Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell v.27 n.1 Mendoza ene./abr. 2003
Distribution of pectins in the pollen apertures of Oenothera hookeri.velans ster/+ster:
I. Noher de Halac1, 2, I.A. Cismondi2, M.I. Rodriguez-Garcia3, G. Famá
1. Centro de Estudio de las Metabolapatías Congénitas (CEMECO), Cátedra de Clínica Pediátrica, Facultad de Ciencias Médicas, Universidad Nacional de Córdoba. 5000 Córdoba, Argentina. Member of the research career of the Argentinean Science Council (CONICET).
2. Cátedra de Biología Celular, Facultad de Odontología, Universidad Nacional de Córdoba. 5000 Córdoba, Argentina.
3. Estación Experimental del Zaidín, CSIC, Profesor Albareda 1, Granada, Spain.
Address correspondence to: Dra. Inés Noher de Halac. CEMECO, Cátedra de Clínica Pediátrica, Facultad de Ciencias Médicas, Universidad Nacional de Córdoba. Ferroviarios 1250, (5000) Córdoba, ARGENTINA. Fax: (+54-351) 468 3282. E-mail: rhalac@arnet.com.ar
Key words: pectins, monoclonal antibodies, JIM5, JIM7, immunogold, pollen, sporoderm, Oenothera, cytochemistry.
ABSTRACT: Cell wall pectins are some of the most complex biopolymers known, and yet their functions remain largely mysterious. The aim of this paper was to deepen the study of the spatial pattern of pectin distribution in the aperture of Oenothera hookeri.velans ster/+ster fertile pollen. We used "in situ" immunocytochemical techniques at electron microscopy, involving monoclonal antibodies JIM5 and JIM7 directed against pectin epitopes in fertile pollen grains of Oenothera hookeri.velans ster/+ster. The same region was also analyzed by classical cytochemistry for polysaccharide detection. Immunogold labelling at the JIM7 epitope showed only in mature pollen labelling mainly located at the intine endo-aperture region. Cytoplasmic structures near the plasma membrane of the vegetative cell showed no labelling gold grains. In the same pollen stage the labelling at the JIM5 epitope was mostly confined to a layer located in the limit between the endexine and the ektexine at the level of the border of the oncus. Some tubuli at the base of the ektexine showed also an accumulation of gold particles. No JIM5 label was demonstrated in the aperture chamber and either in any cytoplasmic structure of the pollen grains. The immunocytochemical technique, when compared with the traditional methods for non- cellulose polysaccharide cytochemistry is fare more sensitive and allows the univocal determination of temporal and spatial location of pectins recognized by the JIM7 and JIM5 MAbs.
Introduction
Cell wall pectins are some of the most complex biopolymers known (Fry, 1986; Jarvis, 1984; Schols et al., 1995; Geitmann et al., 1995; Albersheim et al., 1996; Willats et al., 2001). Pectin is a family of complex polysaccharides present in all plant primary cell walls. The complicated structure of pectic polysaccharides, and the retention by plants of the large number of genes required to synthesize pectin, suggests that pectins have multiple functions in plant growth and development (Ridley et al., 2001). They play important roles in cell wall hydration, adhesion of adjacent cells, wall plasticity during growth and recognition reactions between plant cells and bacterial and fungal pathogens. In pollen grains and pollen tubes the involvement of molecules like pectin and other non cellulose polysaccharides and glycoproteins in phenomena like compatibility and incompatibility reactions (Geitmann et al., 1995) and pollen tube growth (Li et al., 1994), are particularly relevant. The role of pectin esterification and the ability to form gels remain unclear in terms of pectin function within plant cell walls. The de-methylesterification of homogalacturonan by pectin methyl esterases is emerging as a key process for the local modulation of matrix properties. Polyanionic galacturonan backbones are capable of binding calcium, resulting in their aggregation and the formation of gel. The methylesterification of the carboxyl groups prevents the formation of calcium bridges.
