of high molybdenum intake on 1,2-dimethylhydrazine-induced intestinal tumors in rats
M.A. Montenegro1, M. Sánchez Negrette1, E.J. Gimeno2, J.T. Borda1
1 Faculty of Veterinary Sciences, National University of Norwest-UNNE- Argentina.
2 Institute of Pathology, Faculty of Veterinary Sciences, National University of La Plata, Argentina.
E.J. Gimeno is a research career member from CONICET.
Address correspondence to: Dr. Marcial Sánchez Negrette. Cátedra de Patología General y Sistemática, Facultad de Ciencias Veterinarias, Universidad Nacional del Nordeste (UNNE). Sargento Cabral 2139. (3400) Corrientes, ARGENTINA. Tel/Fax: (+54- 3783) 425 753 int. 143. E-mail: Patgral@vet.unne.edu.ar
Key words: Intestinal cancer. Molybdenum. 1,2-dimethylhydrazine. Rats.
Abstract: Wistar male rats, 3 months of age were given ad-libitum a nutritionally adequate diet and demineralized drinking water. The Molybdenum (Mo) and Tungsten (W) were provided in the drinking water at 200 ppm concentration. Intestinal tumors were induced by 1,2-dimethylhydrazine (DMH) given subcuta-neously as 16 weekly doses at 20 mg/kg body weight. Mo in the form of (NH 4 ) 6 Mo 7 O 24 4H 2 O or W in the form of (Na 2 WO 4 ) were provided in the drinking water two months before the first DMH treatment and were continued during 4 months more until the last DMH treatment. Three months after the last carcinogen injec-tion, all animals were sacrificed and examined for intestinal tumors. The number, size and location of the tumors were recorded and the pathology was examined. The addition of Mo to the drinking water induced an increase of hepatic Mo content. At the end of the second month, the hepatic content of Mo was 5.61 ppm, compared with control and W groups (2.18 and 0.96 ppm, respectively). A significantly lower incidence of tumors was observed in the Mo group (47), compared with the control group given DMH alone (105) and W group (113). On the other hand, the Mo group showed a significant decrease in the numbers of multiple tumors per rat.
Introduction
Colorectal cancer is the second most common cancer in terms of incidence and mortality for both men and women in most of developed countries of the world. Although the etiology of the colon cancer is unknown, evidence from epidemiological (Correa and Haenszel, 1978; Wynder and Reddy, 1983), experimental (Free-man et al., 1978; Reddy et al.,1989) and genetic (Willet, 1989; Cannon-Albright et al., 1988) studies suggest that colon cancer genesis may be the product of complex interactions of genetic susceptibility, carcinogens, promoters, and inhibitors. Environmental and dietary factors are considered to be responsible for 85-90% of all cases (Vargas and Alberts, 1992). Among the dietary components suggested as colon cancer promoters are excessive fat and calories and low intake of various dietary fibers, vegetables, and micronutrients such as the antioxidant vitamins (e.g., vitamins C and E, selenium and B-carotene) (Byers and Perry, 1992), and calcium. Laboratory studies in humans (Buset et al., 1986) and animals (Wargovich and Baer, 1989) suggest a protective effect of dietary calcium in colon carcinoma etiology.
Nevertheless, the relationship between colorectal cancer and other elements, such as magnesium, iron, potassium, sodium, manganese, zinc, copper, phosphorous, selenium and germanium was evaluated in humans and laboratory animals. (Jacobs, 1990; Gershbein et al., 1993; Nelson et al.,1994; Yang, 1993; Di Silvestro et al., 1992; Pence, 1991; Mori et al., 1993; Jao et al., 1990).
With reference to molybdenum, epidemiological studies observed that the deficiency is related to esophageal cancer in humans. Thus, low intake of molybdenum is related to the high incidences of esophageal cancer in South Africa among the Bantu of Transkei (Burrell et al., 1966), in China (Luo et al., 1983), and in Russia (Nemenko et al., 1976).
