Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell v.26 n.3 Mendoza ago./dic. 2002
Ultrastructural characteristics of the lung of Melanophryniscus stelzneri stelzneri (Weyenberg, 1875) (Anura, Bufonidae)
Gladys N. Hermida, Alejandro Farías, Luisa E. Fiorito
Laboratorio Histología Animal, Departamento de Biodiversidad y Biología Experimental, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Ciudad Universitaria. Buenos Aires, ARGENTINA.
Address correspondence to: Gladys N. Hermida. Laboratorio Histología Animal. Dpto. de Biodiversidad y Biología Experimental, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires (UBA), Pabellón II, 4 piso, Av. Güiraldes s/n, Ciudad Universitaria, (C1428EHA) Buenos Aires, ARGENTINA. Fax: (+54-11) 4782 0582. E-mail: hermida@bg.fcen.uba.ar
Key words: Lung ultrastructure. Respiratory epithelium. Electronic microscopic. Toad. Amphibian.
Abstract: The lung of the toad, Melanophryniscus stelzneri stelzneri was studied using scanning and transmission electron microscopy. In M.s.stelzneri the parenchyma forms a polygonal network arrangement, therefore the parenchyma is edicular. These spaces are delimited by the interconnection of third order septa which are covered by respiratory epithelium. Small patches of ciliated epithelium without goblet cells appear irregularly distributed on the septa. The respiratory epithelium consists of one type of pneumocyte, which shows characteristics of both type I and type II alveolar cells of higher vertebrates. The pneumocytes are irregular in shape and possess attenuated cytoplasmic processes, which spread around the capillaries to form the outer layer of the air-blood barrier. These cells contain different types of cytoplasmic bodies: electron dense bodies, multivesicular bodies and lamellar bodies. Dense bodies are probably the precursors of lamel-lar bodies and the multivesicular bodies are incorporated into the latter. Neuroepithelial bodies appear ran-domly distributed over the septa. These bodies are separated from the lumen of the lung by thin cytoplasmic processes of neighbouring pneumocytes. The air-blood barrier consists of three layers: epithelium, interstitial space and endothelium. The relatively simple pulmonary structure of M.s.stelzneri is due to a lower degree of partitioning of the pulmonary lumen in comparison to the lung of other bufonid anurans, could be correlated with a well devel-oped cutaneous and buccopharingeal respiration. The testing of this hypothesis awaits further studies.
Introduction
Amphibians were the first vertebrates to colonise land. In this process, they developed anatomical and physiological adaptations to the changing environmental conditions characteristic of transitional habitats. Throughout their evolution, amphibians made use of a wide variety of respiratory organs such as gills, skin, buccopharyngeal mucosa and lungs. The contribution of each of the four kinds of respiratory organs to gas exchange depends on the organism structure, stage of life history and habitat (Duellman and Trueb, 1986; Maina, 1989).
Amphibian lungs have well-vascularized sacs with an internal surface elaboration, which varies widely among the different orders and within each family. The internal surface of the lungs of Gymnophiona is characterised by the presence of only one type of septa that form a network of folds delimiting alveoli (Noble, 1937).
In Anura, the anatomy of the lung is not so diverse. Lungs are strongly folded by first, second and third order septa, which divide the air space into a network of different sized alveoli (Goniakowska-Witalinska, 1995; Hermida and Fiorito, 1994; Hermida et al., 1998). The type of epithelium that covers their apical part defines the three types of septa. A pseudostratified cylindrical ciliated epithelium with goblet cells is located exclusively on the tips of the first septa, while second order septa present the same type of epithelium but without goblet cells. Third order septa are lined exclusively by respiratory epithelium.
The family Bufonidae represent one of the most successful anurans families in terms of their distribution and diversity of species. For our studies about the comparative morphology of the lung among species belonging to the same family, we have chosen the toad, Melanophryniscus stelzneri stelzneri. This is a small toad (size snout-vent: 25-30 mm), which is found especially after the summer storms, alongside the small creeks at 900-2,000 m height in the Trapiche mountain range in the central region of Argentina (Cei, 1980).