In Oenothera species, the peculiarities of the normal sporoderm can be described with the concepts and terminology proposed by Skvarla et al (1976) for the Onagraceae family. The exine consists of two fundamental units, the ektexine and the endexine. The apertures comprise three parts: a) the basal endo-aperture (oncus) composed by the thickened intine at the inside face and sporopollenin materials at its external face; b) the external pore formed by the ektexine layer; c) the apertural chamber, limited on the external face by the ektexine layer and a gradually fainting endexine. The intine layer was assumed to protrude through the pore as the progenitor layer of the pollen tube wall (Suarez-Cervera et al., 2002).The typical viscin threads of Oenothera species are located in the interapertural region of the pollen grains (Noher de Halac and Cismondi, 1994).
The stratigraphy of the pollen wall was traditionally demonstrated by cytochemical tests, based on the affinity of the exine and the intine for dyes binding different kinds of biomolecules like lignin, phenol compounds, lipids and negatively charged groups, polysaccharides, acidic polyanions, and proteins (Erdtman, 1960; Knox and Heslop-Harrison, 1970; Southworth, 1974; Carpita and Gibeaut, 1993; review: Knox, 1984).
Current advances in imaging methods allow direct visualization of the molecular architecture of cell walls and the modifications that occur to the polymers during growth and development (McCann et al., 2001). Immunolabelling with well-defined antibodies demonstrated and characterized the adaptations of methyl esterified pectin in cell walls, in pollen, and during pollen tube interactions with the style tissues. The monoclonal antibodies (MAbs) JIM7 and JIM5 recognize two different pectin epitopes. They have been used for indirect immunofluorescence and immunogold electron microscopy studies in a broad variety of plant tissues and plant species (Knox et al., 1990; Van Aelst and Van Went, 1992; Li et al., 1994; Geitmann et al., 1995; Golaszweska and Bednarska, 1999; Stepka et al., 2000; Lenartowska et al., 2001; Aouali et al., 2001; Suarez-Cervera et al., 2002).
Oenothera hookeri. velans ster/+ster is a laboratory hybrid line obtained by Cornelia Harte in Cologne, Germany. This hybrid was studied morphologically, developmentally and genetically by our group (Noher de Halac et al., 1990; Noher de Halac et al., 1992; Harte and Noher de Halac, 1994; Noher de Halac and Cismondi, 1994; Noher de Halac and Harte, 1994, 1995; Noher de Halac et al., 1999). In the present work we study the spatial pattern of pectins and other polysaccharides in the aperture of Oenothera hookeri.velans ster/+ster fertile pollen using the "in situ" immunolabelling technique at electron microscopy level, with JIM7 and JIM5 MAbs as markers. Several kinds of polysaccharides were also studied in other pollen samples of the same hybrid, using the classical cytochemical methods for light microscopy, which were adapted to react on semithin sections of resin embedded pollen.
Material and methods
Plant material:
The fertile mature pollen of Oenothera hookeri.velans ster/+ster was obtained from plants grown at the green houses of the Plant Morphology and Cytology Department of the Agricultural University Wageningen, The Netherlands. The seeds were provided by Cornelia Harte (University Cologne, Germany), who obtained the genetic lines of this hybrid.
Electron Microscopy:
Mature pollen was fixed in 2.5% glutaraldehyde, 3% paraformaldehyde in 0.05-M phosphate buffer, pH 7.2 for 4 h at room temperature. Then the samples were washed in the same buffer and after that, they were fixed in osmium tetroxide (1% in distilled water) for 1 h at room temperature. The dehydration was in acetone and the embeddment was in Durcupan (Fluka). Ultrathin sections were cut using a Sorvall MT1 ultramicrotome and were collected on 300 mesh copper grids. The specimens were stained with lead citrate (Venable and Coggeshall, 1965) and saturated uranyl acetate. The observation of the samples was done using a Philips 300 Electron Microscope at 60 kV, and the photomicrographs were obtained on 6x9cm Kodak 4486-electron image plates.
Polysaccharide cytochemistry:
The cytochemical methods were carried out on semithin sections (Noher de Halac et al., 1990, 1992; Noher de Halac and Harte, 1994). The embedding resin (Durcupan, Fluka) was previously removed with sodium ethoxide for 5 min (Lane and Europa, 1965). The PAS reaction was used to detect polysaccharides with vicinal glycol hydroxyl groups (O'Brien and Mc Cully, 1981). The alcian blue 8 GX reaction stained acid polysaccharides blue (modified method of Spanhof by Heslop-Harrison, 1979). The ruthenium red method (Jensen, 1962) stained pectic acids red after de- esterification (Sterling, 1970). The toluidine blue stain was used as a general stain for morphological observation (Feder and O'Brien, 1968) and it allowed the demonstration of acidic polyanionic groups, blue; lignin and phenol compounds, green; acidic polysaccharides like pectic acid, red or purple metachromasia (review: Knox, 1984). The photographs were obtained using a Zeiss Standard 14 microscope with a M60 camera on Kodak Gold 100 Asa 35mm film.