Experimentally, several studies have demonstrated the protective effect of molybdenum in experimental carcinogenesis. Sodium molybdate (Na 2 MoO 4 ) administered in drinking water at a concentration of 2 mg/l reduced the incidence of N-nitrososarcosine ethyl ester-induced esophageal and forestomach cancer in male Sprague-Dawley rats (Luo et al., 1983).
Dietary molybdenum at 2 ppm significantly inhibited N-methyl-N-benzylnitrosamine-induced esophagus squamous cell carcinomas in F344 rats (Komada et al., 1990). High levels of molybdenum were found in the esophagus and forestomach tissues. The incidence of mammary gland tumors induced by N-nitroso-N-methylurea (NMU) was lower in female Sprague-Dawley rats receiving 10 mg/l sodium molybdate in drinking water compared with controls (Wei et al., 1985; Seaborn and Yang, 1993).
Molybdenum dichloride inhibits growth of Ehrlich ascites tumors in mice (Kopf-Maier et al., 1979). The present study was designed to develop an animal model to investigate the role of dietary molybdenum in the prevention of intestinal carcinogenesis.
Materials and Methods
Wistar male rats, 3 months old, with average body weight of 180 g were given ad-libitum a nutritionally adequate diet and drinking water with 200 ppm molybdenum (Mo) or 200 ppm Tungsten (W). A total of 120 rats were randomly divided into three experimental groups of 20 rats each group for carcinogen administration (with or without Mo and W), and three controls group without carcinogen injections of 20 rat each group (with or without Mo and W). The body weight was assessed twice a month during the first 6 months and every 30 days until the end of the experience.
Intestinal tumors were induced by 1,2-dimethylhydrazine (DMH) given subcutaneously as 16 weekly doses at 20 mg/kg body weight. The carcinogen (DMH) solution was prepared just before its administration. DMH solution use for injection comprised 400 mg of DMH dissolved in 100 ml of water containing 37 mg of ethylene-diamine tetraacetic acid (EDTA) and the pH was raised to 6.5 with sodium hydroxide. The animals for vehicle treatment were given the same volume of EDTA, pH 6.5.
Mo in the form of (NH 4 ) Mo 7 O 24 4H 2 O and W in the form of (Na 2 WO 4 ) were provided in the drinking water two months before the first DMH treatment and was continued during 4 months more until the last DMH treatment. Three months after the last carcinogen injection, all surviving animals were sacrificed by cervical dislocation after etherization and examined for intestinal tumors.
A full autopsy was performed on each animal, particular attention being paid to the large bowel, which was removed, opened along its length and pinned with its mucosal surface uppermost to a corkboard. The whole large bowel was then fixed in 10% neutral buffered formalin for 24 hs.
For each animal the total length of the fixed colon was recorded, together with a brief description of the naked eye appearances of any tumors, including measurement and site (measured in cm from the anus). The tumors were grossly classified into Polypoid type growth (pedunculated lesions and sessile or broad-based lesions) and Non-Polypoid type growth without intramucosal protuberant growth (flat tumors with plaque-shaped or ulcerative-infiltrative carcinomas).
Transverse blocks of all tumors were taken and processed through to paraffin wax. The rest of the colon was processed according to the Swiss-roll technique (Rubio et al., 1986). Histological sections 5 mm thick were prepared and stained with hematoxylin and eosin.
Histologically, the neoplasms of intestine were classified according to histologic type. The tumors were classified into tubular and tubulovillous adenomas; tubular, villous and tubulovillous adenocarcinomas, signet-ring cell and mucinous carcinomas.
Blood was sampled for analyzing Cu and the liver was sampled for determining Mo and Cu concentration. Both determinations were done by high-pressure liquid chromatography.
Tumor data for each rat and for each treatment group were analyzed by using the Kruskall - Wallis test. Weight gain data for each treatment group were analyzed using a program for analysis of variance (ANOVA) with repeated measures.