Because this species occurs exclusively above 900 m height, Melanophryniscus stelzneri stelzneri may be an interesting model for comparative morphological and ultrastructural studies of the lung. In addition, further insight into the structure of Bufonid lung may be gained. Thus, the object of this paper is not only to contribute to information to amphibian lung morphology but also gain to better understanding of the relationship between structure and function.
Materials and Methods
Adult Melanophryniscus stelzneri stelzneri (Weyenberg, 1875) of 0.9 - 1.4 g body weight, were collected at 1,400 m above sea level in the mountain range within the regions of Trapiche (San Luis province, Argentina) during summer. Once in the laboratory, the animals were anaesthetised by immersion in 0.1 % MS 222 Sandoz, before being sacrificed.
Scanning electron microscopy (SEM):
The lungs of one male and one female were fixed with Bouins fixative by instillation through the glottis. After 1 h the toads were opened ventrally, exposing the lungs. The lungs were removed intact and immersed in the same fixative for 12 h. Then they were dehydrated by conventional methods for SEM. Afterwards, the material was dried (critical point method), coated with gold palladium and observed in a JEOL (Tokyo, Japan) JSM-25 II scanning electron microscope at 25 kV.
Transmission electron microscopy (TEM):
Two adult animals male and female were dissected and small samples of the lungs were fixed overnight with 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4 at 4ºC. Samples were then rinsed in 0.1M cacodylate buffer and postfixed for 1 h in 1% osmium tetroxide in the same buffer. This procedure was followed by dehydration and embedding in araldite resin. Sections (1mm thick) were stained with toluidine blue and examined under the light microscope. Ultrathin sections were double-stained with uranyl acetate - lead citrate (Reynolds, 1963) and observed under a Zeiss (Oberkochen, Germany) EM 109T transmission electron microscope at 80 kV.
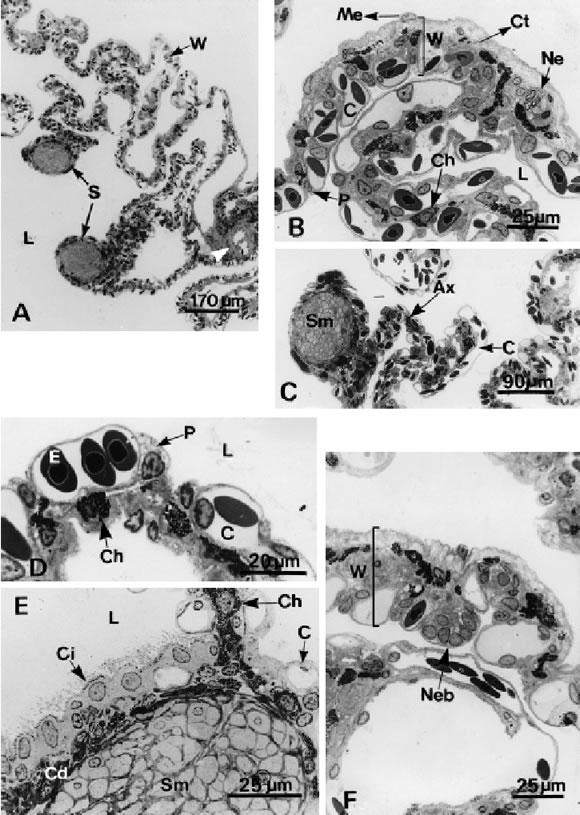
FIGURE 1. Light microscopical section of the lung of Melanophryniscus stelzneri stelzneri. A, Transverse section of the lung showing the internal architecture. Only one type of third order septa (S) is observed protruding into the lumen (L) of the lung. Note the presence of muscle fibers forming thickenings associated to blood vessels (arrowhead) in the lung wall (W). B, Detail of the lung wall (W) with external mesothelium (Me), a central layer of loose connective tissue (Ct) and an internal layer of pneumocytes (P) associated to blood capillaries (C). Note the presence of chromatophores (Ch) and nerves (Ne) in the lung wall. L.: lumen. C, Third order septa presenting an axis (Ax) and an apical dilation containing bundles of smooth muscle fibers (Sm). Note the respiratory epithelium at both sides of the septa. C: blood capillaries. D, Detail of the respiratory epithelium composed of pneumocytes (P) located between blood capillaries (C). The cytoplasm of the pneumocytes has small granulation and a foamy appearance. E: erythrocytes, Ch: chromathophore. E, Cross section of septa showing the ciliated epithelium (Ci). C: capillaries, Cd: dense connective tissue, Ch: chromatophores, L: lumen, Sm: smooth muscle fibers. F, The wall of the lung (W) carrying a neuroepithelial body (Neb).