Immunogold labelling for electron microscopy:
The fixation was in 0.25% glutaraldehyde, 3% paraformaldehyde, 1mM mannitol, in 0.025-M caccodylate buffer at pH 7.2 for 2 h at room temperature. The dehydration was in ethanol and the embeddment was in LR White resin medium grade (Sigma). The ultrathin sections were collected on formvar coated nickel grids, incubated in a solution containing 1% bovine serum albumin, 0.01 M PBS pH 7.4 and 0.1M Tween 20 for 10 min for pre-absorption of unspecific binding. The immunolabelling was with the MAbs JIM5 and JIM7 provided by Dr. K.Roberts, John Innes Center of the UK, following the method of Lenartowska et al. (2001).
The MAbs were diluted 1:20 in PBS and the incubation was at 37oC for 60 min. After several washes in PBS the incubation with the gold-labeled second antibody was in goat-anti-rabbit IgG coupled with 10nm colloidal gold (BioCell) for 60 min at 37oC (dilution 1:20 in PBS). The sections were then washed, dried and stained with uranyl acetate (2% in water). Control sections were treated as described, but omitting the primary antibody. The samples were observed with a Zeiss EM 10C transmission electron microscope at 60 kV.
Results
The morphological structure and the staining characteristics of the sporoderm were: a) Configuration in three layers: the endexine, the ektexine and the intine; b) The endexine was smooth looking with electron microscopy (Figs. 4-8) being composed of sporopollenin and other materials reacting greenish with toluidine blue (Fig. 3) and not changing color with ruthenium red (Fig. 2), nor with alcian blue (Fig. 1); c) The ektexine is a delicate network of branching sporopollenin rods, with the same staining properties than the endexine (Figs. 1-3). It has a faintly paracrystalline-beaded appearance at electron microscopy level (Figs. 4-8). d) The intine borders the inner side of the endexine (Figs. 1-3 and was stained with ruthenium red that revealed pectins (Fig. 2). The alcian blue and the toluidine blue reactivity allowed not a clearly delimitation of the staining properties of the intine that appeared undistinguishable Figs.6-8. Transmission electron microscopy of mature pollen grains labelled with the monoclonal antibody JIM5. Sections pass trough the oncus in the site marked with an arrow in Fig. 2.
Figs.1-3. Semithin sections of anthers treated with cytochemical reactions for polysaccharide material:

FIGURE 1. Vacuolated microspore stained with alcian blue. The oncus (arrowhead), and the ektexine are stained blue, while the endexine remains unstained (arrow). The intine is absent.

FIGURE 2. Vacuolated pollen stained with ruthenium red. The intine is stained red, demonstrating pectins at the interapertural region and in the oncus (arrow head). The exine layers, ektexine and endexine (arrow), remain unstained. Notice red staining at the anther walls and at the remnants of the secretory tapetum.

FIGURE 3. Vacuolated pollen stained with toluidine blue. The intine and the cytoplasm of the vegetative cell are not distinguishable, showing both purple metachromasia. The large arrowhead points the generative nucleus. The small arrowhead points the oncus showing purple metachromasia that indicates polysaccharide material. The thin arrow points the ektexine. The exine layers, ektexine and endexine, show greenish colour denoting sporopollenin materials.
Abbreviations: ac, apertural chamber; e, exine; en, edothecium; ep, epidermis; in, intine; n, nucleus, p, parietal layers; t, tapetum; v, vacuole; vn, vegetative nucleus. Magnification: 800X.
