Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell v.26 n.3 Mendoza ago./dic. 2002
Microanatomical characteristics of marginal ommatidia in three different size-classes of the semi-terrestrial Isopod Ligia exotica (Crustacea; Isopoda)
Essi Keskinen1, Yasuharu Takaku2 , V. Benno Meyer-Rochow3, TakahikoHariyama4
1. Department of Biology, University of Oulu, P.O. Box 3000, SF-90014 Oulu, Finland.
2. Department of Developmental Genetics, National Institute of Genetics, Mishima 411- 8540, Japan.
3. School of Engineering and Science, International University Bremen, D-28725 Bremen, Germany.
4. Department of Biology, Hamamatsu University School of Medicine, 1-20-1 Handayama, Hamamatsu, 431-3192, Japan.
Address correspondence to: Dr. V.B. Meyer-Rochow. School of Engineering and Science, International University Bremen, P.O.Box 75 05 61, 28725 Bremen, GERMANY. Fax: (+49 - 421) 200 49 3242. E-mail: B.Meyer-Rochow@iu-bremen.de
Key words: Crustaceans. Eye. Retina. Vision. Growth. Moulting. Ultrastructure.
Abstract: The aims of this paper have been (a) to characterize marginal ommatidia from different eyeregions through a detailed description of their distinct ultrastructural features in three different size-classes ofL. exotica, and (b) to compare microanatomical characteristics of the marginal ommatidia with those of ommatidia of the same eye, but located further centrally. On the basis of transverse as well as longitudinal sections we conclude that new ommatidia are added from a crescentic dorso-anterio-ventral edge of the eye and that maturing ommatidia go through a sequence in which originally the nuclei of cone -, pigment-, and retinula cells are arranged in three separate layers. At the beginning of the microvillar development, the organization of the corresponding rhabdomeres is still quite different (much less regular) from that of those rhabdomeres that make up the mature rhabdom. Marginal ommatidia always possess smaller diameters than more centrally located ones and retinal screening pigment granules are most apparent in the retinula cells only after the first microvilli have appeared. The diameters of rhabdom microvilli (approx. 55 nm) do not differ in ommatidia from the five investigated eye regions in small specimens (< 1.5 cm body length), but show a tendency to be slightly wider in the anterior (=frontal or rostral) regions of the eye (approx. 65 nm) in larger specimens (> 2.0 cm body length).
Introduction
Recent observations on cell differentiation in the developing compound eye of a variety of crustacean species, e.g., Triops longicaudatus (Harzsch and Walossek, 2001), Homarus americanus (Hafner and Tokarski, 2001), Procambarus clarkii (Hafner and Tokarski, 1998), Hyas araneus and Carcinus maenas (Harzsch and Dawirs, 1995), have led to new insights and a re-appreciation of some older ontogenetic studies on isopod (Idothea sp.: Peabody, 1939), prawn (Penaeus duorarum: Elofsson, 1969 and Palaemon serratus: Fincham, 1984), as well as anomuran species (Fincham, 1988). Comparisons with the eyes of insects have, furthermore, demonstrated that the mechanisms of neurogenesis, leading to the formation of compound eyes in crustaceans and hexapods, is essentially similar, if not identical in the two taxa (Harzsch et al., 1999; Melzer et al., 2000; Hafner and Tokarski, 2001; Harzsch and Walossek, 2001). However, since crustaceans, unlike insects, never cease to grow and throughout their lives keep increasing in size with every moult, some important differences ought to be present as well, especially with regard to the ommatidial accretion in adulthood.
One consequence of the life-long growth in crustaceans is that the compound eyes of most species never stop growing and massive increases in the number of facets have indeed been reported from, to name but a few, isopods (Meyer-Rochow, 1982), lobsters (Meyer-Rochow, 1975; Shelton et al., 1981), anomurans (Bernard, 1937; Meyer-Rochow et al., 1990), and brachyuran crabs (Bernard, 1937; Eguchi et al., 1989; Harzsch and Dawirs, 1995). The new ommatidia, which are added during each moult, are integrated into the existing system of visual pathways leading from the eye to the optic centres of the brain, but the processes by which this is achieved are still poorly understood. Because of the extra ommatidia that a crustacean gains each time it moults, the eye not only gets progressively larger, but in some species it actually changes its shape, its internal organization, and even its function. A growth-related re-organization of eye optics and anatomy from an apposition to a superposition system (for functional characteristics of arthropod eye designs, see Warrant and McIntyre, 1993) have been described in Palinurus longipes (Meyer-Rochow, 1975), mysids (Nilsson et al., 1986), anomurans (Fincham, 1988), and carideans (Fincham, 1984; Douglas and Forward, 1989; Gaten and Herring, 1995).