Results
Light microscopy
The internal view of the lung surface reveals numerous septa protruding into the lumen (Fig. 1A). The wall of the lung is thin and composed of three layers: (1) pleuroperitoneum, formed by a layer of mesothelial cells overlaying a thin sheet of dense connective tissue, (2) a middle layer of loose connective tissue containing scarce fibrocytes, chromatophores, and thin elastic fibers. Smooth muscle appear either as isolated cells or as slight thickenings associated with blood vessels. The inner layer (3) consists of pneumocytes and respiratory capillaries (Fig. 1B). The pulmonary artery is located opposite the pulmonary vein.
In cross section, third order septa present an axis and an apical thickening oriented towards the centre of the lung lumen. A thin network of collagenous and elastic fibers, isolated smooth muscle fibers and mixed nerves forms the axis. The apical extreme is round or oval in section and shows a dilation comprising a bundle of smooth muscle fibres, elastic fibers, loose connective tissue and a vein (Fig. 1C). Both sides of the septa as well as the extreme display a network of pulmonary capillaries on both sides, which, in turn, are covered by pneumocytes. Ciliated cells and intra-epithelial groups of cells occasionally interrupt the respiratory epithelium. These clusters of cells have a clear cytoplasm with spherical or oval nucleus and rest on a basal lamina (Fig. 1D, E, F).

FIGURE 2. Scanning electron microscopy of the inner surface of the lung of Melanophryniscus stelzneri stelzneri. A, Third order septa (S) divide the lumen into edicular spaces (Ed). B, Respiratory epithelium. The network of capillaries is covered by thin cytoplasmic process of pneumocytes (Pp). Cell bodies (Cb) are located deep in the capillary meshes or at the side of capillaries. Longer microvilli (arrowhead) are located near the border between two cells. C, Portion of the septum (S) with small area of ciliated epithelium (Ci). D, Dome-shape protrusions (arrows) are located on the septum (S) of the lung.
Scanning electron microscopy
The paired lungs of M. s. stelzneri are symmetrical, spongy saccular organs. The parenchyma forms a polygonal network arrangement that divides the lung lumen into different sized edicular spaces (Fig. 2A). These spaces are defined by the interconnection of third order septa. The internal surface of the lung is covered by pneumocytes. The free surfaces of the pneumocytes present numerous short microvilli (Fig. 2B). Pneumocytes show thin cytoplasmic processes that cover the capillaries. Occasionally small and irregular patches of ciliated epithelium interrupt the respiratory epithelium (Fig. 2C). Dome-shaped protrusions, completely covered by processes of neighbouring pneumocytes, appear distributed over the septa surface, mainly at the junction of two septa (Fig. 2D).
Transmission electron microscopy
The respiratory epithelium is composed of a single layer of pneumocytes. Pneumocytes are irregular cells with a thick portion that contains the nucleus and a thin cytoplasmatic process that overlies the capillaries. Cell bodies mainly lie in the intercapillay spaces. The free surface of the respiratory cell bears short microvilli (Fig. 3A). Neighbouring pneumocytes are interconnected by interdigitations of the cell membrane and apical junction complexes constituted by a superficial zonula occluder, an intermediate zonula adherens and a desmosome (Fig. 3B).