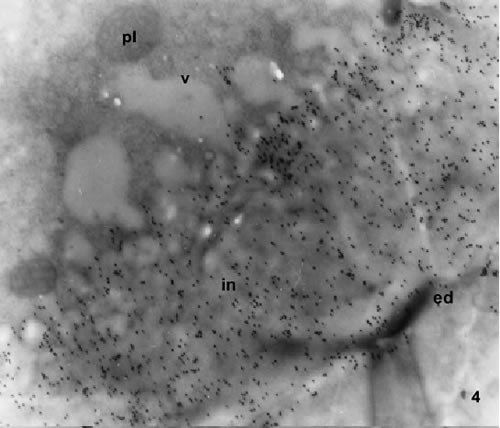

FIGURES 4-5. Transmission electron microscopy of mature pollen grains labelled with the monoclonal antibody JIM 7. Sections pass through the oncus in the site marked with an arrow in Fig 2. The gold particles are homogeneously distributed on the intine. Gold is more abundant in the specimen shown in Fig. 4 than in the specimen of Fig. 5, which is a mature pollen grain. The lipid electron dense material is accumulated at the outer limit of the intine (arrows). The vacuoles are free of gold particles, the same as any other cytoplasm components.
Abbreviations: ed, endexine; in, intine; lipids; pl, plastid; v, vacuole. Magnification: 4, X 8000; 5, X 10000.

FIGURE 6. The gold particles are accumulated at the internal limit of the ektexine and on the tubular structures of the base of the ektexine (arrows). Few gold particles are distributed on the underlining endexine and intine.

FIGURE 7. The gold particles are accumulated at the internal limit of the ektexine (arrow). The endexine, the intine, the vacuoles and the other cytoplasm structures show no gold particles.

FIGURE 8. The gold particles are accumulated at the base of the ektexine and on the external limit of the endexine (arrow). Scarce gold particles are distributed on the intine from the adjacent cytoplasm (Figs. 1-2). At electron microscopy the intine at the aperture region showed reticular structure (Figs. 4-8). Abbreviations: ed, endexine, ek, ektexine; in, intine; v, vacuole. Magnification: 6, X 10000; 7,X8000; 8, X8000
The triaperturate pollen grains showed in each aperture, a basal endo-aperture (oncus), an external pore formed by the ektexine and, a prominent apertural chamber. The endo-aperture had coarsely lamellated and tubular endexine material with the staining properties of sporopollenin (greenish with toluidin blue and not stained through the other reactions). The reactivity to polysaccharide cytochemistry of the endo-aperture was positive in all the cytochemical tests applied for polysaccharides (Figs. 1-3 and Table 1).

The vacuolated microspore showed the aperture region with few gold particles with the JIM7 MAbs, considered to be background, and no label with the JIM5 MAbs (data not shown). At the vacuolated pollen stage the immunogold labelling of the JIM7 epitope (Figs. 4-5, Table 1) was not present at the base of apertures. Later, in mature pollen the mark with JIM7 mainly concentrated at the thickened intine with less mark on the endexine of the oncus. The cytoplasmic vesicles near the plasma membrane of the vegetative cell showed no labelling gold grains at this stage (Figs. 4-5). The labelling at the JIM5 epitope was absent in the young pollen stage (Table 1). At the mature pollen stage, the JIM5 MAb marked the endo-aperture, being the gold particles confined to the limit of the outer and the inner part of the sporopollenin layer (ektexine + endexine in Table 1). (Figs.6-8). The intine layer showed few gold particles, being the label considered dubious in Table 1. Some tubuli situated in the base of the ektexine had a remarkable accumulation of gold particles (Fig. 6). No JIM5 label was detected inside the aperture chamber and in any cytoplasmic structure of the pollen grain.
Discussion
The interpretation of the cytochemical results (Table 1) was done according to the review of Knox (1984). The ultrastructure and the general cytochemistry of the sporoderm layers in the pollen during development were described in earlier papers published by our group (Noher de Halac and Cismondi, 1994; Noher de Halac and Harte, 1994; Noher de Halac et al., 1990, 1992, 1999). The sporoderm layers of pollen grains are chemically, morphologically, developmentally, and genetically distinct (Knox, 1984; Heslop-Harrison, 1975).