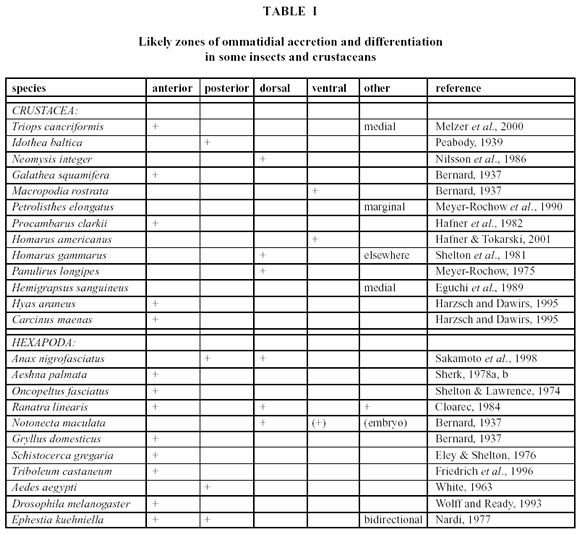
Although little is still known about the way a crustacean is able to functionally and behaviourally accommodate the changes that take place in its eye during a moult, there is a general consensus that so-called growth- or proliferation zones exist around the margin of the eye, in which new ommatidia are generated and mature (this does not happen in the eyes centre). The precise locations of the growth- or proliferation zones, however, appear to vary widely between taxa (Table 1). Since eye researchers, who use the terms anterior, posterior, rostral, caudal, dorsal, ventral, etc. do not always agree in whether they refer to the eye alone or relate the positional terms to the whole animal (especially when used in connection with eyes in different species), some of the variation in the reported proliferation zone directions in crustacea could be due to this problem. Another difficulty is that some investigators appear to use directional terminologies that have been borrowed from the designation of wind directions (the direction from where something originates), while others use designations commonly employed in describing water-current directions (the direction in which something moves). A statement like cells proliferate in an anterior direction may, thus, not be understandable unless accompanied by a clearer definition of anterior direction or by an unambiguous and labelled illustration.
In this investigation of marginal ommatidia in several eye regions of Ligia exotica, we used the directional terms frontal, caudal, ventral, and dorsal, as indicated in Fig. 1. Marginal ommatidia in both insects and crustaceans are known to frequently differ from those located more centrally and in some species the reasons for the different structural organizations are known (e.g., the polarization-sensitive dorsal rim ommatidia in many insects: Labhart, 1980, or the large dorsal ommatidia in some euphausiids: Land, 1981), but in others, marginal ommatidia appear to be aberrant in shape for no known reason other than that they are perhaps rudimentary or still developing. In L. exotica marginal ommatidia have not hitherto been examined and in view of the fact that considerable background information exists on the visual capacity of this semi-terrestrial isopod (Hariyama et al., 1986; 1993; 2001; Keskinen et al., 2002), we felt that such a study was more than overdue.
We are aware that the elucidation of the role of marginal ommatidia is a complex problem, which needs to be tackled from several angles (e.g., emphasizing interspecific phylogenetic relationships, neurogenesis and development, connectivity patterns to optic ganglia, or structure and function of the ommatidia in question). For future research into this problem, however, it seems essential to have a basic understanding of the kinds of marginal ommatidia one is likely to encounter. In this first report, which is qualitative in nature, we therefore decided (a) to characterize marginal ommatidia from different eye regions through detailed descriptions of their distinct ultrastructural features in three different size-classes of L. exotica and (b) to compare the microanatomical characteristics of the marginal ommatidia with those of ommatidia of the same eye, but located further centrally. It is hoped that such a study might shed light on the location of the proliferation zone and the path the new ommatidia take in order to be integrated into the compound eye.
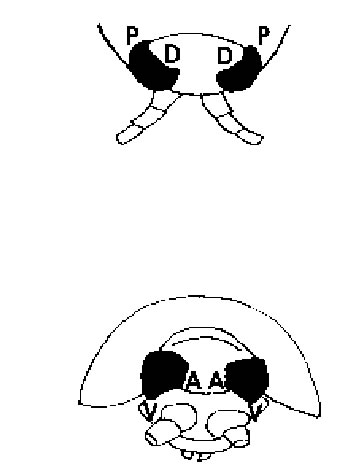
FIGURE 1. Schematic drawing of the head region of Ligia exotica viewed from above (top) and seen from head-on (bottom). A = anterior (frontal or rostral); D = dorsal; P = posterior (caudal); V = ventral.
Materials and Methods
1. Preparation Details
Several hundred individuals of both sexes and various sizes of Ligia exotica were collected on the seashore of Yokohama (Japan). The animals were transported in plastic containers from Yokohama to the laboratory of Tohoku University in Sendai. During transport the containers were shielded from direct light and not allowed to get warmer than 25ºC. In the laboratory the animals were maintained at a temperature of +24ºC and a light/dark regimen of 12:12h. One week following capture, 30 randomly chosen individuals of both sexes and various sizes were used for the observations.
The selected 30 animals were decapitated with a sharp razor blade during the day and under electric light, and the isolated heads were immersed in prefixative solution (2% paraformaldehyde and 2% glutaraldehyde in 0.1 M sodium cacodylate buffer at pH 7.4). The eyes were dissected out and kept in ice-cold prefixative solution overnight. The samples were then rinsed three times for 10 minutes with phosphate buffer and fixed thereafter in ice-cold 2% OsO 4 for 2 h. After rinsing the samples another three times for 10 minutes, they were dehydrated in a graded series of ethanol and finally immersed in propylene oxide twice for 10 min. The samples were then passed through a graded series of Epon and, at last, embedded in 100% Epon. Ultrathin sections, prepared on an ultramicrotome with a glass knife, were first stained in 2% uranyl acetate for 6 min, washed in distilled water for 30 s, and secondarily stained in 0.4% lead citrate for 6 min. Ultimately the sections were observed under a transmission electron microscope and photographed.