Results
The average body weights of the animals from experimental and control groups were all very similar, regardless of their dietary treatment and carcinogen administration. The addition of Mo or W to the drinking water provoked an increasing or decreasing respectively of hepatic Mo contents (P < 0.001) and no modification of hepatic Cu content was observed. High Mo intake did not cause any general DMH toxicity as assessed by body weight gain. At the end of first month, the hepatic content of Mo was 4.65 ppm and at the second month was 5.61 ppm, compared with the control group 2.61 ppm and 2.18 ppm and W group 0.70 ppm and 0.96 ppm. The hepatic content of Cu was similar in all groups: Mo + DMH group = 12.16 ppm; DMH alone group = 11.80 ppm; W + DMH group = 11.16 ppm. The levels of Cu in serum were no different between three groups.
Gross and histopathologic examinations:
No histopathologic change was found in the intestines of control groups rats (with or without Mo and W administration) without carcinogen injection.
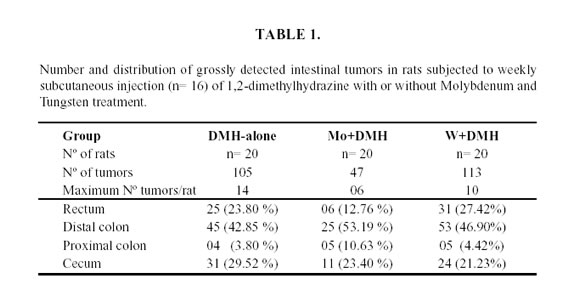
The addition of Mo to the drinking water at 200 ppm provoked a significant 50% decrease of intestinal tumors frequency. The number and location of the tumors is showed in Table 1.
In the Mo group a significant lower incidence of tumors was observed (47), compared with the W group (113) and in the DMH group (105). The small difference in tumor incidence observed between W+DMH group and DMH group was not statistically significant. A major number of tumors were observed in the microscopic study, the DMH group showed 14 tumors, the Mo+DMH group 5 tumors, and the W+DMH group 4 tumors.
No difference in the size and location of tumors was observed between three experimental groups. The intestinal tumors were more commonly found in the distal colon and rectum in the three experimental groups.
The frequency of polypoid and non-polypoid growth is showed in Table 2.
No difference in the frequency of polypoid and no polypoids type was found between Mo+DMH and W+DMH groups. However, the DMH group showed a major number of polypoid tumors.
With reference to ulcerative-infiltrative type, the percentage was similar between DMH and W+DMH group. However, the percentage was minor in the Mo+DMH group (X 2 = 27.5; P < 0,001).
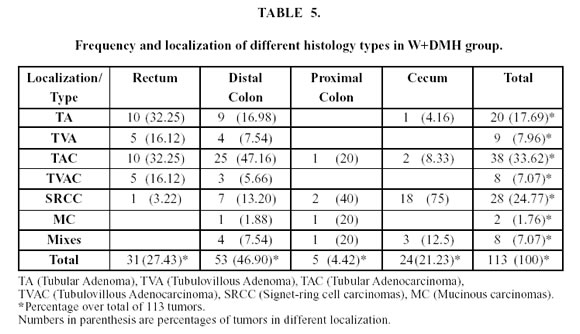
The frequency and localization of different histology types of tumors are showed in Tables 3, 4 and 5. No difference was observed between the histological types in the three experimental groups.
The tubular adenomas and adenocarcinomas were more commonly found in the distal colon and rectum in the three groups and the signet-ring cell carcinomas were more common in the cecum.
The frequency between benign and malignant tumors was similar in the three experimental groups. In the Mo+DMH group 6 adenomas (12.76%) and 41 (87.24%) malignant tumors were observed, compared with the DMH group with 10 adenomas (9.55%) and 95 (90.45%) malignant tumors.