The exine is made of sporopollenin- a wall polymer remarkable for its resistance to biodegradation. Shaw (1971) and Brooks and Shaw (1978) considered sporopollenin to be formed by the oxidative polymerization of carotenoids and carotenoid esters and Prahl et al. (1985) suggested other complex polymers. The intine was considered to be the pectocellulosic cell wall of the vegetative cell with highly specialized functions at the germinal apertures, and later on during pollen tube growth. The intine structure was assumed to consist of a rigid skeleton of cellulose microfibrils and a gel-like matrix built up of pectin, different kinds of non-cellulose polysaccharides, and glycoproteins (Fry, 1986). Our results showed that the alcian blue reaction (Heslop-Harrison, 1979), as the marker of acidic polysaccharides (including pectins), and the ruthenium red reaction, as the marker of un-sterified pectin acid (Sterling, 1970), were clearly positive at the oncus. The detected compounds were assumed to impregnate the cellulosic compartment of the intine layer and the sporopollenin materials at the endo-aperture in mature pollen. The PAS reactivity, and the toluidine blue metachromasia, typical for acidic polysaccharides, showed not so clear results, possibly due to the fact that the reactivity of the intine was indistinguishable of that of the undelying cytoplasm.
The JIM5 and JIM7 MAbs, directed against the respective pectin epitopes were assumed to supply a qualitative picture of the methyl-esterification of pectins at electron microscopy level (Willats et al., 2000), a fact with relevant functional implication. The JIM7 antibody recognized pectins with a high level of esterification (ranging from 35% to 90%); meanwhile the JIM5 antibody, specific for an acidic pectin epitope, reacts with pectin with only up to 50% esterification (Knox et al., 1990). Nowadays it is well established that the epitopes recognized by the JIM7 antibody belong to polymers of galacturonic acid with a methyl-esterification range of about 15 to 80% (regardless of whether esterification displays a random or blockwise pattern of distribution). The anti-pectin antibody JIM5 binds more efficiently polymers with a degree of esterification between 31% and slightly over 40% of methyl-esterification (Willats et al., 2000). On the other side, in other species the labeling properties of the JIM7 and JIM5 epitopes at the base of the aperture chambers during pollen tube emission (Figs. 4-8, Table 1) have been related to the rol of pectins in the hydration process preceding pollen germination (Suarez-Cervera et al., 2002). The labeled pectins have been assumed to play also a rol in the plasticity and elasticity needs of the apical cell wall during pollen tube growth (Li et al., 1994).
Concluding, the immunocytochemical technique, when compared with the traditional methods for non-cellulose polysaccharide cytochemistry, was fare more sensitive and allowed the definition of some temporal and spatial properties of the labeled macromolecules. The thickening intinous endo-aperture of Oenothera was recognized as homogeneously impregnated with pectins with a relatively higher degree of methyl-esterification (binding to JIM7 MAbs) only in mature pollen. The lower methyl-esterified pectins (binding to JIM5 MAbs) showed, at the same stage a more restricted distribution pattern, forming a kind of inter-phase layer inside the sporopollenin/ polysaccharide endo-aperture structure.
Acknowledgements
We dedicate the present work to the memory of Professor Dr. Cornelia Harte who devoted great efforts of her scientific work in Germany to Oenothera as a model plant for genetics and development. She obtained the hybrids used in the present work and she encouraged and oriented our study. We thank Prof. Dr. M.T.M. Willemse for the culture of our plants at the green house of the Department of Plant Cytology and Morphology of the Agricultural University Wageningen, The Netherlands. We are grateful for the grant of the Research Council of Argentina (CONICET). The study was also supported by project no. PGC BMC 2000-1484 from the Spanish Ministerio de Ciencia y Tecnología. We acknowledge Prof. Dr. Raquel Dodelson de Kremer from the National University Córdoba, Argentina for the facilities used in her Laboratory.