2. Assessment Procedure
Three size classes were established and defined as: small (animals with body lengths ranging from 0.7 cm to 1.4 cm), medium (animals with body lengths ranging from 2.0 cm to 2.8 cm), and large (animals with body lengths exceeding 3.5 cm). When not stated otherwise, three animals in each size class had their eyes examined. Since it was known from an earlier anatomical study of the eye of L. exotica (Keskinen et al., 2002) that male and female eyes did not differ, the sex of the individuals could be ignored. Individuals with body lengths that fell between two size classes were eliminated. In each eye, ommatidia of the central region (middle), and frontal as well as caudal margins were observed in cross section. In the small group, moreover, ventral ommatidia were also examined in cross as well as longitudinal sections, but dorsal ommatidia could be gleaned only as longitudinal sections. In the middle group, ventral as well as dorsal ommatidia could be studied in cross section in addition to the frontal, middle, and caudal ones. In each case, only the left eyes were used for observations of the marginal areas; the right eyes served as controls and were used only for examinations of the central region.

FIGURE 2. Transverse section at low magnification through central region of light-adapted (=daytime) L. exotica retina, showing orientation and arrangement of rhabdomeres in several ommatidia. Magnification X 1,000.
The diameters of the microvilli, present in the three size classes, were determined from photographic prints of anterior (=frontal), posterior (=caudal), ventral, dorsal, and central eye regions of middle-sized individuals, anterior, posterior, ventral, and central eye regions of small individuals, and anterior, posterior, and central ommatidia of large specimens. Approximately 38 randomly chosen microvilli were measured in each photograph. Rhabdom areas of the first and second marginal rows of ommatidia were measured from photographs taken of the caudal and the dorsal marginal areas of middle-sized L. exotica. In order to arrive at a figure for the total area of the rhabdom of a single ommatidium, each of the seven rhabdomeres making up one ommatidium (Hariyama et al., 1986) was first measured separately, using a Scion computer programme, and then added together. In this way, total rhabdom areas of 14 first row and 9 second row ommatidia were obtained.
For analyses of statistical significance between samples an Independent samples t-test with the SPSS was used. Box plots as well as p-values for regression relationships were determined for first versus second row ommatidia and microvillus diameters in different eye regions.
Results
Ommatidia as well as rhabdomeres in the eyes of all three size classes were found to be regularly shaped and fully differentiated anywhere in the eye except for extreme dorsal, frontal, ventral, and caudal marginal areas, where considerably smaller and often highly irregular (and seemingly immature) ommatidia dominated (see further below). A fully differentiated ommatidium of the eye (cf. also Hariyama et al., 1986) consisted of seven retinula cells with six rhabdomeres, which, in transverse section, were arranged in a somewhat elliptical fashion around a centrally-located, yet open rhabdom (Figs. 2 and 3). Microvilli of rhabdoms belonging to non-marginal ommatidia were straight and regular, but in those seemingly immature ommatidia close to the aforementioned margins, on the other hand, the arrangement of the retinula cells was anything but regular, the number of the rhabdomeres varied between 1 and 7, and rhabdom microvilli were neither straight nor aligned (Figs. 4 – 6). Furthermore, the microvilli were not always pointing toward the centre of the rhabdom. The cross sectional areas of the rhabdomeres in the immature ommatidia varied from a few square micrometres to dimensions seen in large and fully mature rhabdomeres.
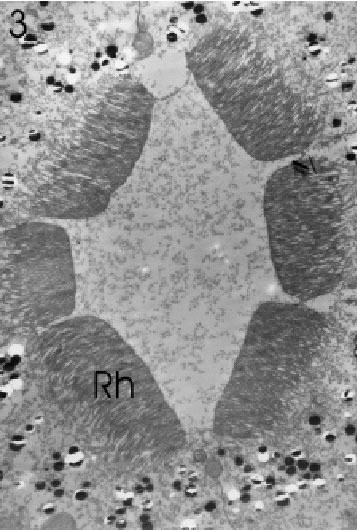
FIGURE 3. Transverse section of rhabdomeres (Rh) of a single central ommatidium at higher magnification, showing orientation of microvilli and arrangement of retinula cells in an Ôopen-typeÕ rhabdom. Magnification X 4,400.
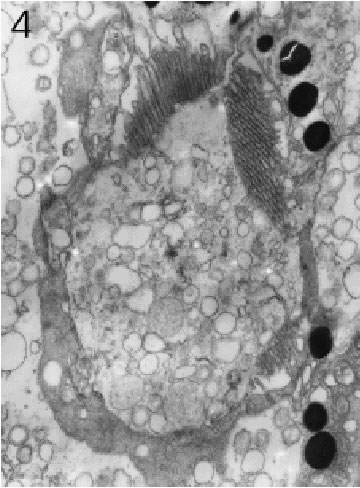
FIGURE 4. Transverse section through a marginal ommatidium from the ventral eye edge, showing that not all rhabdomeres are present and that the rhabdoms do not yet resemble those found in the eyeÕs centre. Magnification X 10,000.