Results of histology examination demonstrate that in the experimental groups there were usually a correlation between the macroscopic form and the microscopic structure of epithelial intestinal tumors. The poly-poid tumors consisted of tubular adenomas or tubular adenocarcinomas with minimal frequency of invasion of the intestinal wall. The highly invasive signet-ring cell and mucinous carcinomas were mainly non-poly-poid lesions.
In many cases, the tubular adenocarcinoma exhibited the features of mucus-secreting adenocarcinomas which in some areas can be transformed into mucinous or signet-ring cell carcinomas; in the latter case, metastases were very common.
Discussion
Molybdenum is ubiquitous in foodstuff and in plant and animal tissues. Shellfish have high concentrations of molybdenum because the plankton they eat concentrate the element from sea water. Humans ingest an average of 350 mg molybdenum per day in food (Hammond and Beliles, 1980). The daily requirement of molybdenum for humans is estimated to be 0.1 to 0.5 mg, but exact requirements are not known (Venugopal and Lukey, 1978; National Research Council, 1980).
In rats, diets that contained approximately 0.020 mg/kg of molybdenum (approximately 0.2 mg per rat per day) appeared to be adequate to support the normal growth of the male Sprague-Dawley rats (Higgins et al., 1956; Luo et al., 1983).
In our experience the presence of 200 ppm Mo or 200 ppm W in drinking water exerted no detrimental effects as evidenced by the data on body weight and histopathologic examinations.
The addition of Mo or W to the drinking water provoked an increasing and decreasing respectively of hepatic Mo content. At the end of second month, the hepatic content of Mo was 5.61 ppm in the Mo group, 0.70 ppm in the W group and 2.18 ppm in the control group. These results are coincident with those obtained by Luo et al. (1983), who supplemented the drinking water with Mo and W in Sprague Dawley rats.
High intake of Mo has been reported to interfere with the metabolism of Cu (Underwood, 1977). In the present study, the supplementation with Mo or W in the drinking water showed no interference on the metabolism of Cu in the body, because no significant difference in either hepatic Cu and serum Cu content were observed.
In our experience the addition of Mo to the drinking water decreased the tumor intestinal incidence in rats treated with 1,2-dimethylhydrazine. Nevertheless, the supplemented Mo+DMH group showed a significant decrease in the number of multiple tumor per rats. Tumor size and location were unaffected by the treatment.
The relationship between molybdenum deficiency and the incidence of esophageal cancer in humans was reported in Southern Africa and China (Burrell et al., 1966; Luo et al., 1982). Levels of Mo in the serum, hair, and urine of the inhabitants of the high-risk area in esophageal cancer were lower than those in the low-risk areas (Department of Chemical Etiology and Carcinogenesis, 1978; Nemenko et al., 1976).
Sodium molybdate administered in drinking water at a concentration of 2 mg/L reduced the incidence of N-nitrososarcosine ethyl ester-induced esophageal and forestomach cancer in male Sprague-Dawley rats (Luo et al., 1983).
In other experience, dietary molybdenum at 2 ppm significantly inhibited N-methyl-N-benzylnitrosamine-induced esophagus squamous cell carcinomas in F-344 rats (Komada et al., 1990). In this experience high levels of molybdenum were found in the esophagus and forestomach tissues.
The incidence of mammary gland tumors induced by N-nitroso-N-methylurea (NMU) was lower in female Sprague-Dawley rats receiving 10 mg/L sodium molybdate in drinking water compared with controls (Wei et al., 1985; Seaborn and Yang, 1993).
Luo et al. (1983), demonstrated that the diet supplemented with Mo increased the activities of the Mo-containing enzyme xanthine oxidase in the liver, intestine, and kidneys of the animals.