The air - blood barrier is basically composed by three constituents: an outer layer consisting of the narrow cytoplasmic process of the pneumocyte, an intermediate layer corresponding to the interstitial space and the internal layer formed by endothelial cells. The endothelial cells contain numerous micropinocytotic vesicles underlining both the luminal and basal plasma membrane surfaces. A zonula occludens and inter-digitations of membranes join the endothelial cells. The interstitial space contains an extracellular matrix and collagen fibers (Fig. 3C).
The nucleus of the pneumocytes shows irregularities in its contour and occupies a great part of the cell volume. The cytoplasm presents abundant free ribosomes and numerous cisterns of rough endoplasmatic reticulum. Vesicles of varying size are observed next to a well developed Golgi apparatus. Mitochondria with ovoid profile are scattered in the cytoplasm (Fig. 3D).
There are three different types of cytoplasmic bodies in the pneumocytes: lamellar bodies (LBs), electron dense bodies (EDBs), and multivesicular bodies (MVBs). LBs are spherical or ovoid and formed by numerous parallel membranes disposed in a concentric arrangement. These membranes are smooth and display a wavy course around a core of homogeneous electron-dense material. Another type of LBs contains parallel membranes immersed in a lucent material. A third type of LBs, is characterized by additional incorporation of MVBs to LBs. Multilamellar filled with groups of small LBs separated from each other by an electron-lucent substance. After that the LBs are released into the lung lumen by a merocrine secretion, gradually the membranes begin to dissociate. No tubular myelin figures have been observed in the lung of M. s. stelzneri. MVBs contain varying numbers of small vesicles with a moderately dense content embedded in a lucent matrix. EDBs are spherical and contain homogeneous electron-dense material. The three kinds of cytoplasmic bodies described above are enclosed by single membranes (Fig. 3 E,F,G).
TEM observations reveal that the dome-shaped protrusions are composed of clusters of clear cells characterised by the presence of dense-core vesicles in the basal portion of the cytoplasm. The basal cells are associated with nerve endings. These corpuscles are separated from the lumen of the lung by thin cytoplasmic processes of the neighbouring pneumocytes (Fig. 4A).
The ciliated cells are irregularly shaped, and possess small microvilli on their luminal surface. The nucleus is pleomorphic occupying a great part of the cell volume and the nuclear envelope shows several invaginations (Fig. 4B). There are numerous mitochondria associated to the two striated rootlets of basal bodies, scarce dense bodies and a Golgi complex mostly located in the apical part of the cytoplasm. Each ciliated cell is joined to its neighbour by a tight junction and interdigitations of the cell membrane (Fig. 4C).
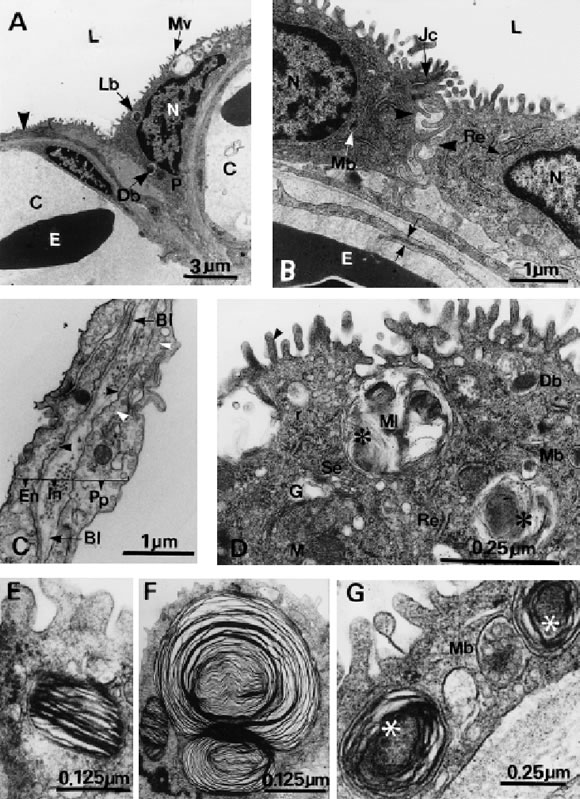
FIGURE 3. Transmission electron microscopy. A, Adjacent capillary (C) covered by a thin cytoplasmic process of the pneumocyte (arrowhead). The apical part of the cytoplasm contains microvilli (Mv), nucleus (N), lamellar bodies (Lb), and a dense body (Db). E: erythrocytes, L: lumen, P: pneumocyte. B, Junctional complex (Jc) and interdigitations of the cell membrane (arrowhead) of neighbouring pneumocytes. Note the join between two endothelial cells (arrows). E: erythrocytes, L: lumen, Mb: multivesicular body, N: nuclei, Re: rough endoplasmic reticulum. C, The air-blood barrier comprised by a thin cytoplasmic process of the pneumocytes (Pp), an interstitial space (In) containing collagen fibers and endothelium (En). Note the micropinocytotic pits (black arrowhead) and