The marginal areas of the eyes caudal and dorsal regions were found to consist of only regularly shaped ommatidia, but as measurements on 14 first and 9 second row dorsal ommatidia showed (Fig. 7), the first row did contain cells of considerably smaller dimensions than the second row (p < 0.008). Comparisons of ommatidia from central, dorsal, and ventral regions in three different individuals of small L. exotica (Fig. 8) showed that significant differences in the widths of the retinal layers (p < 0.016) existed only between central ommatidia (60 – 78 mm) on the one hand and dorsal as well as ventral ommatidia (16 – 24 mm) on the other.
On account of the great variations seen in the retinal widths of both dorsal and ventral ommatidia no significant difference between these two eye regions was detectable (p = 0.76). Regarding crystalline cone diameters, only ventral ommatidia with values of around 25 mm, were statistically different (p < 0.018) from the diameters encountered in central and dorsal ommatidia (33 – 44 mm). At corneal level, facets were found to be regularly shaped hexagons in middle, caudal, and dorsal areas, but not near frontal and ventral margins. Retinula cells and rhabdomeres of aberrantly-shaped, marginal ommatidia never exhibited the same pattern that was characteristic of ommatidia occupying less peripheral rows under their corneal layers.
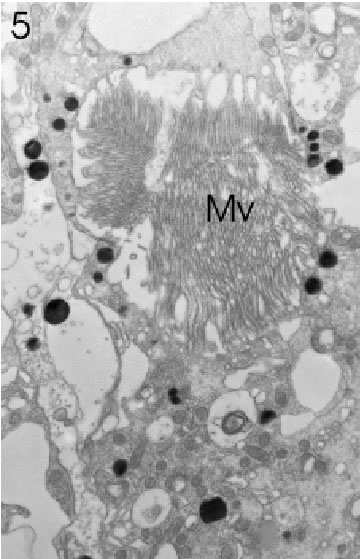
FIGURE 5. Transverse section through a marginal ommatidium from the anterior (frontal) eye edge, showing that not all rhabdomeres are present and that the rhabdom with its microvilli (Mv) has not yet acquired its characteristically open structure. Magnification X 5,000.
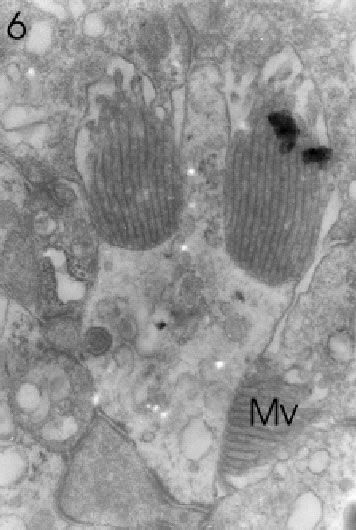
FIGURE 6. Transverse section through a marginal ommatidium from the ventral eye edge, showing a degree of development (only three rhabdomeres with microvilli: Mv) that differs from that of other marginal and, of course, central ommatidia. Magnification X 20,000.
Since the outermost ommatidia in the frontal and ventral regions were found to be most variable in shape and to consist of seemingly immature rhabdomeres with irregularly-arranged microvilli, the compound eye of L. exotica appears to grow either entirely or at least pre-dominantly from the frontal and ventral margins. In these regions the edge of the eye was rather flat and shallow and not round and bulging as in the more dorsal and caudal areas. From longitudinal ultrathin sections in the frontal and ventral areas (Fig. 9) we could determine that in the zone of immature ommatidia the cell nuclei were arranged in three distinct horizontal cell layers and that the proliferation zone at these marginal areas involved several (at least 5) ommatidial rows.
In those areas in which the outermost ommatidia were clearly still immature (like the ventral marginal zone), the ommatidia of the first marginal row had shallower retinal layers than the mature ommatidia further away, i.e., inward, from the edge of the eye. Furthermore, since crystalline cones were either absent or still very small in the most marginal ommatidia, the dioptric apparatus was still in a state of development (Fig. 9). On the other hand, along the dorsal marginal area, as mentioned above, even the outermost ommatidia were actually fully formed, but they were smaller than those of the centre. It may be more accurate to describe the proliferation zone in the eye of L. exotica as crescentic, reaching from dorsal to ventral via the eyes frontal edge.
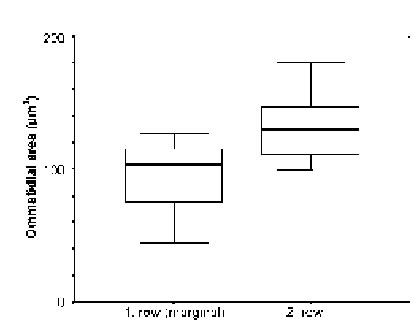
FIGURE 7. Comparison (with widths of standard deviation indicated by horizontal bars) of ommatidial areas (ordinate) between 14 ommatidia from the first and 9 from the second dorsal marginal row (abscissa) in medium-sized (= 2.0 Ð 2.8 cm long) individuals of L. exotica.