References
1. Albersheim P, Darvill AG, O'Neill MA, Schols HA, Voragen AGJ (1996). A hypothesis: the same six polysaccharides are components of the primary cell walls of all higher plants. In: Pectins and Pectinases. J. Visser and A.G.J. Voragen, Eds. Elsevier Science BV, Amsterdam, pp. 47-55. [ Links ]
2. Aouali N, Laporte P, Clement C (2001). Pectin secretion and distribution in the anther during pollen development in Lilium. Planta 213: 71-79. [ Links ]
3. Brooks J, Shaw G (1978). Sporopollenin: A review of its chemistry, palaeochemistry, and geochemisry. Grana 17: 91-97. [ Links ]
4. Carpita NC, Gibeaut DM (1993). Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the wall during growth. Plant J. 3: 1-30. [ Links ]
5. Erdtman G (1960). The acetolysis method. A revised description. Sven Bot Tidskr 54: 531-564. [ Links ]
6. Feder N, O'Brien TP (1968). Plant microtechnique. Some principles and new methods. Amer J Bot 55: 123-142. [ Links ]
7. Fry SC (1986). Cross-linking of matrix polymers in the growing cell walls of angiosperms. Ann Rev Plant Physiol 37: 165-186. [ Links ]
8. Geitmann A, Hudák J, Vennigerholz F, Walles B (1995). Immunogold localization of pectin and callose in pollen grains and pollen tubes of Brugmansia suaveolens. Implications for the self-incompatibility reaction. J Plant Physiol 147: 225-235. [ Links ]
9. Golaszweska B, Bednarska E (1999). Immunocytochemical localization of pectins in the maturing anther of Allium cepa L. Folia Histochem Cytobiol 37: 199-208. [ Links ]
10. Harte C, Noher de Halac I (1994). Gene-dependent male sterility and plastomes in Oenothera. Theor Appl Genet 88: 249-254. [ Links ]
11. Heslop-Harrison J (1975). Pysiology of the pollen grain surface. Proc R Soc Lond B Biol Sci. 190: 275-300. [ Links ]
12. Heslop-Harrison J (1979). Aspectos of the structure, cytochemistry and germination of the pollen of Rye (Secale cereale L). Ann Bot 44: 1-47. [ Links ]
13. Jarvis MC (1984). Structure and properties of pectin gels in plant cell walls. Plant Cell Environ 7: 153-164. [ Links ]
14. Jensen WA (1962). Botanical histochemistry. Freeman, San Francisco. [ Links ]
15. Knox RB, Heslop-Harrison J (1970). Pollen-wall proteins: localization and enzymatic activity. J Cell Sci. 6: 1-27. [ Links ]
16. Knox JP, Linstead PJ, King J, Cooper C, Roberts K (1990). Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 181: 512-521. [ Links ]
17. Knox RB (1984). The pollen grain. In: Embryology of Angiosperms. B.M. Johri, Ed. Springer Verlag, Berlin Heidelberg New York Tokyo, pp. 197-271. [ Links ]
18. Lane BP, Europa DL (1965). Differential staining of ultrathin sections of epon embedded tissues for light microscopy. J Histochem Cytochem 13: 579-582. [ Links ]
19. Lenartowska M, Rodriguez-Garcia MI, Bednarska E (2001). Immunocytochemical localization of sterified and un-sterified pectins in unpollinated and pollinated styles of Petunia hybrida Hort. Planta 213: 182-191. [ Links ]
20. Li YQ, Chen F, Linskens HF, Cresti M (1994). Distribution of unesterified and esterified pectins in cell walls of pollen tubes of flowering plants. Sex Plant Reprod 7: 145-152. [ Links ]
21. McCann MC, Bush M, Milioni D, Sado P, Stacey NJ, Catchpole G, Defernez M, Carpita NC, Hofte H, Ulvskov P, Wilson RH, Roberts K (2001). Approaches to understanding the functional architecture of the plant cell wall. Phytochemistry 57: 811-821. [ Links ]
22. Noher de Halac I, Cismondi IA (1994). Gametophyte ontogeny an overview based on Oenothera. In: Growth patterns in Vascular Plants. M. Iqbal, Ed. Portland, Oregon, Dioscorides Press, pp. 344-371. [ Links ]
23. Noher de Halac I, Cismondi IA, Famá G, Harte C (1999). Lipid histochemistry and fine structure of microspores and tapetal cells in the male sterility phenotype of the hybrid Oenothera h hookeri. Velans. Biocell 23: 1-12. [ Links ]