pinocytotic vesicles (white arrowhead). Bl: basal lamina of the pneumocytes and endothelium. D, A fragment of cytoplasm with the Golgi complex (G), surrounded by vacuoles of different size, rough (Re) and smooth (Se) endoplasmic reticulum, free ribosomes (r), mitochondria (M) and different kinds of cytoplasmic bodies, an electron dense body (Db), a multivesicular body (Mb) and a multilamellar body (Ml). Some of them contain a multivesicular body (asterisk). Microvilli appear filled with actin microfilaments (arrowhead). E, Lamellar body with parallel membranes. F, Multilamellar body. G, Multivesicular body (Mb) in contact with an immature lamellar body. Small vesicles (asterisk) are observed inside the lamellar body.
Discussion
The inner architecture of the lung of Melano-phryniscus stelzneri stelzneri is different from that of other anuran species such as Rana temporaria, R. esculenta (Dierichs, 1975), Hyla arborea (Goniakowska-Witalinska, 1986) and Chiromanthis petersi (Maina, 1989). In the above listed anurans, the lungs present a more complex morphology: interconnected first, second and third order septa, protruding into the lumen of the lung divide the internal air space into numerous ediculae of varying size.
The general structure of the lung of this study species is less complex than that described above, and also compared with other species belonging to the same family such as B. marinus (Smith and Campbell, 1976), B. arenarum (Hermida and Fiorito, 1994; Hermida et al., 1998), due to the presence of only one type of septum, analogue to third order septa of other species, but in this species the structural unit of the parenchymal lung is also the ediculae. On the other hand, the location of pneumocytes with regard to that of the capillaries does not differ from that of other anurans (Goniakowska-Witalinska, 1995).
Different investigators tend to refer to the amphibian respiratory units with the term alveoli. Dunker (1978) coined the terms ediculae or faveoli to describe the unit structure of the reptilian parenchymal, which are analogous to mammalian alveoli, but are up to 1,000 times larger than alveoli of similar sized mammals. The trabeculae are raised above the inner lung surface, differentiating these parenchymal types from trabecular parenchyma (Perry, 1998), in which the trabeculae lie directly on the lung wall.
We suggest to use the term ediculae for the unit structure of the parenchymal of M. s. stelzneri instead of alveoli, because the primary cubicles are not deeper than they are broad.
In the lungs of M.s.stelzneri as well as in the rest of amphibians studied a unique type of neumocyte appears to accomplish both functions, gaseous exchange and production of surface active agents. Pneumocytes types I and II of amniotes have likely evolved and originated from this unique type of cell.
The ultrastructure of the pneumocyte is basically similar to that of the anuran species. The role played by the apical microvilli of pneumocytes is not clearly understood. However some authors consider that pneumocyte microvilli are involved in the maintenance of the surface fluid layer (Meban, 1973). A fact sup-porting this hypothesis is that pneumocytes of M. s. stelzneri as well as those of other anurans that possess few goblet and ciliated cells or even lacking them, are rich in cytoplasmic vesicles. These vesicles supply mucopolysaccharides for mucus secretion as reported by Goniakowska-Witalinska (1980 a, b; 1986). As another function of these microvilli we suggest they provide a reserve of plasma membrane for the distension of the lung. The membrane interdigitations between the lateral walls of neighbouring pneumocytes may also contribute to the distension of the lung allowing changes in cell shape to occur.