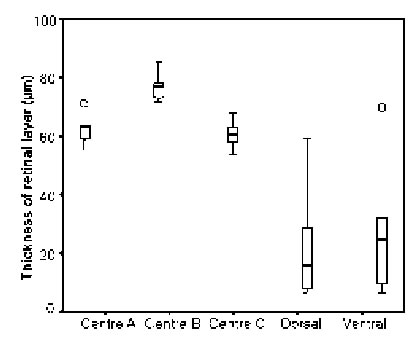
FIGURE 8. Comparison of the widths of the retinal layers in centrally-located three regions 1, 2, and 3), dorsal, and ventral ommatidia of small L. exotica, i.e., animals with body lengths < 1.4 cm. Statistical outliers indicated by circles.
Electron-opaque screening pigment granules were abundant in mature ommatidia, but appeared to remain absent from the retinula cells of maturing ommatidia until the formation of microvilli had begun. Numerous mitochondria occurred in both mature and immature retinal cells, but their density was higher in the immature cells (Fig. 10), as was that of electron-translucent vesicles, measuring up to ten times the size of a mitochondrion. Throughout the ommatidial maturation process, a stretching (elongation) of the ommatidium along its longitudinal axis took place (Fig. 9).
The diameters of the microvilli (n = 38) did not statistically vary in mature rhabdoms of the different eye regions of small individuals (p = 0.131), but in the middle-sized and large specimens, the rhabdoms of the frontal region contained wider microvilli than those in a more caudal, ventral, dorsal, and central position (in that order: cf. Figs. 11 - 13). The differences in both cases were significant (p < 0.0001).
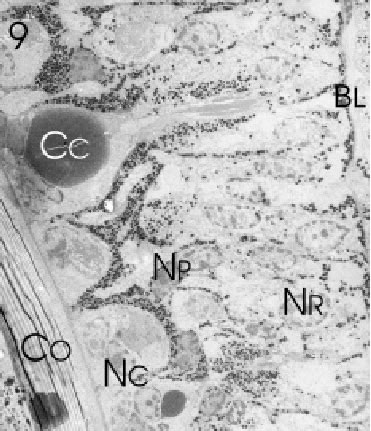
FIGURE 9. Longitudinal section through several marginal rows of immature ommatidia along the fronto-ventral edge of the eye of L. exotica. Retinula (NR )-, crystalline cone (NC ) -, and pigment cell nuclei (NP ) of the differentiating ommatidia occupy different positions in the developing retina above the basal lamina (BL). The direction of differentiation is from the lower immature (NC ) to the upper mature crystalline cones (Cc) underneath the corneal cuticle (Co). Magnification X 700.
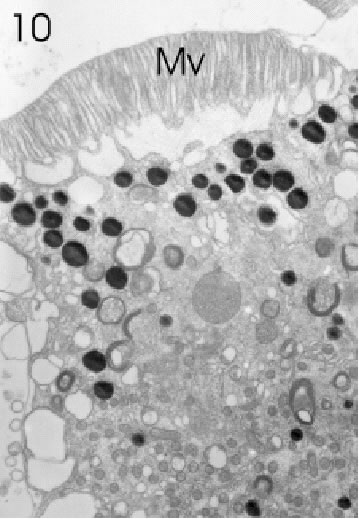
FIGURE 10. Retinula cell of a marginal ommatidium from the anterior (=frontal) eye edge of L. exotica. The cytoplasm of this cell is rich in small mitochondria, screening pigment granules, and a variety of vesicles. Rhabdom microvilli (Mv) are fully developed. Magnification X 5,000.
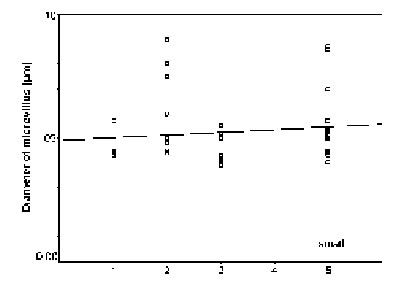
FIGURE 11. Comparison of microvillar diameters (ordinate) in rhabdoms from anterior (1), posterior (2), ventral (3), and central (5) eye regions of small specimens (for definition of ÔsmallÕ see Materials and Methods) of L.exotica. The regression is not significant.
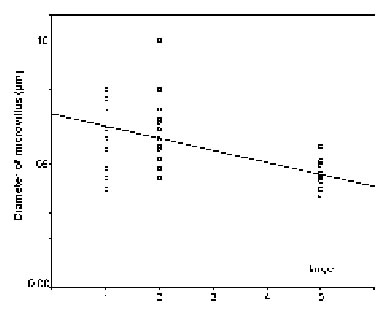
FIGURE 12. Comparison of microvillar diameters (ordinate) in rhabdoms from anterior (1), posterior (2), ventral (3), dorsal (4), and central (5) eye regions of middle-sized specimens (for definition of middle-sized see Materials and Methods) of L. exotica. The regression is significant.

FIGURE 13. Comparison of microvillar diameters (ordinate) in rhabdoms from anterior (1), posterior (2), and central (5) eye regions of large sized specimens (for definition of large see Materials and Methods) of L. exotica. The regression is significant.