In our experience we used dimethylhydrazine, a procarcinogen; that is, it requires metabolic activation within the host to an active carcinogen. The metabolic activation of dimethylhydrazine first involves its oxidation to azomethane, a gas at body temperature which appears in the expired air of dimethylhydrazine-treated rats (Fiala, 1975; Fiala et al., 1976). A second oxidation converts azomethane to azoxymethane which is then N-hydroxylated to methylazoxymethanol. These metabolic steps probably occur in the liver and possibly in other tissues (La Mont, 1978). Methylazoxymethanol is chemically unstable at body temperature and decomposes spontaneously in vitro to formaldehyde, water, and nitrogen (Nagasawa et al., 1972). During this decomposition, the alkylating agent methyldiazonium is formed, which generates a reactive carbonium ion capable of methylating DNA, RNA, or protein (Matsumoto and Higa, 1966). The carcinogenic action of dimethyl-hydrazine involves methylation of colonic epithelial cell DNA (Hawks et al., 1971, Hawks and Magee, 1974). Grab and Zedek (1977) have presented evidence that methylazoxymethanol is converted to methylazoxy-formaldehyde by the enzyme alcohol dehydrogenase. That is very important because this enzyme is present in high concentration in rat liver and colon, which are target organs for this carcinogen.
In our experience the minor amount of tumors in rats supplemented with Mo, may be because Mo interferes in the DMH metabolism. The molybdenosis possibly enhance the enzyme concentrations where the Mo is a cofactor, such as xanthine oxidoreductase, sulfide oxidase and aldehyde oxidase. These enzymes probably inactivate some steps in the DMH metabolism, and in this way the alkylating agent methyldiazonium is not formed.
The protective action of molybdenum is considered to be enhanced detoxification by denitrosation of nitroso compounds rather than the activation reaction of dealkylation (Koizumi et al., 1995).
By the other hand, due to the enzyme alcohol dehydrogenase is present in high concentration in rat colon, which converts methylazoxymethanol to methylazoxyformaldehyde, and the Mo is a cofactor to enzyme aldehyde oxidase, we suggest that this enzyme could be able to oxide aldehyde groups and convert to methylazoxyformaldehyde in a innocuous to organism.
In conclusion, in our experience the addition of Mo to the drinking water decreased the tumor intestinal incidence and the number of multiple tumors per rats treated with 1,2-dimethylhydrazine. These data strengthen the theory that diverse components of the diet play an important role in the cause and in the prevention of colon cancer, in human beings and in experimental animals.
Acknowledgements
This research was supported by the Secretaría de Ciencia y Técnica de la Universidad Nacional del Nordeste (UNNE), Corrientes, Argentina, and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), the National Research Council of Argentina. We thank Dr. Miguel A. Quiroga (Faculty of Veterinary Sciences, UNCPBA) and Lic. Susana Cseh (INTA, Balcarce) for Cu and Mo determinations. We thank Professor Mirian Molina for the English-language revision.



References
BURRELL RJ, ROACH WA, SHADWELL A (1966). Esophageal cancer in the Bantu of the Transkei associated with mineral deficiency in garden plants. J Natl Cancer Ins 36: 201-209. [ Links ]