Pneumocytes also contain numerous cytoplasmic lamellar bodies, which are likely homologous to the lamellate osmiophilic bodies observed in mammalian type II pneumocytes. The surfactant is produced in pneumocytes and stored in dense and lamellar bodies. According to our observations, the different types of osmiophilic inclusion bodies, seem to represent different steps in the lung surfactant secretion cycle. Lamellar bodies, following their exocytosis, are gradually transformed into the monolayer film over the epithelium. The surfactant thus reducing the surface tension at the air-epithelium interface. Without adequate secretions of surfactant, the ediculae would collapse. In non-mammals, surfactant appears to act as anti-glue preventing the adhesion of adjacent respiratory surfaces that occur when the lungs collapse and could not be rein-flated. Without an anti-glue to lower the surface tension of the fluid intervening between the contacting epithelial surfaces, inspiration after lung collapse would be more difficult (Daniels et al., 1998).
The existence of distinctive groups of cells with specific morphological characteristics of endocrine cells and particular innervation are identified in the respiratory epithelium of M. s. stelzneri. These cells, called neuroendocrine cells (NE) are observed in small groups of four or six cells forming a round or oval corpuscle, denominated by Lauweryns and Peuskens (1972) as neuroepithelial body (NEB). Generally, NEBs are separated from the lung lumen by a single layer of ciliated epithelium as in Bufo marinus (Rogers and Cutz, 1978) or by a single layer of pneumocytes as in Hyla arborea (Goniakowska-Witalinska, 1986) and M. s. stelzneri.
The few ciliated cells present in the lung of M. s. stelzneri lack of lamellar bodies, electron dense bodies and vacuoles in contrast to those described for Urodela (Goniakowska-Witalinska, 1980a, b) and Anurans (Goniakowska-Witalinska, 1986). Thus these cells are only functional for clearing and transporting mucus, surfactants and debris. Since M. s. stelzneri lives at 900- 2,000 m height, we are likely to suppose that the increase in the proportion of respiratory cells at the expense of ciliated and goblet cells may be an adaptation to the oxygen uptake at low PO 2 without increasing the total lung surface area.
Concluding, the reduction of the complexity in the pulmonary structure of M. s. stelzneri due to a lower partition of the pulmonary lumen in comparison to the lung of other bufonid anurans, such as B.arenarum and B. marinus, could be due to that the cutaneous and buccopharingeal respiration may compensate the pulmonary respiration; consequently, the lung would be structurally more simple.
Considering that the studied species lives above 1,000 m height, we can suppose that the low O 2 availability may produce changes at anatomical as well as at a physiological level. To assure a greater distribution of oxygen to tissues there could be a proliferation of pulmonary capillaries or an increase of the respiratory surface, may be at the expense of the ciliated epithelium, as well as a reduction in the thickness of the gas exchange barrier. Morphometric data regarding the surface area, air-blood diffusion distance and the distribution of respiratory surfaces are needed to achieve with the purpose to corroborate the above mentioned adaptations.

FIGURE 4. Transmission electron microscopy. A, A cluster of granulated cells (Gc) separated from the lumen (L) of the lung by a thin process (Pp) of the neighbouring pneumocytes (P). B, Ciliated cells. N: nuclei. C, The supranuclear cytoplasm contains numerous mitochondria (M) associated to ciliary rootlets (Cr) and a prominent Golgi complex (G). Note a junction complex (Jc) between two cells. N: nuclei.
Acknowledgements
The authors are grateful to Prof. Dr. S.F. Perry (Institut für Zoologie, Universität Bonn, Germany) for the useful criticisms of the manuscripts, and to Mrs. Isabel Farías for their technical assistance.