Discussion
It is obvious from the comparison of identified proliferation zones in the compound eyes of crustaceans and immature insects (Table I) that places of ommatidial accretion, despite similar mechanisms of neurogenesis (Melzer et al., 2000; Hafner and Tokarski, 2001; Harzsch and Walossek, 2001) vary considerably between taxa. However, it is rare to find examples in which ommatidia are added along all sides of the compound eye: usually a particular, specialized border zone of the eye appears to function meristematically to generate new ommatidia. Within the frame set by phylogeny, the location of this zone appears to depend on head-morphological constraints and eye-functional limitations.
Although frequently the outer appearance, i.e., size and shape of a facet, can provide clues about the internal organization of an ommatidium (we have demonstrated in this paper that aberrantly-shaped facets usually cover an abnormal arrangement of retinula cells), the conclusions one can draw from this relationship are far from unambiguous. Facets in the dorsal eye region, for instance, may be smaller than those of other regions not necessarily because they are maturing and under the influence of the proliferation zone, but because functional constraints demand that they be smaller. In some crustaceans, e.g. midwater euphausiids (Land et al., 1979), not even the relationship between number of corneal lenses and number of underlying ommatidial clusters is constant.
In order to identify the proliferation zone properly, ingenious experiments have been carried out: in Oncopeltus fasciatus, for example, ommatidial transplants from pigmented donor individuals to white-eyed hosts were carried out and the fate of the differently-coloured transplant was monitored from moult to moult (Shelton and Lawrence, 1974). In the water stickinsect Ranatra linearis and nymphs of the dragonfly Aeshna palmata cuts, made into the eye from the outside by Cloarec (1984) and Sherk (1978a, b), respectively, were traced over several moults, so that from the changing positions of these cuts conclusions could be drawn as to which direction the newly added ommatidia took when they were incorporated into the eye. Most commonly, however, marginal ommatidial cell clusters were identified, counted, and compared with other less marginal ones.
Employing this latter technique, we conclude that the eye of L. exotica grows by accretion of new ommatidia, which differentiate along the fronto-ventral margin of the eye. No differences in microvillar diameters of rhabdomeres from the various eye regions were detected in young specimens, but as the animals grew and got older (like in Petrolisthes elongates (Meyer-Rochow and Reid, 1996), a size difference between microvilli of the frontal (larger) and other regions (smaller) became apparent. Although, at first glance, this does not appear to agree with observations made by Roach and Wiersma (1974), who reported that newly added ommatidia in the crayfish eye displayed microvilli of closer and more regular packing, there may no longer be a discrepancy, when it is remembered that our animals, in contrast to the nocturnal crayfish Roach and Wiersma (1974) had worked with, were diurnally active crustaceans. It is well documented that in crustaceans the diameters of rhabdom microvilli frequently vary, depending on day/night activity, rhythmicities, and exposures to light and darkness (Meyer-Rochow, 1999).
It was noticed that in agreement with observations on other developing compound eyes (e.g., Bernard, 1937; Friedrich et al., 1996; Hafner and Tokarski, 2001), initially undifferentiated ommatidia in L. exotica consisted of cell clusters, in which the nuclei of potential retinula cells and crystalline cone cells were arranged in different planes. Major alterations then took place to turn the immature into a mature ommatidium. Whether, as has been suggested by Shelton and Lawrence (1974) and Ready et al. (1976), the differentiated retina provides an inductive influence that organizes unpatterned cells into functional ommatidia or whether the epithelial cells ahead of the growth zone possess an inherent quality to differentiate into cone, retinula, and pigment cells without an inductor (Nowel and Shelton, 1980; Lebovitz and Ready, 1986), remains an unresolved question, although it has to be said that the latter scenario has received more general support (Wolff and Ready, 1993).
What is certain, is that an ommatidium cannot ful-fil its role as an optical device as long as the inner structures of the photoreceptive cell arent organized into perceiving elements (i.e., photoreceptive membranes with visual pigments) and conducting elements (axons with neurotubules and synapses). Retinula cell screening pigments appeared to be absent from a cell as long as microvilli were not yet differentiated, but even after the appearance of the latter, it is questionable whether such cells would have been capable of transmitting information on photic stimulation, because (a) rhabdoms were generally very irregular and (b) synaptic contacts with second order neurons would have had to be established and functional at that stage. However, as with the effects of light-induced damage (Meyer-Rochow and Eguchi, 1986), a connection between degree of microvillar development and screening pigment granule function is clearly apparent, but the nature of this relationship requires further investigation.
The way newly added ommatidia link up with second order neurons and then channel and integrate information into an existing network of fibres, are also questions that need to be tackled in L. exotica. That the larger number of ommatidia and the size increases of the compound eye in older individuals of L. exotica affect their visual behaviour, has been tested (Hariyama et al., 2001; Keskinen et al., 2002). Evidence from newly generated neurons in the brains of fishes, whose eyes also possess proliferation areas and have to accommodate additional photoreceptor cells as they grow (Johns, 1977; Johns and Easter, 1977), suggests that apoptotic events play an important role in ageing, maturation, and repair processes (Zupanc, 1999; Dezawa et al., 2001), but whether we can extend that observation to the eye of crustaceans generally (cf. Harzsch et al., 1999) and to that of L. exotica, in particular, remain important, but as yet unanswered questions.