BUSET M, LIPKIN M, WINAWER S, SWAROOP S, FRIEDMAN E (1986). Inhibition of human colonic epithelial cell proliferation in vivo and vitro by calcium. Cancer Res 46: 5626-5630. [ Links ]
BYERS T, PERRY G (1992). Dietary carotenes, vitamin C, and vitamin E as protective antioxidants in human cancers. Annu Rev Nutr 12: 139-159. [ Links ]
CANNON-ALBRIGHT LA, SKOLNICK MH, BISHOP DT, LEE RG, BURT RW (1988). Common inheritance of susceptibility in colonic adenomatous polyps and associated colorectal cancers. N Engl J Med 319: 533-537. [ Links ]
CORREA P, HAENSZEL W (1978). The epidemiology of large bowel cancer. In: Advances in cancer research. Klein, G.; Weinhouse, S., editors. New York. Academic Press. 26: 1-141. [ Links ]
DEPARTMENT OF CHEMICAL ETIOLOGY AND CARCINOGENESIS, CANCER INSTITUTE, CHINESE ACADEMY OF MEDI-CAL SCIENCES (1978). Molybdenum content in serum, urine and hair samples among inhabitants of high and low incidence areas of esophageal cancer in Henan Province. Res Cancer Prevent Treat 4: 19-24. [ Links ]
DI SILVESTRO RA, GREENSON JK, LIAO Z (1992). Effects of Copper intake on dimethylhydrazine-induced colon cancer in rats. Proc Soc Exp Biol Med 201: 94-97. [ Links ]
FIALA ES (1975). Investigations into the metabolism and mode of action of the colon carcinogen 1,2 dimethylhydrazine. Cancer 36: 2407-2412. [ Links ]
FIALA ES, KULAKIS C, BOBOTAS G (1976). Detection and estimation of Azomethane in expired air of 1,2-dimethylhydrazine treated rats. J Natl Cancer Inst 56: 1271-1273. [ Links ]
FREEMAN HJ, SPILLER GA, KIM YS (1978). A double blind study on the effect of purified cellulose dietary fiber on 1,2-dimethylhy-drazine-induced rat colonic neoplasia. Cancer Res 38: 2912. [ Links ]
GERSHBEIN LL, REZAI VK, AMIR-MOKRI E, RAO KC (1993). Adenocarcinoma production in rats administered 1,2-dimethylhydra-zine and fed iron salt and guar gum diets. Anticancer Res 13: 2027-2030. [ Links ]
GRAB DJ, ZEDEK MS (1977). Organ-specific effects of the carcinogen methylazoximethanol related to metabolism by nicotinamide adenine dinucleotide dependent dehydrogenase. Cancer Res 37: 4182-4189. [ Links ]
HAMMOND PB, BELILES RP (1980). Metals. In: Casarett and Doull´s Toxicology: The Basic Science of Poisons (J. Doull, C.D. Klaassen and M.O. Amdur, Eds), 2 nd ed., 409-467. Macmillan Publishing Co., Inc., New York. [ Links ]
HAWKS A, MAGEE PN (1974). The alkylations of nucleic acids of rat and mouse in vivo by the carcinogen 1,2- dimethylhydrazine. Br J Cancer 30: 440-446. [ Links ]
HAWKS A, SWANN PF, MAGEE PN (1971). Probable methylation of nucleic acids of mouse colon by 1,2-dimethylhydrazine in vivo. Biochem Pharmacol 21: 432-435. [ Links ]
HIGGINS ES, RICHERT DA, WESTERFELD WW (1956). Molybdenum deficiency and tungstate inhibition studies. J Nutr 59: 539. [ Links ]
JACOBS MM (1990). Potassium inhibition of DMH-induced small intestinal tumors in rats. Nutr Cancer 14: 95-101. [ Links ]
JAO SW, LEE W, HO YS (1990). Effects of germanium on 1,2-dimethylhydrazine induced intestinal cancer in rats. Dis Colon Rectum 33: 99-104. [ Links ]
KOIZUMI T, TAJIMA K, EMI N, HARA A, SUZUKI KT (1995). Suppressive effect of molybdenum on hepatotoxicity of N-nitrosodiethylamine in rats. Biol Pharm Bull 18: 460-462. [ Links ]
KOMADA H, KISE Y, NAKAGAWA M, YAMAMURA M, HIOKI K, YAMAMOTO M (1990). Effect of dietary molybdenum on esophageal carcinogenesis in rats induced by N-methyl-N-benzyl-nitrosamine. Cancer Res 50: 2418-2422. [ Links ]