24. Noher de Halac I, Cismondi IA, Harte C (1990). Pollen ontogenesis in Oenothera: a comparison of genotypically normal anthers with the male-sterile mutant sterilis. Sex Plant Reprod 3: 41-53. [ Links ]
25. Noher de Halac I, Famá G, Cismondi IA (1992). Changes in lipids and polysaccharides during pollen ontogeny in Oenothera anthers. Sex Plant Reprod 5: 110-116. [ Links ]
26. Noher de Halac I, Harte C (1994). Histochemical analysis of male sterile anthers of the genotype fr/fr in progenies of the hybrid Oenothera hookeri De Vries x strigosa De Vries. Biocell 18: 31-41. [ Links ]
27. Noher de Halac I, Harte C (1995). Genetics and development of morphological and physiological characters of male sterility in Oenothera. Protoplasma 187: 22-30. [ Links ]
28. O'Brien JP, Mc Cully ME (1981). The study of plant structure. Principles and selected methods. Termacharpi PTY Ltd. Melbourne. [ Links ]
29. Prahl AK, Springstubbe G, Wiermann R (1985). Studies on sporopollenin biosynthesis: the effect of inhibitors of carotenoid biosynthesis: the effect of inhibitors of carotenoid biosynthesis on sporopollenin accumulation. Z Naturf 40: 621-626. [ Links ]
30. Ridley BL, O'Neill MA, Mohnen D (2001). Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 57: 929-967. [ Links ]
31. Schols HA, Bakx EJ, Schipper D, Voragen AGJ (1995). A xylogalacturonan subunit present in the modified hairy regions of apple pectin. Carbohydr Res 279: 265-279. [ Links ]
32. Shaw G (1971). The chemistry of sporopollenin. In: Sporopollenin. J. Broks, P.R. Grant, M. Muir, P. van Gijse and G. Shaw, Eds. Academic Press, London New York, pp. 305-350. [ Links ]
33. Skvarla JJ, Raven PH, Praglowski J (1976). Ultrastructural survey of Onagraceae pollen. In: The Evolutionary Significance of the Exine. I.K. Ferguson IK and J. Muller, Eds. London: Linnean Soc. pp. 447-479. [ Links ]
34. Southworth D (1974). Solubility of pollen exines. Am J Bot 61: 36-44. [ Links ]
35. Suarez-Cervera M, Arcalís E, Le Thomas A, Seoane-Camba J (2002). Pectin distribution pattern in the apertural intine of Euphorbia peplus L. (Euphorbiaceae) pollen. Sex Plant Reprod 14: 291-298. [ Links ]
36. Stepka M, Ciampolini F, Charzynska M, Cresti M (2000). Localization of pectins in the pollen tube wall of Ornithogalum virens L. Does the pattern of pectin distribution depends on the growth rate of the pollen tube?. Planta 210: 630-635. [ Links ]
37. Sterling C (1970). Crystal-stucture of ruthenium red and stereochemistry of its pectic stain. Amer J Bot 57: 172-175. [ Links ]
38. Thiéry JP (1967). Mise en évidence des polysaccharides sur coupes fines en microscopie électronique. J Microsc 6: 987-1018. [ Links ]
39. Van Aelst AC, Van Went JL (1992). Ultrastructural immuno-localization of pectins and glycoproteins in Arabidopsis thaliana pollen grains. Protoplasma 168: 14-19. [ Links ]
40. Vanderbosh KA, Bradley DJ, Knox JP, Perotto S, Butcher GW, Brewin NJ (1989). Common components of the infection thread matrix and the intercellular space identified by immunocytchemical analysis of pea nodules and uninfected roots. EMBO J. 8: 335-342. [ Links ]
41. Venable JH, Coggeshall R (1965). A simplified lead citrate stain for use in electron microscopy. J Cell Biol 25: 407-408. [ Links ]
42. Willats WG, Limberg G, Buchholt HC, van Alebeek GJ, Benen J, Christensen TM, Visser J, Voragen A, Mikkelsen JD, Knox JP (2000). Analysis of pectic epitopes recognised by hybridoma and phage display monoclonal antibodies using defined oligosaccharides, polysaccharides, and enzymatic degradation. Carbohydr Res 327: 309-320. [ Links ]
43. Willats WG, McCartney L, Mackie W, Knox JP (2001). Pectin: cell biology and prospects for functional analysis. Plant Mol Biol 47: 9-27. [ Links ]
Received on April 24, 2002.
Accepted on December 2, 2002.