Acknowledgments
This work was supported in part by a grant-in-aid to T.H. for Scientific Research on Priority Areas (A) (12048204) and a grant to VBM-R from the Oskar Öflunds Stiftelse. EK gratefully acknowledges the support received from the Jenny ja Antti Wihurin Rahasto.
References
BERNARD F (1937). Recherches sur la morphogénèse des yeux composés darthropodes: développement, croissance, reduction. Suppl Bull Biol France Belg 23: 1-166. [ Links ]
CLOAREC A (1984). Development of the compound eyes of the water stick insect Ranatra linearis. Physiol Entomol 9: 253-262. [ Links ]
DEZAWA M, MO X, Oshitari T, TAKANO M, MEYER-ROCHOW VB, SAWADA H, EGUCHI E (2001). Effects of light and darkness on cell deaths in damaged retinal ganglion cells of the carp retina. Acta Neurobiol Exp 61: 85-91. [ Links ]
DOUGLAS JK, FORWARD RB (1989). The ontogeny of facultative superposition optics in a shrimp eye: hatching through metamorpho-sis. Cell Tiss Res 258: 289-399. [ Links ]
EGUCHI E, ARIKAWA K, ISHIBASHI S, SUZUKI T, MEYER-ROCHOW VB (1989). Growth-related biometrical and biochemical studies of the compound eye of the crab, Hemigrapsus sanguineus. Zool Sci 6: 241-250. [ Links ]
ELEY J, SHELTON PMJ (1976). Cell junctions in the developing compound eye of the desret locust Schistocerca gregaria. J Embryol Exp Morph 36: 409-423. [ Links ]
ELOFSSON R (1969). The development of the compound eyes of Penaeus duorarum (Crustacea: Decapoda) with remarks on the nervous system. Z Zellforsch 97: 323-350. [ Links ]
FINCHAM AA (1984). Ontogeny and optics of the eyes of the common prawn Palaemon serratus (Pennant, 1777). Zool J Linn Soc 81: 89-113. [ Links ]
FINCHAM AA (1988). Ontogeny of anomuran eyes. Symp Zool Soc Lond 59: 123-155. [ Links ]
FRIEDRICH M, RAMBOLD I, MELZER RR (1996). The early stages of ommatidial development in the flour beetle Tribolium castaneum (Coleoptera; Tenebrionidae). Dev Genes Evol 206: 136-146. [ Links ]
GATEN E, HERRING PJ (1995). Morphology of the reflecting superposition eyes of larval oplophoroid shrimps. J Morphol 225: 19-29. [ Links ]
HAFNER GS, TOKARSKI TR (1998). Morphogenesis and pattern formation in the retina of the crayfish Procambarus clarkii. Cell Tiss Res 293: 535-550. [ Links ]
HAFNER GS, TOKARSKI TR (2001). Retinal development in the lobster Homarus americanus. Comparisons with compound eyes of insects and other crustaceans. Cell Tiss Res 305: 147-158. [ Links ]
HAFNER GS, TOKARSKI T, HAMMOND-SOLTIS G (1982). Development of crayfish retina: a light and electron microscope study. J Morphol 173: 101-118. [ Links ]
HARIYAMA T, MEYER-ROCHOW VB, EGUCHI E (1986). Diurnal changes in structure and function of the compound eye of Ligia exotica (Crustacea, Isopoda). J Exp Biol 123:1-26. [ Links ]
HARIYAMA T, MEYER-ROCHOW VB, KAWAUCHI T, TAKAKU Y, TSUKAHARA Y (2001). Diurnal changes in retinal cell sensitivi-ties and receptive fields (two-dimensional angular sensitivity function) in the apposition eyes of Ligia exotica (Crustacea, Isopoda). J Exp Biol 204: 239-248. [ Links ]
HARIYAMA T, TSUKAHARA Y, MEYER-ROCHOW VB (1993). Spectral responses, including a UV-sensitive cell type, in the eye of the isopod Ligia exotica. Naturwissenschaften 80: 233-235. [ Links ]
HARZSCH S, BENTON J, DAWIRS RR, BELTZ B (1999). A new look at embryonic development of the visual system in decapod crustaceans: neuropil formation, neurogenesis, and apoptotic cell death. J Neurobiol 39: 294-306. [ Links ]
HARZSCH S, DAWIRS RR (1995). Maturation of the compound eyes and eyestalk ganglia during larval development of the brachyuran crustaceans Hyas araneus L. (Decapoda, Majidae) and Carcinus maenas L. (Decapoda, Portunidae). Zool 99: 189-204. [ Links ]
HARZSCH S, WALOSSEK D (2001). Neurogenesis in the developing visual system of the branchiopod crustacean Triops longicaudatus (LeConte, 1846): corresponding patterns of compound eye formation in Crustacea and Insecta? Dev Genes Evol 211:37-43. [ Links ]
JOHNS PR (1977). Growth of the adult goldfish eye. III. Source of the new retinal cells. J Comp Neurol 176: 343-358. [ Links ]
JOHNS PR, EASTER SS (1977). Growth of the adult goldfish eye. II. Increase in retinal cell number. J Comp Neurol 176: 331-342. [ Links ]