KOPF-MAIER P, LEITNER Z, VOIGTLANDER R, KOPF H (1979). Molybdocene dichloride as an anti-tumor agent. Z Naturforsch 34c, 1174-1176. [ Links ]
LA MONT JT, OGORMAN TA (1978). Experimental colon cancer. Gastroenterology 75: 1157-1169. [ Links ]
LUO XM, LU SM, LIU YY (1982). The multiple correlation study on esophageal cancer mortality and contents of chemical elements in drinking water and grains from 50 Peoples Communes in Henan Province. Chin J Epidemiol 3: 91-96. [ Links ]
LUO XM, WEI HJ, YANG SP (1983). Inhibitory effects of molybdenum on esophageal and forestomach carcinogenesis in rats. J Natl Cancer Inst 71: 75-78. [ Links ]
MATSUMOTO HT, HIGA HH (1966). Studies on methylazoxymethanol, the aglicone of cycasin: methylation of nucleic acids in vitro. Biochem J 98: 20-22. [ Links ]
MORI H, MORISHITA Y, SHINODA T, TANAKA T (1993). Preventive effect of magnesium hydroxide on carcinogen-induced large bowel carcinogenesis in rats. Basic Life Sci 61: 111-118. [ Links ]
NAGASAWA HT, SHIROTA FN, MATSUMOTO H (1972). Decomposition of methylazoxymethanol, the aglicone of cycasin in D20. Nature 236: 234-235. [ Links ]
NATIONAL RESEARCH COUNCIL (1980). Recommended Dietary Allowances. 9 th ed. National Academy of Sciences, Washington, DC. [ Links ]
NELSON RL, DAVIS FG, SUTTER E, SOBIN LH, KIKENDALL JW, BOWEN P (1994). Body iron stores and risk of colonic neoplasia. J Natl Cancer Inst 86: 455-460. [ Links ]
NEMENKO BA, MOLDAKULOVA MM, ZORINA SN (1976). Rasprostranenie raka pishchevoda V Gur'evskoi blasti v zavisimosti ot mineral'nogo sostava pit'evoi vody. Vopr Onkol 22: 75-76. [ Links ]
PENCE BC (1991). Dietary selenium and antioxidant status: toxic effects of 1,2-dimethylhydrazine in rats. J Nutr 121: 138-144. [ Links ]
REDDY BS, ENGLE A, KATSIFIS S, SIMI B, BARTRAM HS, PERRINO P (1989). Biochemical epidemiology of colon cancer: effect of types of dietary fiber on fecal mutagens, acid and neutral sterols in healthy subjects. Cancer Res 49: 4629-4635. [ Links ]
RUBIO CA, NYLANDER G, SVEANDER M, DUVANDER A, ALUN ML (1986). Minimal invasive carcinoma of the colon in rats. Am J Pathol 123: 161-165. [ Links ]
SEABORN CD, YANG SP (1993). Effect of molybdenum supplementation on N-nitroso-N-methylurea-induced mammary carcinogenesis and molybdenum excretion in rats. Biol Trace Elem Res 39: 245-256. [ Links ]
UNDERWOOD EJ (1977). Trace elements in human and animal nutrition. 4 th ed. New York. Academic Press, pp. 123-127. [ Links ]
VARGAS PA, ALBERTS DS (1992). Primary prevention of colorectal cancer through dietary modification. 70: 1229-1235. [ Links ]
VENUGOPAL B, LUKEY TD (1978). Metal Toxicity in Mammals. 2. Chemical Toxicity of Metals and Metalloids. Plenum Press, New York. [ Links ]
WARGOVICH MJ, BAER AR (1989). Basic and clinical investigations of dietary calcium in the prevention of colorectal cancer. Prev Med 18: 672-679. [ Links ]
WEI HJ, LUO XM, YANG SP (1985). Effects of Molybdenum and Tungsten on mammary carcinogenesis in SD rats. J Natl Cancer Inst 74: 469-473. [ Links ]
WILLET W (1989). The search for the causes of breast and colon cancer. Nature 338: 389-394. [ Links ]
WYNDER EL, REDDY BS (1983). Dietary fat and fiber and colon cancer. Semin Oncol 10: 264-272. [ Links ]
YANG G (1993). Relationship between colorectal cancer and ten inorganic elements. Chung Hua Yu Fang Y Hsuch Tsa Chih. 27: 282-285. [ Links ]
Received on March 24, 2002.
Accepted on July 1, 2002.