References
CEI JM (1980). Amphibians of Argentina. Monitore Zoologico Italiano. N.S. Monograf ia 2: 1-609. [ Links ]
DANIELS CB, LOPATKO OV, ORGEIG S (1998). Evolution of surface activity related functions of vertebrate pulmonary surfactant. Clinical and Experimental Pharmacology and Physiology 25: 716-721. [ Links ]
DIERICHS R (1975). Electron microscopic studies of the lung of the frog. II. Topography of the inner surface by scanning and transmis-sion electron microscopy. Cell and Tissue Research 160: 399-410. [ Links ]
DUELLMAN WE, TRUEB L (1986). Biology of Amphibians. McGraw-Hill Publishing Company, New York. pp. 217. [ Links ]
DUNKER HR (1978). Funktionsmorphologie des Atemapparates und Coelom-Gliederung bei Reptielien, Vögeln und Säugern. Verh Dtsch Zool Ges 1978: 99-132. [ Links ]
GONIAKOWSKA-WITALINSKA L (1980 a). Ultrastructural and morphometric changes in the lung of newt, Triturus cristatus cranifex Laur. during ontogeny. Journal of Anatomy 130: 571-583. [ Links ]
GONIAKOWSKA-WITALINSKA L (1980 b). Scanning and transmission electron microscopic study of the lung of the newt, Triturus alpestris Laur. Cell and Tissue Research 205: 133-145. [ Links ]
GONIAKOWSKA-WITALINSKA L (1986). Lung of the tree frog, Hyla arborea L. A scanning and transmission electron microscopic study. Anatomy and Embryology 174: 379-389. [ Links ]
GONIAKOWSKA-WITALINSKA L (1995). The histology and ultrastructure of the Amphibian lung. In: Histology, Ultrastructure and Immunohistochemistry of the Respiratory Organs in non-Mammalian Vertebrates. Pastor LM (Ed.). Secretariado de Publicaciones, Universidad de Murcia, Spain. pp. 77-112. [ Links ]
HERMIDA GN, FIORITO LE (1994). Estereoultraestructura del pulmón de anuros bufónidos. I. Bufo arenarum. Cuadernos de Herpetología 8(1): 25-29. [ Links ]
HERMIDA GN, FIORITO LE, FARÍAS A (1998). The lung of the common toad, Bufo arenarum (Anura: Bufonidae). A light and electron microscopy study. Biocell 22(1): 19-26. [ Links ]
LAUWERYNS JM, PEUSKENS JC (1972). Neuroepithelial bodies (neuroreceptor or secretory organs?) in human infant bronchial and bronchiolar epithelium. The Anatomical Record 172: 471-482. [ Links ]
MAINA JN (1989). The morphology of the lung of the east African tree frog Chiromantis petersi with observations on the skin and the buccal cavity as secondary gas exchange organs. A TEM and SEM study. Journal of Anatomy 165: 29-43. [ Links ]
MEBAN C (1973). The pneumocyte in the lung of Xenopus laevis. Journal of Anatomy 114: 235-244. [ Links ]
NOBLE GK (1937). The Biology of Amphibian. Ed. McGraw-Hill Publishing Company, New York. pp. 158-178. [ Links ]
PERRY SF (1998). Lungs: comparative anatomy, functional morphology, and evolution. In: Biology of the Reptilia Morpohology G: Visceral Organs vol. 19. Gans, C (Ed.). American Press, New York, London. pp. 1-92. [ Links ]
REYNOLDS ES (1963). The use of lead citrate at high pH as electron - opaque stain in electron microscopy. Journal of Cell Biology 17: 208-212. [ Links ]
ROGERS DC, CUTZ E (1978). Innervation and cytochemistry of the neuroepithelial bodies in the ciliated epithelium of the toad lung (Bufo marinus). Cell and Tissue Research 195: 395-410. [ Links ]
SMITH DG, CAMPBELL G (1976). The anatomy of the pulmonary vascular bed in the toad Bufo marinus. Cell and Tissue Research 165: 199-213. [ Links ]
Received on April 29, 2002.
Accepted on July 7, 2002.