KESKINEN E, TAKAKU Y, MEYER-ROCHOW VB, HARIYAMA T (2002). Postembryonic eye growth in the seashore isopod Ligia exotica (Crustacea, Isopoda). Biol Bull, in press. [ Links ]
LABHART T (1980). Specialized photoreceptors at the dorsal rim of the honeybees compound eye: polarizational and angular sensitiv-ity. J Comp Physiol 141: 19-30. [ Links ]
LAND MF (1981). Optics and vision in invertebrates. In: Handbook of Sensory Physiology, vol. VII/6B. H.Autrum, Ed., Springer, Berlin, New York, pp. 471-592. [ Links ]
LAND MF, BURTON FA, MEYER-ROCHOW VB (1979). The optical geometry of euphausiid eyes. J Comp Physiol 130: 49-62. [ Links ]
LEBOVITZ R, READY DF (1986). Ommatidial development in eye disc fragments. Dev Biol 117: 663-671. [ Links ]
MELZER RR, MICHALKE C, SMOLA U (2000). Walking on insect paths? Early ommatidial development in the compound eye of the ancestral crustacean, Triops cancriformis. Naturwissenschaften 87: 308-311. [ Links ]
MEYER-ROCHOW VB (1975). Larval and adult eye of the Western rock lobster Panulirus longipes. Cell Tiss Res 162: 439-457. [ Links ]
MEYER-ROCHOW VB (1982). The divided eye of the isopod Glyptonotus antarcticus: effects of unilateral dark adaptation and tempera-ture elevation. Proc Roy Soc Lond B 215: 433-450. [ Links ]
MEYER-ROCHOW VB (1999). Compound eye: circadian rhythmicity, illumination, and obscurity. In: Atlas of Arthropod Sensory Re-ceptors. E.Eguchi and Y.Tominaga, Eds., Springer, Tokyo, New York, pp. 97-124. [ Links ]
MEYER-ROCHOW VB, EGUCHI E (1986). Do disintegrating microvilli in the eye of the crayfish Procambarus clarkii contribute to the synthesis of screening pigment granules? Z Mikrosk Anat Forsch 100: 39-55. [ Links ]
MEYER-ROCHOW VB, REID WA (1996). Does age matter in studying the crustacean eye? J Comp Physiol B 166: 319-324. [ Links ]
MEYER-ROCHOW VB, TOWERS D, ZIEDINS I (1990). Growth patterns in the eye of the halfcrab Petrolisthes elongates Milne-Edwards (Crustacea; Decapoda; Anomura). Exp Biol 48: 329-340. [ Links ]
NARDI JB (1977). The construction of the insect compound eye: the involvement of cell displacement and cell surface properties in the positioning of cells. Dev Biol 61: 287-298. [ Links ]
NILSSON DE, HALLBERG E, ELOFSSON R (1986). The ontogenetic development of refracting superposition eyes in crustaceans: transformation of optical design. Tissue Cell 18: 509-519 [ Links ]
NOWEL MS, SHELTON PMJ (1980). The eye margin and compound eye development in the cockroach: Evidence against recruitment. J Embryol Exp Morph 60: 329-343. [ Links ]
PEABODY EB (1939). Development of the eye of the isopod Idothea. J Morph 64: 519-553. [ Links ]
READY DF, HANSON TE, BENZER S (1976). Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol 53: 217-240. [ Links ]
ROACH JLM, WIERSMA CAG (1974). Differentiation and degeneration of crayfish photoreceptors in darkness. Cell Tiss Res 153: 137-144. [ Links ]
SAKAMOTO K, SEKI T, KOSEKI S, KASHIWAGI T, EGUCHI E (1998). Comparative studies on compound eyes of larvae and adults of an aeschnid dragonfly, Anax nigrofasciatus nigrofasciatus (I) Dorsal part. Int J Odonatol 1: 15-31. [ Links ]
SHELTON PMJ, LAWRENCE PA (1974). Structure and development of ommatidia in Oncopeltus fasciatus. J Embryol Exp Morph 32: 337-353. [ Links ]
SHELTON PMJ, SHELTON RGJ, RICHARDS (1981). Eye development in relation to moult stage in the European lobster Homarus gammarus (L.). J Cons Int Explor Mer 39: 239-243. [ Links ]
SHERK TE (1978a). Development of the compound eyes of dragonflies (Odonata). II. Development of the larval compound eyes. J Exp Zool 203: 47-60. [ Links ]
SHERK TE (1978b). Development of the compound eyes of dragonflies (Odonata). IV. Development of the adult compound eyes. J Exp Zool 203: 183-200. [ Links ]
WARRANT EJ, McINTYRE PD (1993). Arthropod eye design and the physical limits to spatial resolving power. Progr in Neurobiol 40: 413-461. [ Links ]
WHITE RH (1963). Evidence for the existence of a differentiation center in the developing eye of the mosquito. J Exp Zool 152: 139-147. [ Links ]
WOLFF T, READY DF (1993). Pattern formation in the Drosophila retina. Cold Spring Harbor Lab Press, New York, pp. 1277-1325. [ Links ]
ZUPANC GK (1999). Neurogenesis, cell death, and regeneration in the adult gymnotiform brain. J Exp Biol 202: 1435-1446. [ Links ]
Received on May 21, 2002.
Accepted on September 9, 2002.














